Clustered Occurrence of Osteitis Condensans Ilii in Patients with Symptomatic Hip Dysplasia
Abstract
1. Introduction
2. Methods
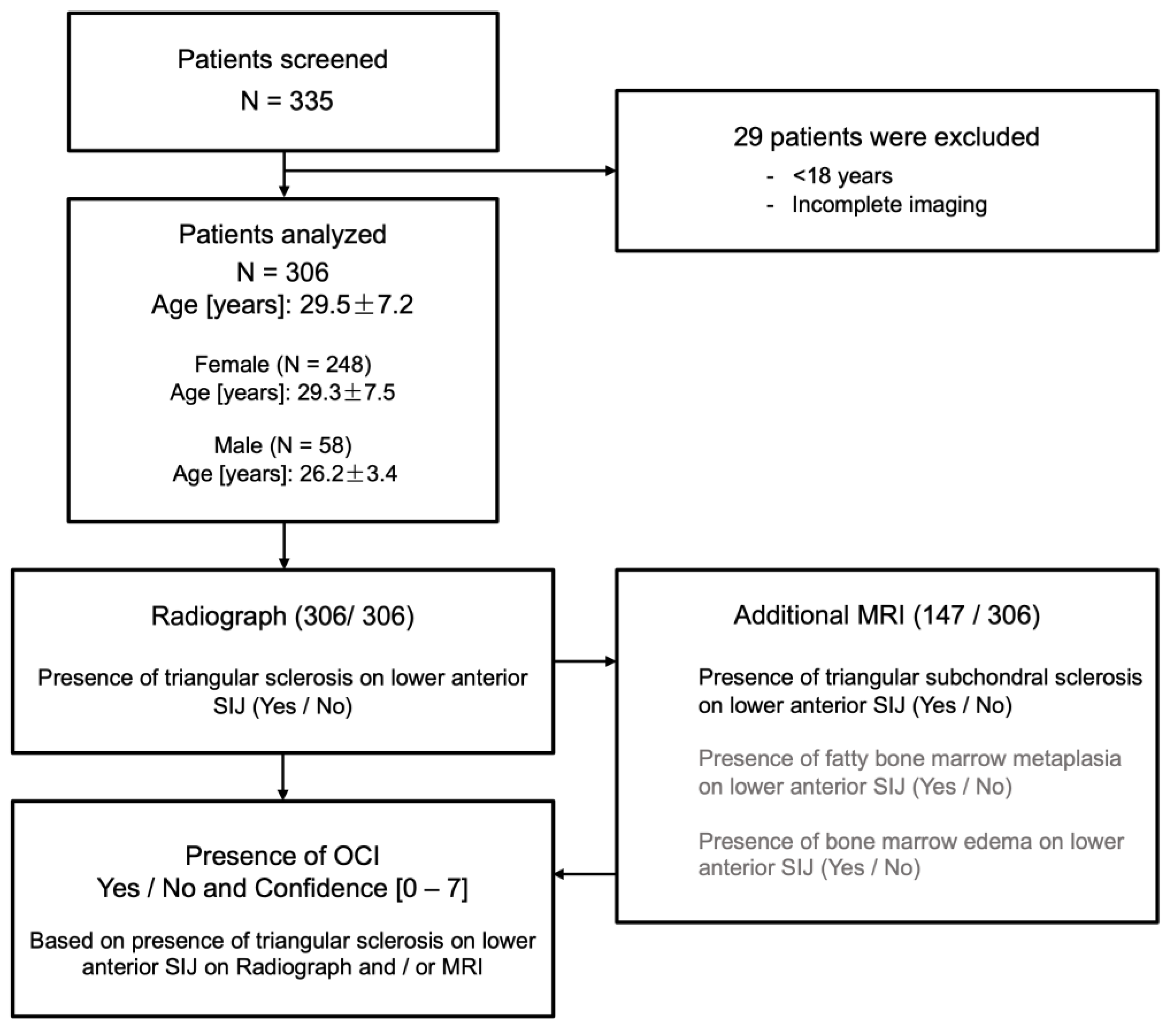
2.1. Subjects
2.2. Radiographic Assessment
2.3. Statistical Analysis
3. Results
3.1. Patients
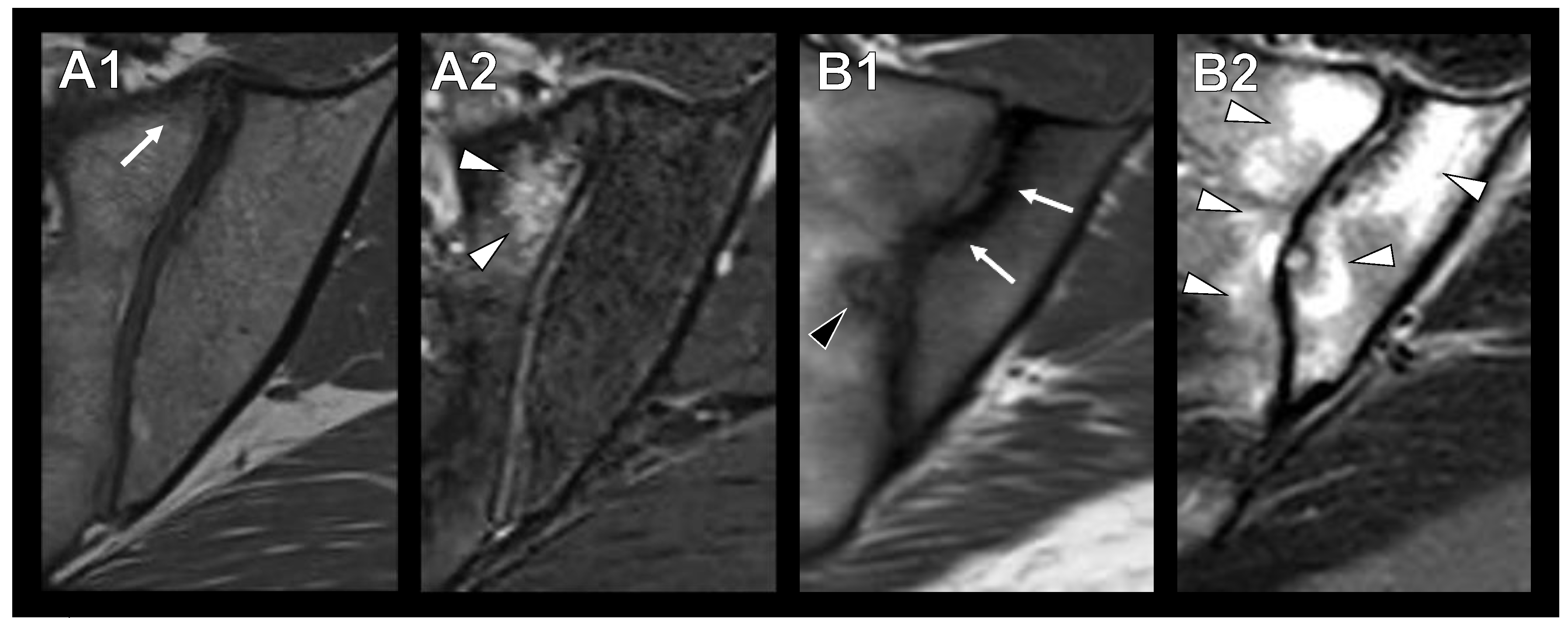
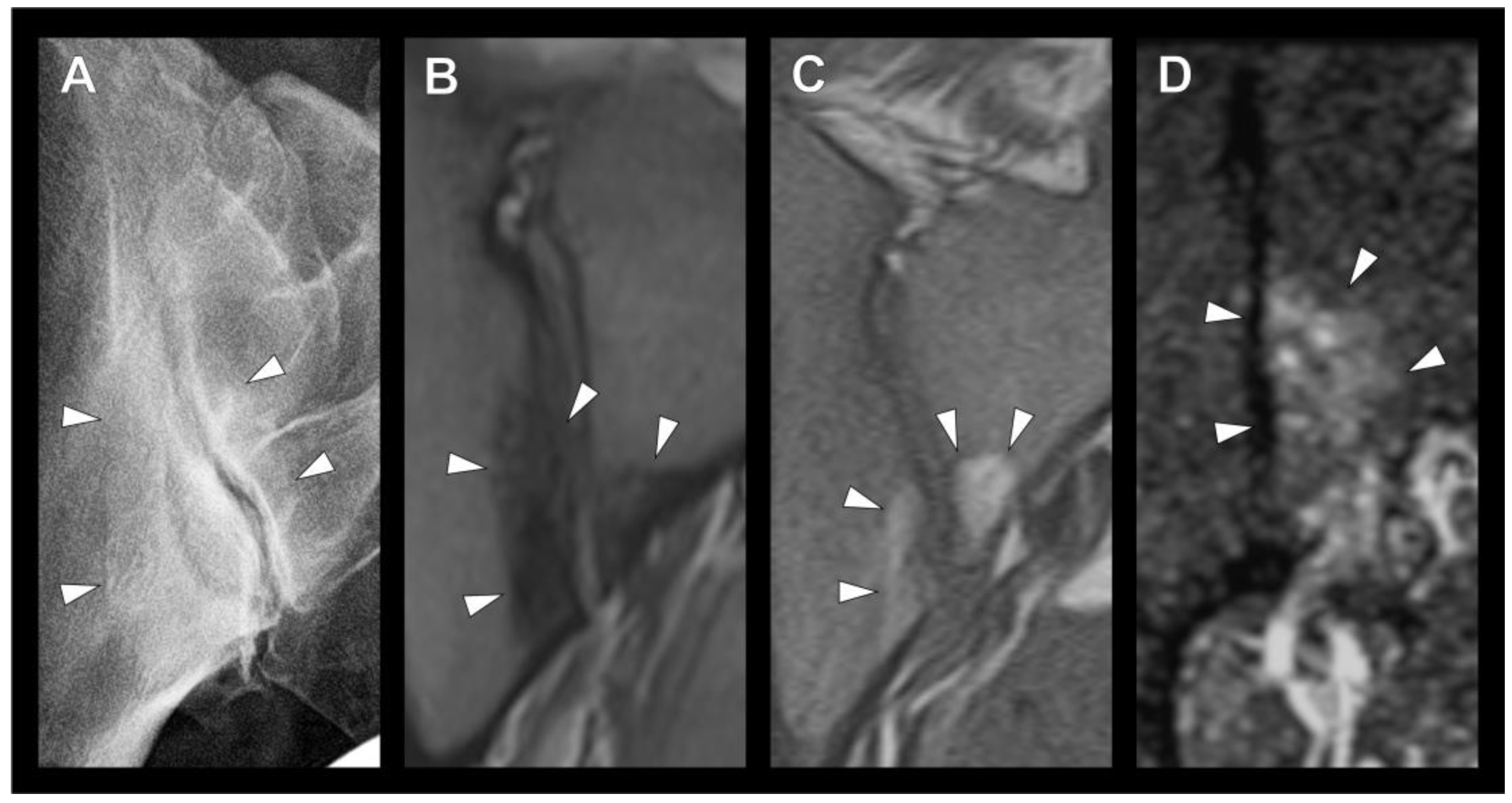
3.2. Sclerotic Lesions in Typical Location of Osteitis Condensans Ilii
3.3. Imaging Modality and Inter-Rater Reliability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wu, A.; March, L.; Zheng, X.; Huang, J.; Wang, X.; Zhao, J.; Blyth, F.M.; Smith, E.; Buchbinder, R.; Hoy, D. Global low back pain prevalence and years lived with disability from 1990 to 2017: Estimates from the Global Burden of Disease Study 2017. Ann. Transl. Med. 2020, 8, 299. [Google Scholar] [CrossRef]
- Dagenais, S.; Caro, J.; Haldeman, S. A systematic review of low back pain cost of illness studies in the United States and internationally. Spine J. 2008, 8, 8–20. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Bain, C.; Williams, G.; March, L.; Brooks, P.; Blyth, F.; Woolf, A.; Vos, T.; Buchbinder, R. A systematic review of the global prevalence of low back pain. Arthritis Rheum. 2012, 64, 2028–2037. [Google Scholar] [CrossRef] [PubMed]
- Knezevic, N.N.; Candido, K.D.; Vlaeyen, J.W.S.; Van Zundert, J.; Cohen, S.P. Low back pain. Lancet 2021, 398, 78–92. [Google Scholar] [CrossRef] [PubMed]
- Pai, S.; Sundaram, L.J. Low back pain: An economic assessment in the United States. Orthop. Clin. N. Am. 2004, 35, 1–5. [Google Scholar] [CrossRef]
- Thiese, M.S.; Hegmann, K.T.; Wood, E.M.; Garg, A.; Moore, J.S.; Kapellusch, J.; Foster, J.; Ott, U. Prevalence of low back pain by anatomic location and intensity in an occupational population. BMC Musculoskelet. Disord. 2014, 15, 283. [Google Scholar] [CrossRef]
- Maher, C.; Underwood, M.; Buchbinder, R. Non-specific low back pain. Lancet 2017, 389, 736–747. [Google Scholar] [CrossRef]
- Biswas, S.; Konala, V.M.; Adapa, S.; Amudala, P.; Naramala, S. Osteitis Condensans Ilii: An Uncommon Cause of Back Pain. Cureus 2019, 11, e4518. [Google Scholar] [CrossRef]
- Sicard, J.A.; Gally, L.; Haguenau, J. Ostéites condensantes: Une étiologie inconnue. J. Radiol. Electrol. 1926, 10, 506–507. [Google Scholar]
- Parperis, K.; Psarelis, S.; Nikiphorou, E. Osteitis condensans ilii: Current knowledge and diagnostic approach. Rheumatol. Int. 2020, 40, 1013–1019. [Google Scholar] [CrossRef]
- Shipp, F.L.; Haggart, G.E. Further experience in the management of osteitis condensans ILII. J. Bone Jt. Surg. Am. 1950, 32, 841–847. [Google Scholar] [CrossRef]
- Diekhoff, T.; Lambert, R.; Hermann, K.G. MRI in axial spondyloarthritis: Understanding an ‘ASAS-positive MRI’ and the ASAS classification criteria. Skelet. Radiol. 2022, 51, 1721–1730. [Google Scholar] [CrossRef] [PubMed]
- Nunley, R.M.; Prather, H.; Hunt, D.; Schoenecker, P.L.; Clohisy, J.C. Clinical presentation of symptomatic acetabular dysplasia in skeletally mature patients. J. Bone Jt. Surg. Am. 2011, 93, 17–21. [Google Scholar] [CrossRef]
- Eshed, I.; Lidar, M. MRI Findings of the Sacroiliac Joints in Patients with Low Back Pain: Alternative Diagnosis to Inflammatory Sacroiliitis. Isr. Med. Assoc. J. 2017, 19, 666–669. [Google Scholar] [PubMed]
- Mitra, R. Osteitis Condensans Ilii. Rheumatol. Int. 2010, 30, 293–296. [Google Scholar] [CrossRef] [PubMed]
- Gillespie, H.W.; Lloydroberts, G. Osteitis Condensans. Brit. J. Radiol. 1953, 26, 16–21. [Google Scholar] [CrossRef]
- Younus, A.; Kelly, A. Exploring the link between Post-partum backache and Osteitis Condensans Ilii- A case report and literature review. Interdiscip. Neurosur. 2020, 22, 100870. [Google Scholar] [CrossRef]
- Cidem, M.; Capkin, E.; Karkucak, M.; Karaca, A. Osteitis condensans ilii in differential diagnosis of patients with chronic low back pain: A review of the literature. Mod. Rheumatol. 2012, 22, 467–469. [Google Scholar] [CrossRef]
- Clarke, D.P.; Higgins, J.N.; Valentine, A.R.; Black, C. Magnetic resonance imaging of osteitis condensans ilii. Br. J. Rheumatol. 1994, 33, 599–600. [Google Scholar] [CrossRef]
- Jacqueline, F.; Arlet, J. Osteitis condensans ilii; a comparison with the acetabular condensation observed in cases of osteoarthritis secondary to subluxation of the hip. Arthritis Rheum 1959, 2, 8–15. [Google Scholar] [CrossRef]
- Toyohara, R.; Kaneuji, A.; Takano, N.; Kurosawa, D.; Hammer, N.; Ohashi, T. A patient-cohort study of numerical analysis on sacroiliac joint stress distribution in pre- and post-operative hip dysplasia. Sci. Rep. 2022, 12, 14500. [Google Scholar] [CrossRef] [PubMed]
- Borlandelli, E.; Ciaffi, J.; Festuccia, G.; Facchini, G.; Miceli, M.; Brusi, V.; Mancarella, L.; Lisi, L.; Di Martino, A.; Faldini, C.; et al. Osteitis condensans ilii: Prevalence and characteristics of a neglected mimic of sacroiliitis. Clin. Rheumatol. 2022, 41, 483–490. [Google Scholar] [CrossRef] [PubMed]
- Poddubnyy, D.; Weineck, H.; Diekhoff, T.; Redeker, I.; Gobejishvili, N.; Llop, M.; Rodriguez, V.R.; Proft, F.; Protopopov, M.; Haibel, H.; et al. Clinical and imaging characteristics of osteitis condensans ilii as compared with axial spondyloarthritis. Rheumatology 2020, 59, 3798–3806. [Google Scholar] [CrossRef] [PubMed]
- Offierski, C.M.; Macnab, I. Hip Spine Syndrome. Spine 1983, 8, 316–321. [Google Scholar] [CrossRef]
- Harada, T.; Hashizume, H.; Taniguchi, T.; Iidaka, T.; Asai, Y.; Oka, H.; Muraki, S.; Akune, T.; Kawaguchi, H.; Nakamura, K.; et al. Association between acetabular dysplasia and sagittal spino-pelvic alignment in a population-based cohort in Japan. Sci. Rep. 2022, 12, 12686. [Google Scholar] [CrossRef] [PubMed]
- Abola, M.V.; Teplensky, J.R.; Cooperman, D.R.; Bauer, J.M.; Liu, R.W. Pelvic Incidence Is Associated with Sacral Curvature, Sacroiliac Joint Angulation, and Sacral Ala Width. Spine 2018, 43, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Muellner, M.; Kreutzinger, V.; Becker, L.; Diekhoff, T.; Pumberger, M.; Schomig, F.; Heyland, M.; Ziegeler, K. Unexpected Sex Differences in the Relationship of Sacroiliac Joint and Lumbar Spine Degeneration. Diagnostics 2022, 12, 275. [Google Scholar] [CrossRef]
- Tauviqirrahman, M.; Ammarullah, M.I.; Jamari, J.; Saputra, E.; Winarni, T.I.; Kurniawan, F.D.; Shiddiq, S.A.; van der Heide, E. Analysis of contact pressure in a 3D model of dual-mobility hip joint prosthesis under a gait cycle. Sci. Rep. 2023, 13, 3564. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Santoso, G.; Sugiharto, S.; Supriyono, T.; Kurdi, O.; Tauviqirrahman, M.; Winarni, T.I.; Jamari, J. Tresca stress study of CoCrMo-on-CoCrMo bearings based on body mass index using 2D computational model. J. Tribologi. 2022, 33, 31–38. [Google Scholar]
- Ammarullah, M.I.; Afif, I.Y.; Maula, M.I.; Winarni, T.I.; Tauviqirrahman, M.; Akbar, I.; Basri, H.; van der Heide, E.; Jamari, J. Tresca Stress Simulation of Metal-on-Metal Total Hip Arthroplasty during Normal Walking Activity. Materials 2021, 14, 7554. [Google Scholar] [CrossRef]
- Ammarullah, M.I.; Hartono, R.; Supriyono, T.; Santoso, G.; Sugiharto, S.; Permana, M.S. Polycrystalline Diamond as a Potential Material for the Hard-on-Hard Bearing of Total Hip Prosthesis: Von Mises Stress Analysis. Biomedicines 2023, 11, 951. [Google Scholar] [CrossRef] [PubMed]
| All | Female | Male | |
|---|---|---|---|
| N | 306 | 248 | 58 |
| Age [years] | 29.5 ± 7.2 | 29.3 ± 7.5 | 26.2 ± 3.4 |
| BMI [kg/m2] | 24.0 ± 4.0 | 23.5 ± 4.0 | 30.5 ± 5.7 |
| Presence of SIJ alterations N (%) | |||
| OCI/Sclerosis | 65 (21.2) | 56 (22.6) | 9 (15.5) |
| Edema | 21 (6.9) | 19 (7.7) | 2 (3.4) |
| Local fat deposit | 11 (3.6) | 11 (4.4) | 0 (0) |
| PAO N (%) | |||
| Right side | 165 (53.9) | 135 (54.4) | 30 (51.7) |
| Left side | 141 (46.1) | 113 (45.6) | 28 (48.3) |
| Additional intervention N (%) | |||
| Femoral osteoplasty | 102 (33.3) | 63 (25.4) | 39 (67.2) |
| Femoral osteotomies | 2 (0.6) | 1 (0.4) | 1 (1.7) |
| Femoral osteoplasty and osteotomy | 1 (0.3) | 1 (0.4) | 0 (0) |
| Femoral osteoplasty and surg. hip dislocation | 3 (0.9) | 0 (0) | 3 (5.1) |
| Comorbidities N (%) | |||
| Asthma | 5 (1.6) | 5 (2.0) | 0 (0) |
| Diabetes mellitus | 1 (0.3) | 1 (0.4) | 0 (0) |
| Depression | 4 (1.3) | 1 (0.4) | 3 (5.2) |
| Anxiety disorders | 2 (0.6) | 2 (0.8) | 0 (0) |
| Hypertension | 2 (0.6) | 2 (0.8) | 0 (0) |
| Hypothyroidism | 9 (2.9) | 8 (3.2) | 1 (1.7) |
| Infantile cerebral palsy | 1 (0.3) | 1 (0.4) | 0 (0) |
| PCOS | 2 (0.6) | 2 (0.8) | 0 (0) |
| Migraine | 4 (1.3) | 4 (1.6) | 0 (0) |
| Meniere’s disease | 1 (0.3) | 1 (0.4) | 0 (0) |
| Multiple sclerosis | 1 (0.3) | 1 (0.4) | 0 (0) |
| Other | 5 (1.6) | 5 (2.0) | 0 (0) |
| No OCI/Sclerosis | OCI/Sclerosis | p-Value | |
|---|---|---|---|
| N = 306 | 241 | 65 | |
| Side of sclerosis | n/a | Unilateral: 30; Bilateral: 35 | |
| Age [years] | 29.6 ± 7.4 | 29.0 ± 6.5 | 0.545 |
| BMI [kg/m2] | 23.7 ± 3.8 | 25.0 ± 4.4 | 0.044 |
| Sex | F:192; M:49 | F:56; M:9 | 0.286 |
| PAO | |||
| Left side | 114 | 27 | 0.484 |
| Right side | 127 | 38 |
| 95% CI for Odds Ratio | ||||
|---|---|---|---|---|
| Predictors | Odds Ratio | Lower Bound | Upper Bound | p |
| Age [years] | 0.980 | 0.936 | 1.025 | 0.379 |
| BMI [kg/m2] | 1.104 | 1.024 | 1.191 | 0.010 |
| Sex | 2.832 | 1.091 | 7.352 | 0.032 |
| Used Modality | |||||
|---|---|---|---|---|---|
| Rater | Radiograph | MRI | Both | Confidence | ICC (95% CI) |
| I | 164 | 35 | 107 | 5.48 ± 0.73 | 0.716 (0.656–0.767) |
| II | 161 | 27 | 118 | 4.78 ± 1.25 | |
| III | 157 | 45 | 104 | 6.81 ± 0.54 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muellner, M.; Ziegeler, K.; Diekhoff, T.; Haffer, H.; Schömig, F.; Leopold, V.J.; Pumberger, M.; Göhler, F. Clustered Occurrence of Osteitis Condensans Ilii in Patients with Symptomatic Hip Dysplasia. Diagnostics 2023, 13, 1701. https://doi.org/10.3390/diagnostics13101701
Muellner M, Ziegeler K, Diekhoff T, Haffer H, Schömig F, Leopold VJ, Pumberger M, Göhler F. Clustered Occurrence of Osteitis Condensans Ilii in Patients with Symptomatic Hip Dysplasia. Diagnostics. 2023; 13(10):1701. https://doi.org/10.3390/diagnostics13101701
Chicago/Turabian StyleMuellner, Maximilian, Katharina Ziegeler, Torsten Diekhoff, Henryk Haffer, Friederike Schömig, Vincent Justus Leopold, Matthias Pumberger, and Friedemann Göhler. 2023. "Clustered Occurrence of Osteitis Condensans Ilii in Patients with Symptomatic Hip Dysplasia" Diagnostics 13, no. 10: 1701. https://doi.org/10.3390/diagnostics13101701
APA StyleMuellner, M., Ziegeler, K., Diekhoff, T., Haffer, H., Schömig, F., Leopold, V. J., Pumberger, M., & Göhler, F. (2023). Clustered Occurrence of Osteitis Condensans Ilii in Patients with Symptomatic Hip Dysplasia. Diagnostics, 13(10), 1701. https://doi.org/10.3390/diagnostics13101701








