Design and Construct Validity of a Postural Control Test for Pre-Term Infants
Abstract
1. Introduction
2. Materials and Methods
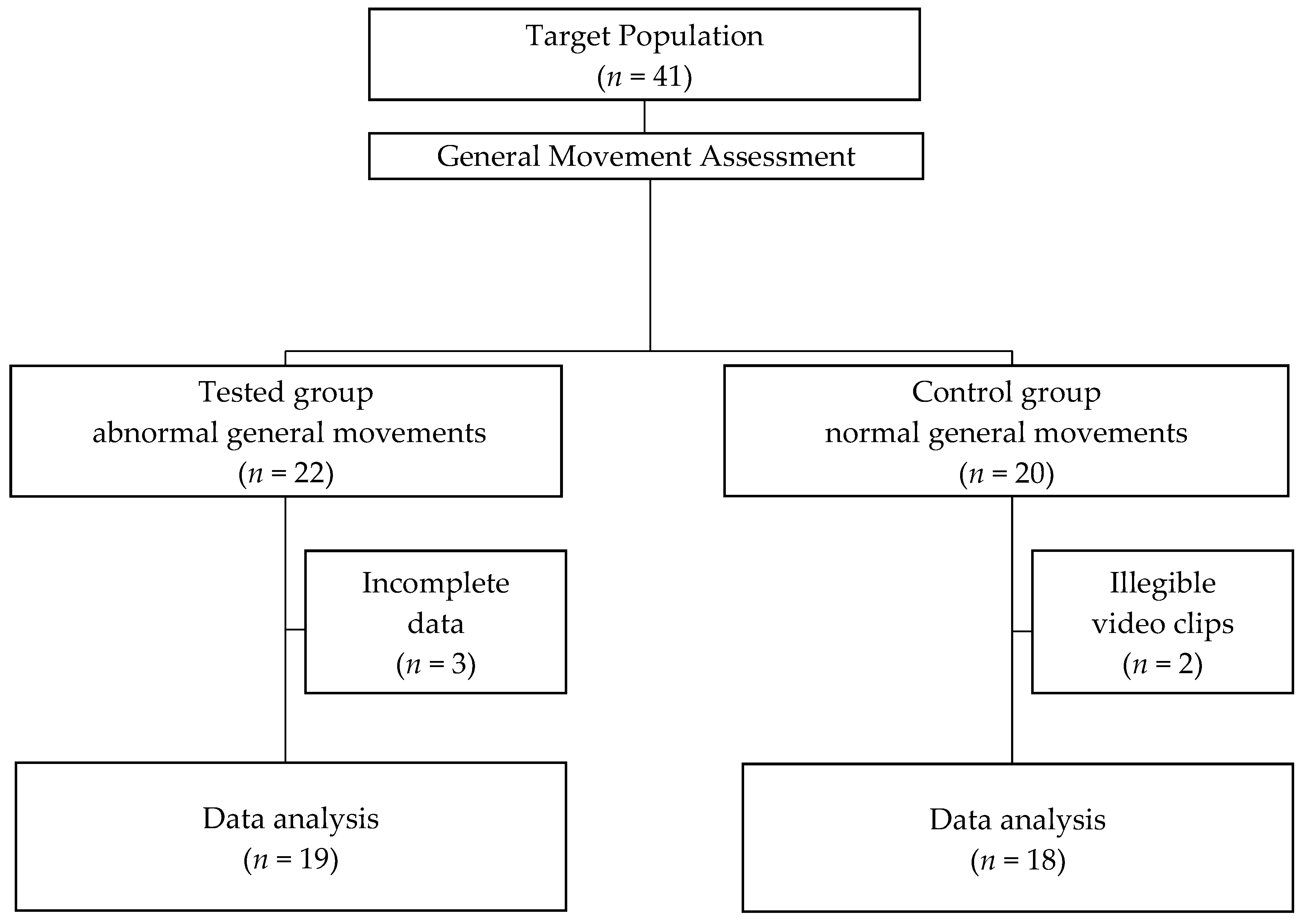
2.1. Participants
2.2. Examinations
2.2.1. GMs Assessment
2.2.2. Experimental Procedure
2.3. Statistical Analysis
3. Results
4. Discussion
5. Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Nash, A.; Dunn, M.; Asztalos, E.; Corey, M.; Mulvihill-Jory, B.; O’Connor, D.L. Pattern of growth of very low birth weight preterm infants, assessed using the WHO Growth Standards, is associated with neurodevelopment. Appl. Physiol. Nutr. Metab. 2011, 36, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Vogel, J.P.; Chawanpaiboon, S.; Moller, A.-B.; Watananirun, K.; Bonet, M.; Lumbiganon, P. The global epidemiology of preterm birth. Best Pract. Res. Clin. Obstet. Gynaecol. 2018, 52, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Aylward, G.P. Neurodevelopmental Outcomes of Infants Born Prematurely. J. Dev. Behav. Pediatr. 2005, 26, 427–440. [Google Scholar] [CrossRef] [PubMed]
- Bracewell, M.; Marlow, N. Patterns of motor disability in very preterm children. Ment. Retard. Dev. Disabil. Res. Rev. 2002, 8, 241–248. [Google Scholar] [CrossRef]
- Salt, A.; Redshaw, M. Neurodevelopmental follow-up after preterm birth: Follow up after two years. Early Hum. Dev. 2006, 82, 185–197. [Google Scholar] [CrossRef]
- Hack, M. Survival and Neurodevelopmental Outcomes of Preterm Infants. J. Pediatr. Gastroenterol. Nutr. 2007, 45, S141–S142. [Google Scholar] [CrossRef]
- Bosanquet, M.; Copeland, L.; Ware, R.; Boyd, R. A systematic review of tests to predict cerebral palsy in young children. Dev. Med. Child Neurol. 2013, 55, 418–426. [Google Scholar] [CrossRef]
- Caesar, R.; Colditz, P.B.; Cioni, G.; Boyd, R.N. Clinical tools used in young infants born very preterm to predict motor and cognitive delay (not cerebral palsy): A systematic review. Dev. Med. Child Neurol. 2020, 63, 387–395. [Google Scholar] [CrossRef]
- Einspieler, C.; Prechtl, H.F.R. Prechtl’s assessment of general movements: A diagnostic tool for the functional assessment of the young nervous system. Ment. Retard. Dev. Disabil. Res. Rev. 2005, 11, 61–67. [Google Scholar] [CrossRef]
- Aizawa, C.Y.P.; Einspieler, C.; Genovesi, F.F.; Ibidi, S.M.; Hasue, R.H. The general movement checklist: A guide to the assessment of general movements during preterm and term age. J. Pediatr. 2020, 97, 445–452. [Google Scholar] [CrossRef]
- Robinson, H.; Hart, D.; Vollmer, B. Predictive validity of a qualitative and quantitative Prechtl’s General Movements Assessment at term age: Comparison between preterm infants and term infants with HIE. Early Hum. Dev. 2021, 161, 105449. [Google Scholar] [CrossRef] [PubMed]
- Xie, K.; Zheng, H.; Li, H.; Zhang, C.; Li, H.; Jin, H.; Ma, B. The Study of Effect for General Movements Assessment in the Diagnosis of Neurological Development Disorders: A Meta-Analysis. Clin. Pediatr. 2015, 55, 36–43. [Google Scholar] [CrossRef] [PubMed]
- Pires, C.D.S.; Marba, S.T.M.; Caldas, J.P.D.S.; Stopiglia, M.D.C.S. Predictive value of the general movements assessment in preterm infants: A meta-analysis. Rev. Paul. Pediatr. 2020, 38, e2018286. [Google Scholar] [CrossRef] [PubMed]
- Peinado-Gorlat, P.; Gómez de Valcárcel-Sabater, M.; Gorlat-Sánchez, B. General movement assessment as a tool for determining the prognosis in infantile cerebral palsy in preterm infants: A systematic review. Rev. Neurol. 2020, 71, 134–142. [Google Scholar]
- Fjørtoft, T.; Einspieler, C.; Adde, L.; Strand, L.I. Inter-observer reliability of the “Assessment of Motor Repertoire—3 to 5 Months” based on video recordings of infants. Early Hum. Dev. 2009, 85, 297–302. [Google Scholar] [CrossRef]
- Katz, J.S. Interrater and Intrarater Reliability Using Prechtl’s Method of Qualitative Assessment of General Movements in Infants. Int. J. Pediatr. Res. 2016, 2, 014. [Google Scholar] [CrossRef]
- Silva, N.; Zhang, D.; Kulvicius, T.; Gail, A.; Barreiros, C.; Lindstaedt, S.; Kraft, M.; Bölte, S.; Poustka, L.; Nielsen-Saines, K.; et al. The future of General Movement Assessment: The role of computer vision and machine learning—A scoping review. Res. Dev. Disabil. 2021, 110, 103854. [Google Scholar] [CrossRef]
- Irshad, M.T.; Nisar, M.A.; Gouverneur, P.; Rapp, M.; Grzegorzek, M. AI Approaches Towards Prechtl’s Assessment of General Movements: A Systematic Literature Review. Sensors 2020, 20, 5321. [Google Scholar] [CrossRef]
- Chen, H.; Xue, M.; Mei, Z.; Oetomo, S.B.; Chen, W. A Review of Wearable Sensor Systems for Monitoring Body Movements of Neonates. Sensors 2016, 16, 2134. [Google Scholar] [CrossRef]
- Adde, L.; Helbostad, J.L.; Jensenius, A.R.; Taraldsen, G.; Grunewaldt, K.H.; Støen, R. Early prediction of cerebral palsy by computer-based video analysis of general movements: A feasibility study. Dev. Med. Child Neurol. 2010, 52, 773–778. [Google Scholar] [CrossRef]
- Adde, L.; Helbostad, J.; Jensenius, A.R.; Langaas, M.; Støen, R. Identification of fidgety movements and prediction of CP by the use of computer-based video analysis is more accurate when based on two video recordings. Physiother. Theory Pract. 2013, 29, 469–475. [Google Scholar] [CrossRef] [PubMed]
- Adde, L.; Yang, H.; Sæther, R.; Jensenius, A.R.; Ihlen, E.; Cao, J.-Y.; Støen, R. Characteristics of general movements in preterm infants assessed by computer-based video analysis. Physiother. Theory Pract. 2018, 34, 286–292. [Google Scholar] [CrossRef] [PubMed]
- Støen, R.; on behalf of the CIMA Norway Study Group; Songstad, N.T.; Silberg, I.E.; Fjørtoft, T.; Jensenius, A.R.; Adde, L. Computer-based video analysis identifies infants with absence of fidgety movements. Pediatr. Res. 2017, 82, 665–670. [Google Scholar] [CrossRef] [PubMed]
- Karch, D.; Kang, K.-S.; Wochner, K.; Philippi, H.; Hadders-Algra, M.; Pietz, J.; Dickhaus, H. Kinematic assessment of stereotypy in spontaneous movements in infants. Gait Posture 2012, 36, 307–311. [Google Scholar] [CrossRef]
- Rahmati, H.; Martens, H.; Aamo, O.M.; Stavdahl, O.; Stoen, R.; Adde, L. Frequency Analysis and Feature Reduction Method for Prediction of Cerebral Palsy in Young Infants. IEEE Trans. Neural Syst. Rehabil. Eng. 2016, 24, 1225–1234. [Google Scholar] [CrossRef]
- Tacchino, C.; Impagliazzo, M.; Maggi, E.; Bertamino, M.; Blanchi, I.; Campone, F.; Durand, P.; Fato, M.; Giannoni, P.; Iandolo, R.; et al. Spontaneous movements in the newborns: A tool of quantitative video analysis of preterm babies. Comput. Methods Programs Biomed. 2020, 199, 105838. [Google Scholar] [CrossRef]
- Emarcroft, C.; Khan, A.; Embleton, N.; Trenell, M.; Plã¶tz, T. Movement Recognition Technology as a Method of Assessing Spontaneous General Movements in High Risk Infants. Front. Neurol. 2015, 5, 284. [Google Scholar] [CrossRef]
- Dusing, S.C.; Kyvelidou, A.; Mercer, V.S.; Stergiou, N. Infants Born Preterm Exhibit Different Patterns of Center-of-Pressure Movement Than Infants Born at Full Term. Phys. Ther. 2009, 89, 1354–1362. [Google Scholar] [CrossRef]
- Dusing, S.C.; Izzo, T.A.; Thacker, L.R.; Galloway, J.C. Postural complexity differs between infant born full term and preterm during the development of early behaviors. Early Hum. Dev. 2014, 90, 149–156. [Google Scholar] [CrossRef]
- Onell, A. The vertical ground reaction force for analysis of balance? Gait Posture 2000, 12, 7–13. [Google Scholar] [CrossRef]
- Kyvelidou, A.; Harbourne, R.T.; Shostrom, V.K.; Stergiou, N. Reliability of Center of Pressure Measures for Assessing the Development of Sitting Postural Control in Infants with or at Risk of Cerebral Palsy. Arch. Phys. Med. Rehabil. 2010, 91, 1593–1601. [Google Scholar] [CrossRef] [PubMed]
- Harbourne, R.T.; Deffeyes, J.E.; Kyvelidou, A.; Stergiou, N. Complexity of postural control in infants: Linear and nonlinear features revealed by principal component analysis. Nonlinear Dyn. Psychol. Life Sci. 2009, 13, 123–144. [Google Scholar]
- Pavão, S.L.; dos Santos, A.N.; Woollacott, M.H.; Rocha, N.A.C.F. Assessment of postural control in children with cerebral palsy: A review. Res. Dev. Disabil. 2013, 34, 1367–1375. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Łukaszewicz, T.; Kania, D.; Kidoń, Z.; Pethe-Kania, K. Posturographic methods for body posture symmetry assessment. Bull. Pol. Acad. Sci. Tech. Sci. 2015, 63, 907–917. [Google Scholar] [CrossRef][Green Version]
- Cunningham, J.B.; McCrum-Gardner, E. Power, effect and sample size using GPower: Practical issues for researchers and members of research ethics committees. Evid. Based Midwifery 2007, 5, 132–136. [Google Scholar]
- Pat-Horenczyk, R.; Kenan, A.M.; Achituv, M.; Bachar, E. Protective Factors Based Model for Screening for Posttraumatic Distress in Adolescents. In Child Youth Care Forum; Springer: New York, NY, USA, 2013; Volume 43, pp. 339–351. [Google Scholar] [CrossRef]

| Parameter | Control Group (n = 18) | Tested Group (n = 19) | ||
|---|---|---|---|---|
| Mean (SD) | Min–Max | Mean (SD) | Min–Max | |
| Birth weight (grams) | 975.83 (273.41) | 690–1850 | 949.68 (209.50) | 650–1430 |
| Gestational age (weeks) | 27.72 (2.16) | 24–32 | 27.15 (1.50) | 25–30 |
| Apgar at 5th minute (score) | 6.33 (2.40) | 1–9 | 5.31 (1.67) | 3–8 |
| Neonatal medical index—NMI (score) | 2.1 (0.9) | 1–5 | 3.6 (1.6) | 1–5 |
| Length of time stay in hospital (days) | 27 (16) | 2–65 | 31 (20) | 5–85 |
| Delivery; Normal, n (%) Caesarean, n (%) | 8 (44) 10 (56) | 6 (32) 13 (68) | ||
| Sex; Girls, n (%) Boys, n (%) | 6 (33) 12 (67) | 6 (32) 13 (68) | ||
| Posturometric Indices Based on CoP Shifts during Lying | |
|---|---|
| Vmax CoP | Maximal velocity of the CoP displacement [cm/s] |
| SPL | Sway path length of the CoP [cm] |
| Posturometric indices, based on the area of the CoP during lying | |
| ACoP | area of CoP shifts under the unrolled trajectory [cm2] where: p(i) is the surface area of a triangle comprised of two successive points of a given trajectory (Tc(i−1), Tc(i)), and the point Tc0 represents the center of that trajectory; l(i) is given by the formula, whereas values of r(i), r(i−1), and ob (i) are calculated using the following equations: |
| MCoPx | Mean medial-lateral linear displacement of the CoP [mm] |
| MCoPy | Mean posterior-anterior displacement of the CoP [mm] |
| PARAMETER | Control Group (n = 18) | Tested Group (n = 19) | Mean Difference | Statistical Test |
|---|---|---|---|---|
| M (SD) | M (SD) | (95% CI) | p-Value | |
| SPL | 358,33.61 (12,702.11) | 157.38 (83.37) | 200.94 (118.27–283.62) | <0.001 |
| VmaxCoP | 11.94 (4.23) | 5.24 (2.77) | 6.69 (3.94–9.45) | <0.001 |
| ACoP | 245.19 (106.28) | 144.71 (68.50) | 10.04 (3.15–16.93) | 0.006 |
| MCoPx | 7.94 (3.45) | 3.37 (1.81) | 4.57 (2.61–6.53) | <0.001 |
| MCoPy | 7.63 (3.44) | 3.37 (1.95) | 4.25 (2.08–6.42) | <0.001 |
| PARAMETER | Control Group (n = 18) | Tested Group (n = 19) | Mean Difference | Statistical Test |
|---|---|---|---|---|
| M (SD) | M (SD) | (95% CI) | p-Value | |
| SPL | 391.64 (144.29) | 245.49 (129.53) | 146.15 (43.14–249.16) | 0.007 |
| VmaxCoP | 13.05 (4.80) | 8.18 (4.31) | 4.87 (1.43–8.30) | 0.007 |
| ACoP | 32.25 (10.89) | 25.11(13.26) | 7.13 (−1.74–16.02) | 0.111 |
| MCoPx | 8.95 (4.45) | 5.36 (2.98) | 3.59 (0.68–6.50) | 0.017 |
| MCoPy | 7.56 (2.80) | 5.74 (2.47) | 1.82 (−0.17–3.80) | 0.071 |
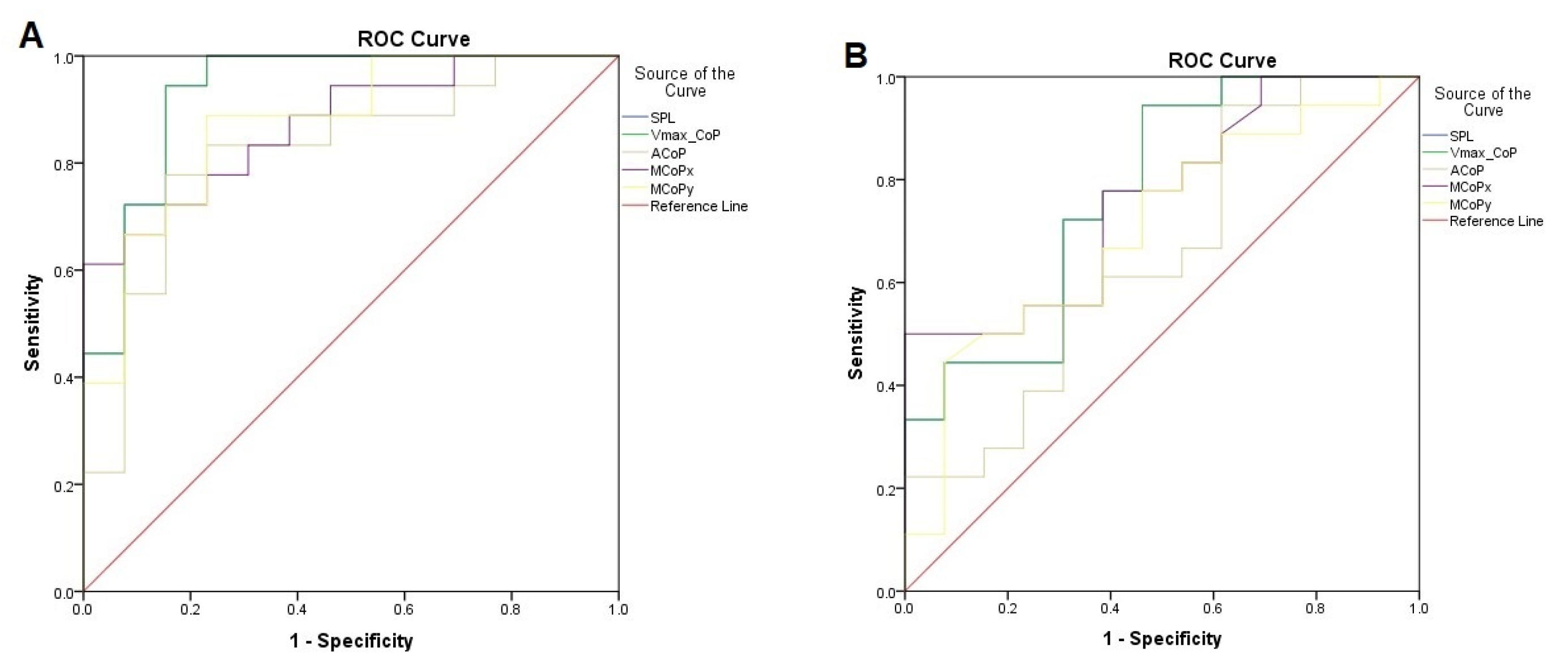
| PARAMETER | Supine Position | Prone Position | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| HDV | SE | SP | AUC | 95% CI | HDV | SE | SP | AUC | 95% CI | |
| SPL | 212.42 | 94% | 85% | 0.932 | 0.836–1.00 | 298.39 | 72% | 69% | 0.774 | 0.604–0.943 |
| VmaxCoP | 7.08 | 94% | 85% | 0.932 | 0.836–1.00 | 9.94 | 94% | 85% | 0.774 | 0.604–0.943 |
| ACoP | 17.22 | 83% | 77% | 0.821 | 0.665–0.976 | - | - | - | - | - |
| MCoPx | 4.52 | 78% | 77% | 0.872 | 0.751–0.992 | 6.13 | 78% | 62% | 0.763 | 0.596–0.930 |
| MCoPy | 4.38 | 89% | 78% | 0.872 | 0.746–0.998 | - | - | - | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kniaziew-Gomoluch, K.; Szopa, A.; Kidoń, Z.; Siwiec, A.; Domagalska-Szopa, M. Design and Construct Validity of a Postural Control Test for Pre-Term Infants. Diagnostics 2023, 13, 96. https://doi.org/10.3390/diagnostics13010096
Kniaziew-Gomoluch K, Szopa A, Kidoń Z, Siwiec A, Domagalska-Szopa M. Design and Construct Validity of a Postural Control Test for Pre-Term Infants. Diagnostics. 2023; 13(1):96. https://doi.org/10.3390/diagnostics13010096
Chicago/Turabian StyleKniaziew-Gomoluch, Katarzyna, Andrzej Szopa, Zenon Kidoń, Andrzej Siwiec, and Małgorzata Domagalska-Szopa. 2023. "Design and Construct Validity of a Postural Control Test for Pre-Term Infants" Diagnostics 13, no. 1: 96. https://doi.org/10.3390/diagnostics13010096
APA StyleKniaziew-Gomoluch, K., Szopa, A., Kidoń, Z., Siwiec, A., & Domagalska-Szopa, M. (2023). Design and Construct Validity of a Postural Control Test for Pre-Term Infants. Diagnostics, 13(1), 96. https://doi.org/10.3390/diagnostics13010096






