Abstract
Measurable residual disease (MRD) is a well-known independent prognostic factor in acute leukemias, and multicolor flow cytometry (MFC) is widely used to detect MRD. MFC is able not only to enumerate MRD accurately but also to describe an antigen expression profile of residual blast cells. However, the relationship between MRD immunophenotype and patient survival probability has not yet been studied. We determined the prognostic impact of MRD immunophenotype in adults with B-cell acute lymphoblastic leukemia (B-ALL). In a multicenter study RALL-2016 (NCT03462095), 267 patients were enrolled from 2016 to 2022. MRD was assessed at the end of induction (day 70) in 94 patients with B-ALL by six- or 10-color flow cytometry in the bone marrow specimens. The 4 year relapse-free survival (RFS) was lower in MRD-positive B-ALL patients [37% vs. 78% (p < 0.0001)]. The absence of CD10, positive expression of CD38, and high expression of CD58 on MRD cells worsened the 4 year RFS [19% vs. 51% (p = 0.004), 0% vs. 51% (p < 0.0001), and 21% vs. 40% (p = 0.02), respectively]. The MRD immunophenotype is associated with RFS and could be an additional prognostic factor for B-ALL patients.
1. Introduction
Relapse of acute lymphoblastic leukemia (ALL) can be explained by the persistence of measurable residual disease (MRD) after the achievement of complete remission (CR) [1]. MRD detection is incorporated in the vast majority of clinical protocols. MRD is the strongest independent prognostic factor in both pediatric and adult ALL [2,3]. According to a meta-analysis for pediatric patients (n = 11,249), 10 year event-free survival (EFS) was better for those who were MRD-negative (77% vs. 32%). For adult patients (n = 2076), the 10 year EFS was 64% in MRD-negative cases vs. 21% for MRD-positive cases [4]. MRD is associated with a high probability of relapse and is used for risk stratification and treatment decision making such as deintensification or escalation of therapy, use of targeted therapy, or allogeneic stem-cell transplantation (ASCT) [5,6,7,8,9,10,11,12].
Multicolor flow cytometry (MFC) and polymerase chain reaction (PCR) are widely used methods for MRD detection with high sensitivity of at least 10−4 (0.01%) [13]. MRD monitoring is performed by real-time quantitative PCR of rearranged immunoglobulins and T-cell receptor genes in numerous studies [10,14,15,16]. This method has been highly standardized by international consortia [17]. However, this method is time- and labor-consuming, and it requires the assessment of an initial diagnostic sample. In about 5% of cases, the PCR target cannot be identified [18] or can be lost during the course of the disease [19,20].
MFC is faster, cheaper, and applicable to most ALL cases in comparison with PCR. MFC allows for the detection of MRD with two different approaches. The first is the determination of the leukemia-associated immunophenotype (LAIP) of blast cells before treatment and tracking it in follow-up samples. The second method called “different-from-normal” (DfN) requires strong knowledge of antigen expression patterns of normal hematologic progenitors, relies on differences between immunophenotype of blast cells and normal cells, and does not require a pretreatment sample [21,22]. However, MFC is less standardized than PCR. The lack of uniformity for MRD assessment between laboratories is one of the important limitations of MFC [1].
MFC allows not only to enumerate MRD accurately but also to describe the antigen expression profile of residual blast cells. However, the relationship between MRD immunophenotype and patient survival had not been studied yet. The aim of the presented study is to assess an immunophenotype significance of MRD in B-ALL patients enrolled in a Russian multicenter study.
2. Materials and Methods
2.1. Patients
From 2016 to 2022, 267 Ph-negative ALL [146 B-ALL, 109 T-ALL, and 12 mixed-phenotype acute leukemia (MPAL)] patients were included in the Russian multicenter study RALL-2016 (Russian Acute Lymphoblastic Leukemia study) (NCT03462095). It was planned to include 350 patients in the study by the end of 2022; however, due to the COVID-19 pandemic, the recruitment rate significantly decreased. Assessment of MRD as a prognostic factor was performed in patients with complete remission and in cases without significant deviations from the therapeutic protocol. Thus, MRD was evaluated in 190 ALL patients (98 B-ALL, 84 T-ALL, and eight MPAL). The median age of B-ALL patients was 33 (range: 18–55). The male-to-female ratio was 54:44. According to EGIL classification [23], 19 patients were diagnosed with B-I (pro-B), 78 were diagnosed with B-II (common) ALL, and five were diagnosed with B-III (pre-B) ALL. MRD was assessed in the bone marrow specimens at different timepoints: at the end of induction II (day 70) in 94 B-ALL patients, at the end of consolidation III (day 133)—n 78 B-ALL patients, and at the end of consolidation V (day 190)—in 76 B-ALL patients. In four patients, MRD was analyzed at 133 or 190 days but not at day 70 because of issues with sample delivery. All cases were Ph-negative, which was confirmed by fluorescence in situ hybridization. Normal karyotype was found in 32 B-ALL patients, hyperploidy was observed in 13 patients, hypoploidy was observed in two patients, complex karyotype was observed in five patients, t(1;19)(q23;p13) was observed in three patients, and other karyotype abnormalities were observed in 20 patients. KMT2A rearrangements were found in five patients. In the RALL-2016 protocol, only mutations of the KMT2A gene were considered as an adverse risk factor. Therapy was not changed depending on MRD status.
2.2. MRD Detection
MRD was carried out at the National Medical Research Center for Hematology (Moscow). Samples were sent from hospitals in Moscow, St. Petersburg, Nizhny Novgorod, Volgograd, Yaroslavl, Kaluga, Kirov, Surgut, and Yekaterinburg. Delivery of samples was carried out within 24 h from the moment of taking the bone marrow specimens. Bone marrow samples were collected in EDTA-treated tubes, lysed with Pharm Lyse solution (BD Biosciences), and centrifuged at 400× g for 3.5 min. The cell pellet was washed with 2 mL of CellWASH solution (BD Biosciences) and centrifuged. Monoclonal antibodies were added to 100 μL of cell suspension containing at least 2 × 106 cells, if possible, according to Table S1 in Supplementary Materials. After incubation, cells were washed and analyzed on a six-color FACSCanto II (BD Biosciences) or 13-color CytoFLEX (Beckman Coulter) flow cytometer. Detection of MRD was performed using the DfN approach previously described [22,24], as the centralized initial immunophenotypic study of blast cells was not included in the RALL-2016 study. Laboratories in local hospitals independently performed immunophenotyping for line verification of acute leukemia. However, LAIP was detected before treatment in patients who were treated at the National Medical Research Center for Hematology, and this LAIP was a starting point in the search for MRD.
MRD was defined as a population that consisted of 20 or more cells with an aberrant immunophenotype. The sensitivity of the method was at least 0.01%.
2.3. MRD Immunophenotype Description
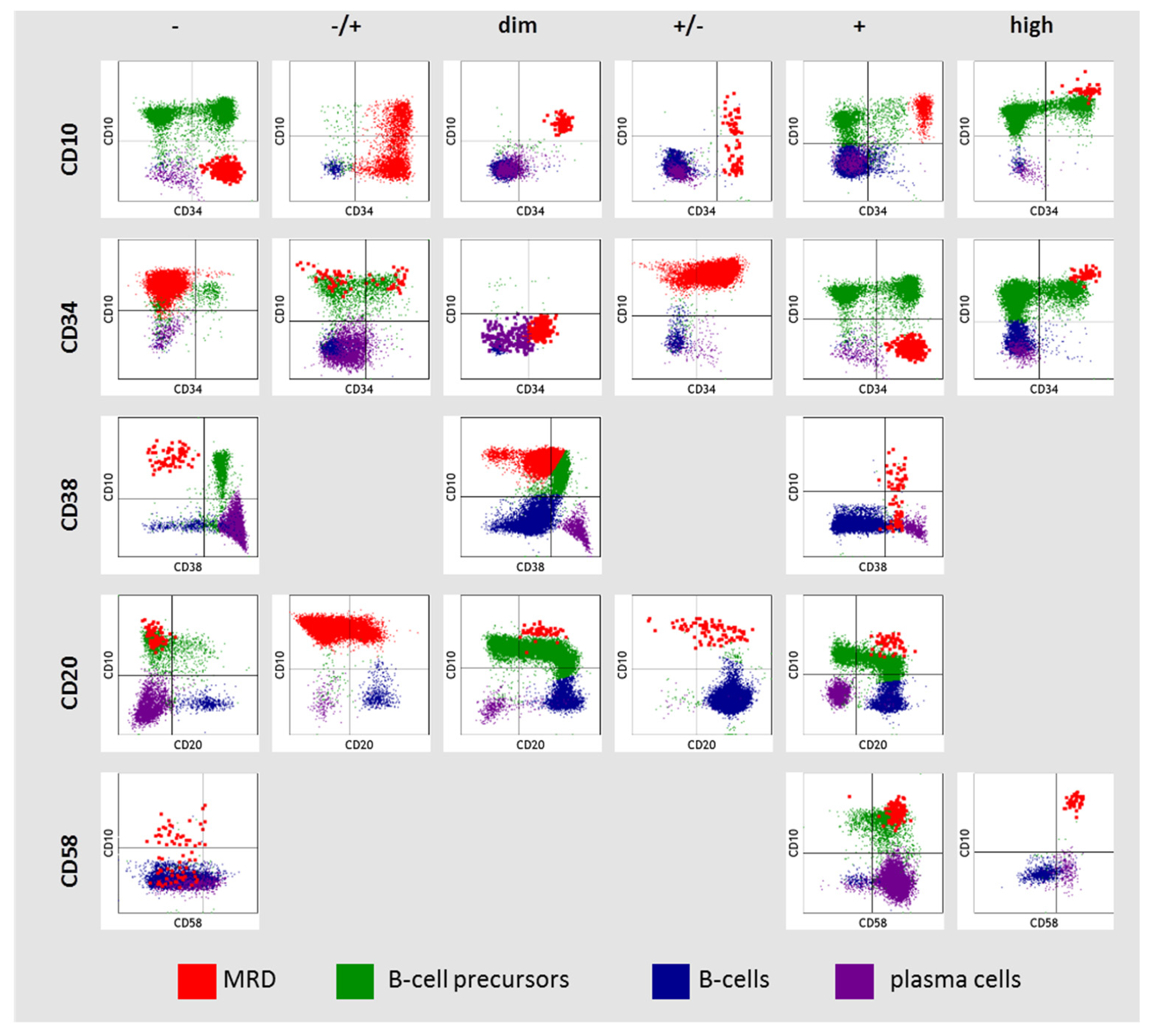
Since MRD was detected on different flow cytometers using three different monoclonal antibody panels, a strategy for qualitative immunophenotype description was adopted from a previously reported study [25]. The expression of antigens was assessed in comparison with normal hematopoietic cells. Six variants of antigen expression were identified: (i) absence of antigen (−); (ii) partly positive (−/+); (iii) low expression (dim); (iv) partly negative (+/−); (v) positive (+); (vi) high expression (high). Partly positive expression was called if the proportion of positive cells was 50% or less and partially negative if more than 50%. The expression of CD10, CD34, CD38, CD20, and CD58 in MRD cells was assessed in comparison with normal B-cell progenitors, mature B cells, and plasma cells (PCs) (Figure 1). High expression of CD10 and CD34 in MRD cells was considered if it was higher than in normal B-cell precursors (hematogones). Dim expression of CD10 and CD34 in MRD cells was considered if it was homogeneous and lower than in hematogones. High expression of CD58 in MRD cells was considered if it was higher than it was in plasma cells (CD19+ cells with the strongest CD38 expression). Negative expression of CD38 in MRD was considered if it was as on mature B cells and did not overlap with hematogones. If MRD cells expressed CD38 higher than mature B cells, but lower than hematogones, this was denoted as “dim” CD38 expression. In this case, MRD and hematogones overlapped partly in CD38 expression. Positive expression of CD38 in MRD was considered if it was as in hematogones or higher.
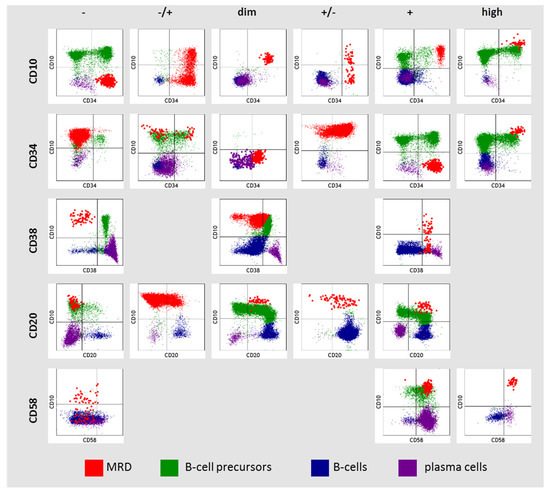
Figure 1.
Description of CD10, CD34, CD38, CD20, and CD58 expression in residual blast cells (MRD—measurable residual disease).
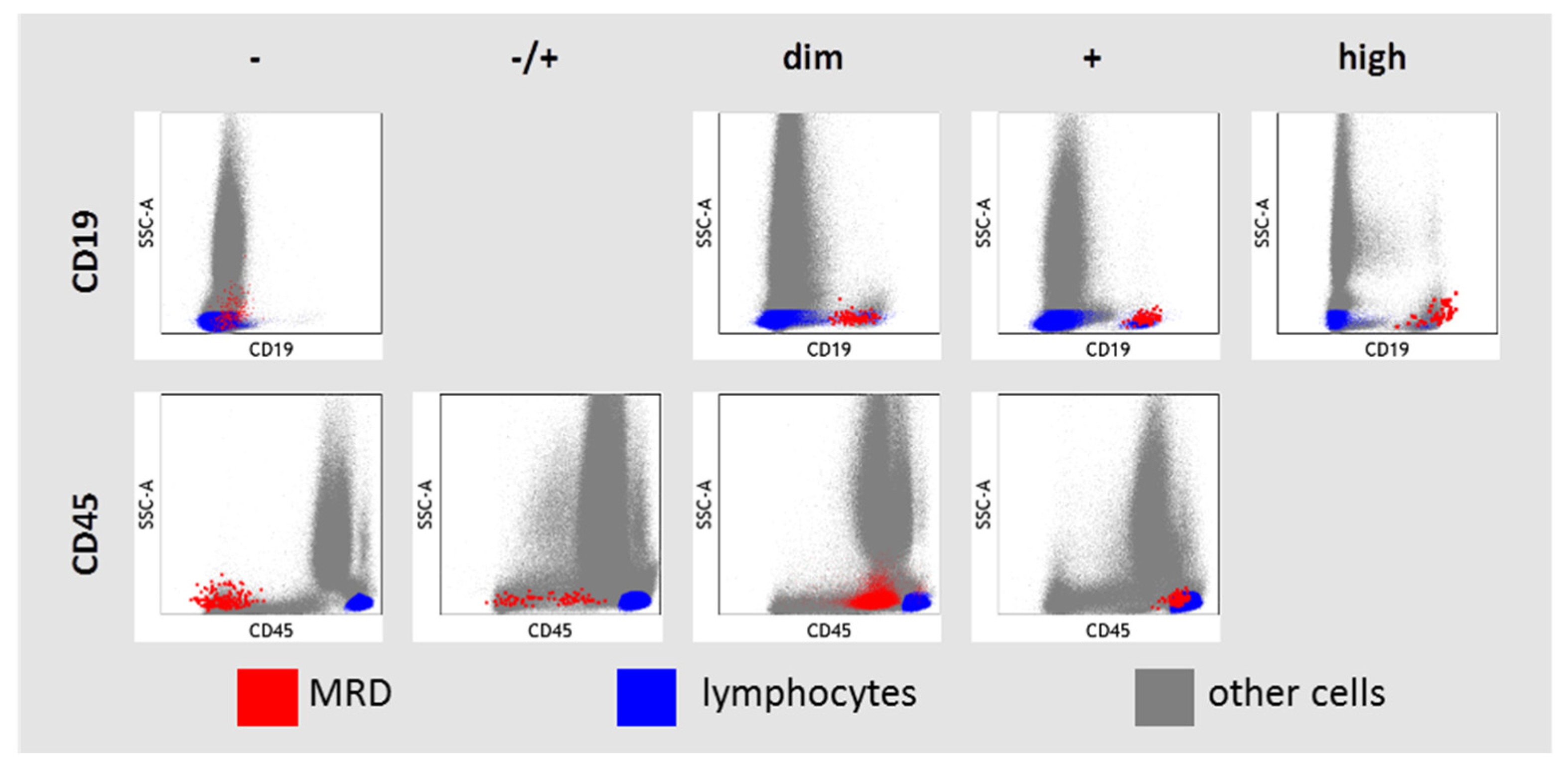
The expression of CD19 and CD45 in MRD cells was assessed in relation to granulocytes, monocytes, and lymphocyte–leukocyte subsets presented in each sample (Figure 2). High CD19 expression was considered if it was higher than in hematogones and even in mature B cells. If MRD cells and mature B cells expressed CD19, the CD19 expression was labeled as “positive”. CD19 expression was considered as dim if it was lower in MRD cells than in mature B cells. Negative expression of CD19 in MRD cells was considered if it did not differ from negative control cells (CD19-negative lymphocytes, monocytes, and granulocytes). There was only one case of CD19-negative B-ALL in our study (initial diagnosis of B-ALL was confirmed by strong expression of CD10, CD22, and cytoplasmic CD79a). CD45 expression was considered negative in MRD cells if it was lower than on granulocytes and the MRD population did not overlap with the left edge of granulocytes in the SSC vs. CD45 dot plot. CD45 expression in MRD cells was considered positive in MRD cells if the blast cell population overlapped with lymphocytes on the SSC vs. CD45 dot plot. Intermediate variants between negative and positive CD45 expressions were “−/+” or “dim”, depending on the size of the negative part of CD45 expression.
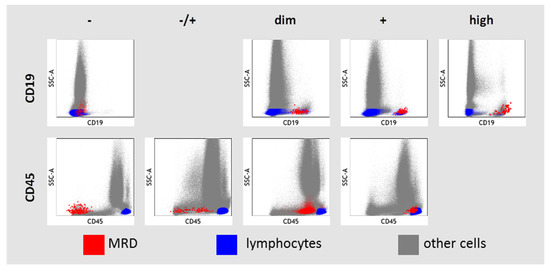
Figure 2.
Description of CD19 and CD45 expression in residual blast cells (MRD—measurable residual disease).
2.4. Statistical Analyses
Statistical analyses were performed using IBM SPSS v.23 and R 3.6.3 software. Analysis of relapse-free survival (RFS) was performed using the Kaplan–Meier method. The initial date was the date of the MRD assessment. The time interval was counted from the initial date to the date of the first adverse event (relapse/death) or the date of the last contact (censoring). Cluster analysis of cases according to immunophenotype MRD was performed using the ward.D method of the dendextend package in R 3.6.3. To do this, a qualitative description of MRD immunophenotype was converted into a numerical semiquantitative equivalent: (i) (−) → 1; (ii) (−/+) → 2; (iii) (dim) → 3; (iv) (+/−) → 4; (v) (+) → 5; (vi) (high) → 6. The comparison of MRD values between two groups was performed using the Mann–Whitney test, while the comparison among three groups was performed using the Kruskal–Wallis test. Nonparametric tests were used due to the non-normal distribution of the data. The normality of distribution was tested using the Shapiro–Wilk test.
3. Results
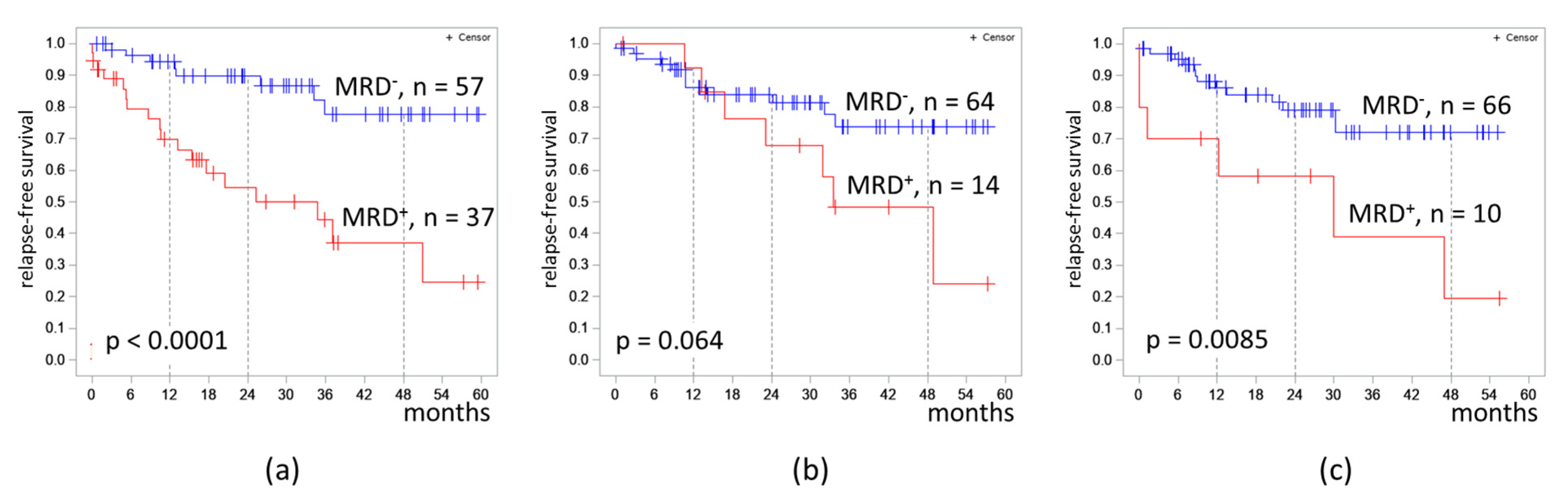
The proportion of MRD-positive cases was 39.4%, 18.0%, and 13.2% in B-ALL patients on days 70, 133, and 190 respectively. The 4 year RFS was lower in MRD-positive patients on day 70 (37% vs. 78%, p < 0.0001) and on day 190 (19% vs. 78%, p = 0.0085) (Figure 3).
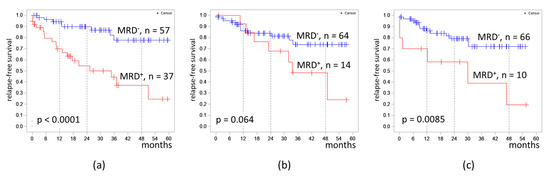
Figure 3.
Relapse-free survival of patients with B-cell acute lymphoblastic leukemia depending on MRD at different timepoints: (a) at the end of induction II (day 70); (b) at the end of consolidation III (day 133); (c) at the end of consolidation V (day 190). The initial date was the date of the MRD assessment.
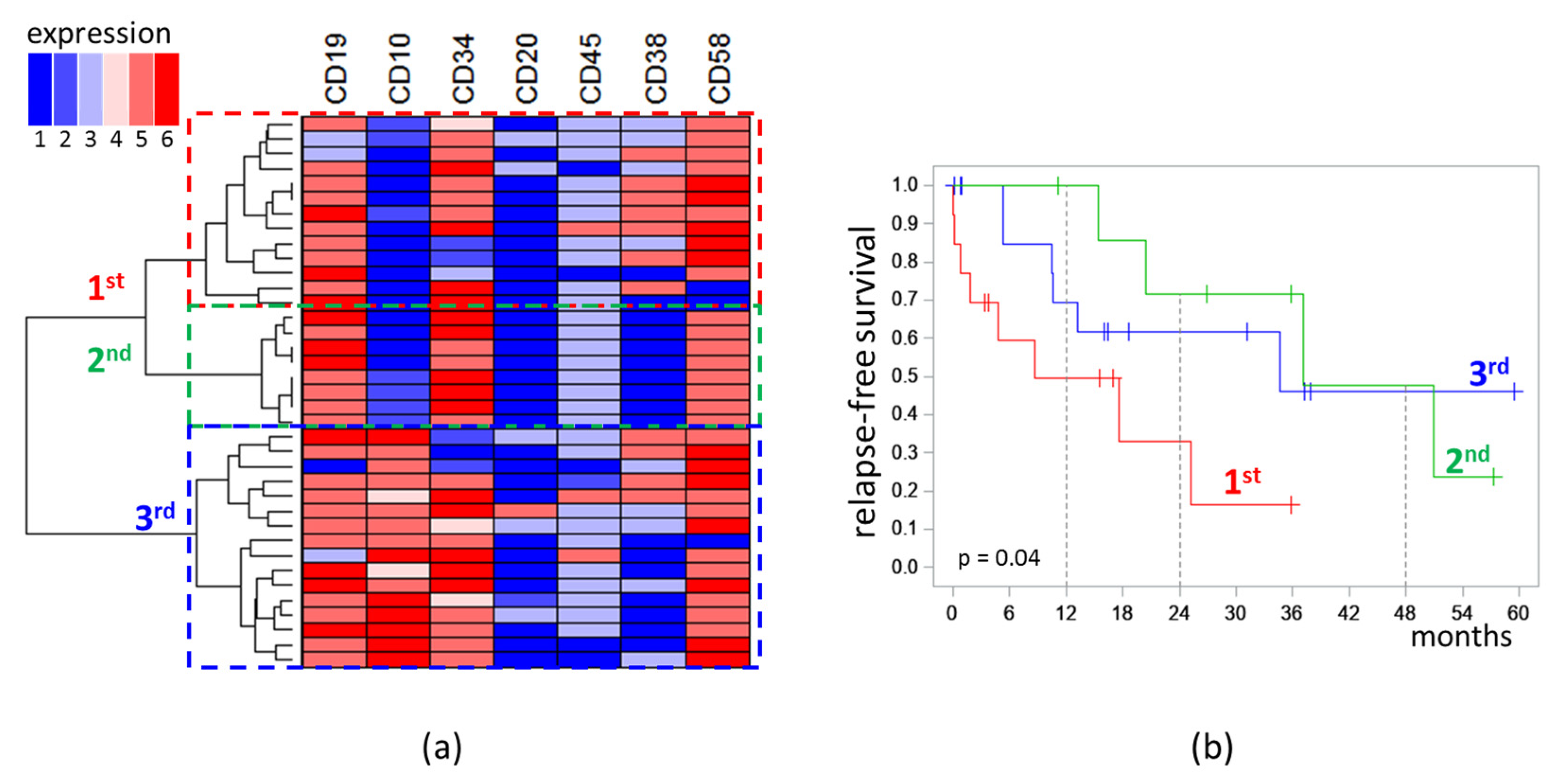
Analysis of the immunophenotype was performed only for MRD detected on day 70. MRD cases formed three distinct clusters on dendrogram. The first (n = 13) and second (n = 8) clusters were characterized by low expression of CD10 (“−” or “−/+”), while the third (n = 16) cluster consisted of CD10-positive cases (“+/−”, “+”, “high”). All cases in the second cluster were CD38-negative, whereas, in the first cluster, seven cases were CD38+, four were CD38dim, and only two cases were CD38−. High expression of CD58 was observed in the first cluster more frequently than in second one (five vs. zero cases) [Figure 4a].
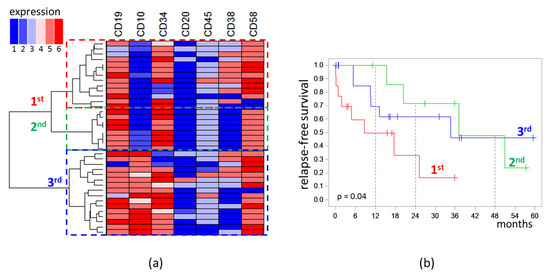
Figure 4.
Analysis of immunophenotype of measurable residual blast cells detected at the end of induction: (a) Dendrogram and heat map of semiquantitative immunophenotype description (1: “−”; 2: “−/+”; 3: “dim”; 4: “+/−”; 5: “+”; 6: “high”); (b) relapse-free survival depending on belonging to the clusters highlighted on the dendrogram. The initial date was the end of induction.
RFS of patients depended on the belonging of MRD (on day 70) to the clusters of the obtained dendrogram [Figure 4b]. In patients with MRD of the first cluster, the median of RFS was 8.7 months; in patients with MRD of the second cluster, it was 37.1 months; in patients with MRD of the third cluster, it was 34.7 months (p = 0.04). MRD amount did not differ significantly across the three clusters. In the first cluster, the median MRD percentage was 0.13%; in the second, it was 0.06%; in the third, it was 0.10% (p = 0.97).
In the first cluster, three patients had KMT2A rearrangements, one had hypoploidy, and five had other karyotype abnormalities (der(21), del(9), del(11), der(7;9), and t(11;14)). The second cluster included two cases with complex karyotype and one case with t(2;14). In the third cluster, two patients had hyperploidy, two patients had complex karyotype abnormalities, and add(6), der(19), der(7) were found in three patients.
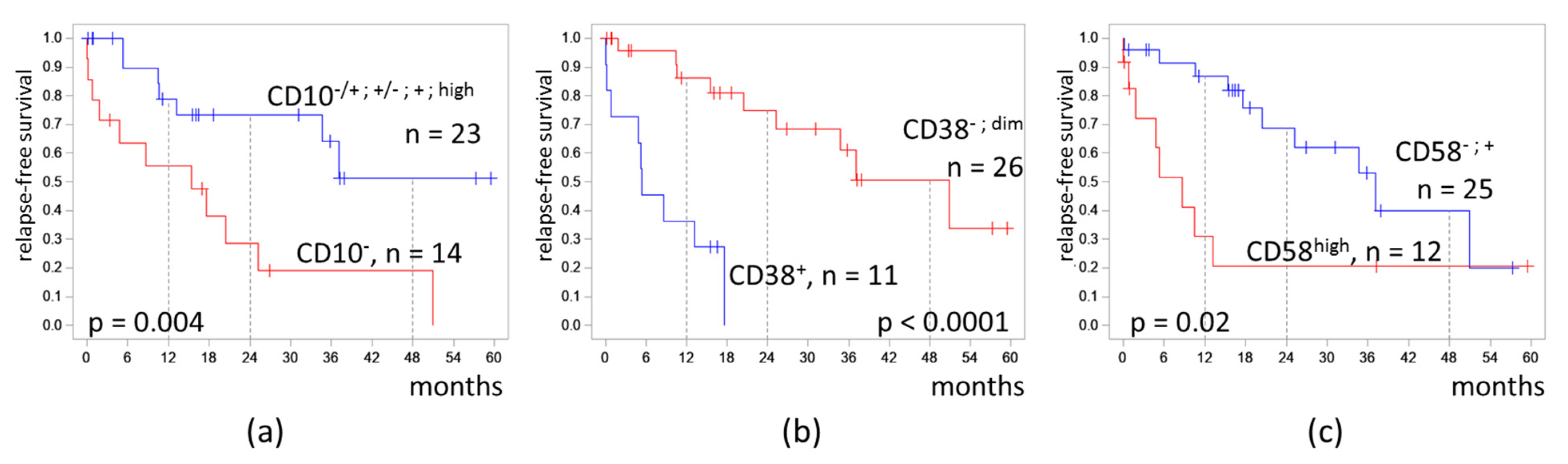
Because the clusters differed mainly in the expression of CD10, CD38, and CD58, the effect of the expression of these antigens on RFS was separately tested (Figure 5).
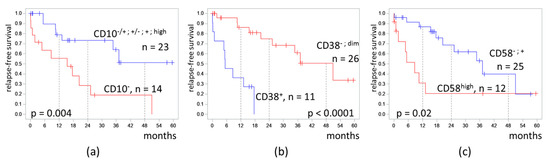
Figure 5.
Relapse-free survival of patients depending on expression of CD10 (a), CD38 (b), and CD58 (c) on measurable residual blast cells detected at the end of induction. The initial date was the end of induction.
In patients with CD10− MRD, the 4-RFS was lower than in patients with MRD with any positive part of CD10 expression (19% vs. 51%, p = 0.004). MRD percentages did not differ in these two groups (medians 0.11% vs. 0.07%, p = 0.67). Despite a CD10-negative immunophenotype of MRD, seven patients had B-II (common) ALL, and seven other patients had B-I (pro-B) ALL.
Patients with CD38-positive MRD had a lower 4-RFS compared to CD38-negative cases (0% vs. 51%, p < 0.0001). MRD amount did not differ in CD38-positive and CD38-low groups (medians 0.17% vs. 0.07%, p = 0.89).
High expression of CD58 in MRD cells also worsened the 4-RFS (21% vs. 40%, p = 0.02). MRD percentages did not differ in cases with or without high expression of CD58 (medians 0.03% vs. 0.16%, p = 0.51).
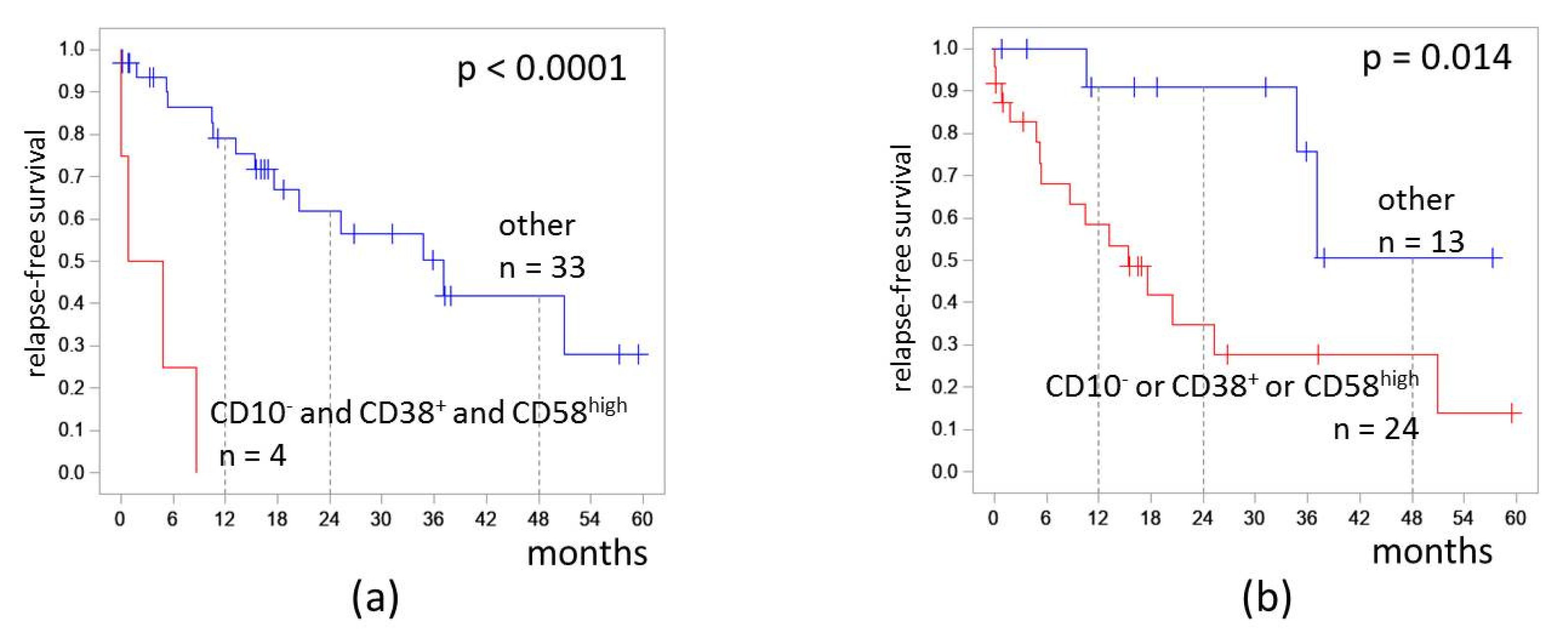
We assessed the influence of CD10, CD38, and CD58 expression combinations on RFS. Patients who had CD10−CD38+CD58high MRD (the most adverse group) had RFS 0%. The hazard ratio was 15.1 (3.7–62.3). The 4-RFS was lower in patients who had at least one of CD10−, CD38+, or CD58high (28% vs. 51%, p = 0.014; Figure 6). The hazard ratio was 4.3 (1.2–14.9).
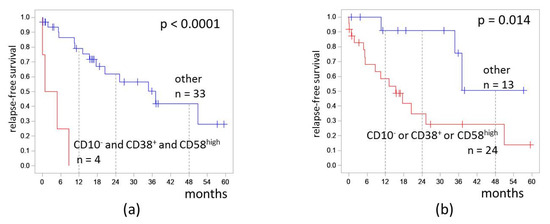
Figure 6.
Relapse-free survival of patients depending on expressions of CD10, CD38, and CD58 on measurable residual blast cells detected at the end of induction: (a) combination of CD10− and CD38+ and CD58high; (b) CD10−, CD38+, or CD58high immunophenotype. The initial date was the end of induction.
4. Discussion
MRD detected by MFC is a strong prognostic marker in ALL, as demonstrated in the presented study and in many others [6,7,8,9,12,26]. Cytostatic intensity and treatment regimen differed between different clinical trials, but comparable MRD negativity between the RALL-2016 study and previously reported data was observed. The proportions of MRD-negative B-ALL patients included in the GMALL (German Multicenter Study Group for Adult Acute Lymphoblastic Leukemia) protocol were 66% and 78% at the end of induction and consolidation, respectively [10]; in the presented study, the proportions of MRD-negative patients were 60.6% and 82.0% at the same time periods. At the end of phase 2 induction, the proportion of MRD-negative non-T-ALL patients was 68.8% in the UKALL XII/ECOG2993 study (United Kingdom arm of the international ALL trial XII/Eastern Cooperative Oncology Group) [16]. At the end of consolidation, the proportion of MRD-negative patients in the presented study was also comparable to the PETHEMA (The Programa Español de Tratamientos en Hematología) ALL-AR-03 trial, there it was 86% [9].
The 4 year RFS was lower in MRD-positive patients on days 70 and 190; however, RFS did not differ significantly depending on MRD measured on day 133. This fact could be explained by a small number of patients who were MRD-positive on days 133 and 190 of the protocol. There was a statistical trend (p = 0.064) in differences in 4 year RFS between patients depending on MRD on day 133. It is likely that, with a larger number of patients enrolled to trial or a longer follow-up period, these differences will become more pronounced.
MFC is able not only to enumerate MRD accurately, but also to describe an antigen expression profile of residual blast cells. We showed that CD10−, CD38+, and CD58high MRD is associated with worse prognosis in B-ALL patients. No studies on the MRD immunophenotype have been published thus far; however, there are a number of studies devoted to the study of the prognostic significance of the initial immunophenotype of blast cells before treatment [27,28,29,30].
CD10 is an antigen expressed by B-cell precursors and a subset of mature neutrophils [31,32]. In B-ALL, CD10 expression allows identifying B-ALL subtypes [23]. CD10-negative BI (pro-B) ALL is associated with KMT2A rearrangement, which is an adverse prognostic marker [33,34]. According to a previous study, CD10-negative patients with BIII (pro-B) ALL had better a prognosis than CD10-positive ones [35]. In the presented study, patients with CD10− MRD had lower RFS; however, only half of them had B-I (pro-B) ALL, and only three patients had KMT2A rearrangements. The other half of these patients had BII (common) B-ALL. We assume that, during therapy, the CD10-negative population of blast cells is selected, which is likely to be more malignant than the CD10-positive population.
One study showed that CD38 positivity of blast cells correlated with the presence of ETV6::RUNX1 rearrangement, and a higher number of B-ALL cells with low CD38 expression could be an early indicator of relapse risk [29,30]. CD38 is a multifunctional transmembrane glycoprotein that operates both as a receptor and as an enzyme. CD38 is involved in the metabolism of nicotinamide dinucleotide and is also a regulator of intracellular calcium homeostasis [36]. Plasma cells have the highest expression of CD38, but B cells, T cells, NK cells, myeloid cells, and precursors also express CD38 with different density [37]. CD38 is involved in B-cell differentiation and proliferation, and the CD38 expression is positive in B-cell precursors. However, B-ALL cells have a reduced expression of CD38 in most cases [38]. In the presented study, in 26 (70%) cases of MRD detected at the end of induction, CD38 expression was dim or negative. However, positive CD38 expression in MRD cells was associated with worse disease-free survival (DFS). We did not find studies on the effect of CD38 expression on the prognosis of B-ALL patients, but the significance of CD38 expression has been well studied in lymphoproliferative diseases. For example, in 27–46% of patients with chronic lymphocytic leukemia (CLL), CD38 expression was found on tumor B cells [39,40]. The presence of CD38 in CLL is associated with a worse survival prognosis, as well as an increase of CD38 expression on tumor cells, which reflects a switch to a more aggressive CLL type [41].
CD58 is an intercellular adhesion molecule that binds to the CD2 antigen which is expressed on cells. CD58 expression is found on many hematopoietic and nonhematopoietic cells, as well as malignant neoplasms, including CLL, Hodgkin’s lymphoma, multiple myeloma, and acute myeloid leukemias [42]. CD58 is often expressed higher on leukemia B lymphoblasts than on normal B-cell progenitors [38,43,44]. It is assumed that a high density of CD58 expression leads to an increase in the adhesive properties of blast cells, their proliferative activity, and the tendency to undergo apoptosis. High proliferative activity of MRD cells with high CD58 expression could explain the deterioration of DFS found in the presented study. However, another study found that CD58 negativity of initial blast cells was a predictor for adverse outcome in B-ALL patients [30]. Interestingly, in patients with pancreatic ductal adenocarcinoma, CD58 was upregulated in cancer tissues and associated with worse overall survival and disease-free survival [45].
The obtained data should be confirmed in subsequent studies, given the limitations of the present investigation, such as the low number of MRD-positive cases. Expanding the spectrum of antigens under study and comparing the immunophenotype with more detailed cytogenetics and molecular features of blast cells seems to be interesting perspective topic of research.
It should be noticed that antigen expression changes occur during treatment. For example, downmodulation of CD10 and CD34 and upmodulation of CD20 and CD45 during induction therapy was confirmed in different studies [46,47]. Thus, the prognostic significance of MRD immunophenotype and initial blast cell immunophenotype could be different.
5. Conclusions
In summary, the present study revealed that MRD cells detected by MFC at the end of induction could be clustered according to their immunophenotype, with CD10, CD38, and CD58 expression making the greatest contribution to clustering. The absence of CD10, the presence of CD38, and the high expression of CD58 by MRD cells were associated with worse DFS. These findings indicate that MRD immunophenotype could be an additional prognostic factor in B-ALL patients.
Supplementary Materials
The following supporting information can be downloaded at https://www.mdpi.com/article/10.3390/diagnostics13010021/s1: Table S1. Monoclonal antibody panels used in the study.
Author Contributions
Conceptualization, Y.D. and N.K.; methodology, Y.D., N.K. and K.N.; software, Y.D., Y.C. and S.K.; validation, I.G., S.K. and E.P.; formal analysis, Y.D. and Y.C.; investigation, Y.D. and N.K.; resources, O.A., G.I., E.K., A.S., V.T. and E.P.; data curation, Y.D., O.A., G.I., E.K., A.S. and V.T.; writing—original draft preparation, Y.D.; writing—review and editing, I.G., N.K. and K.N.; visualization, Y.D. and Y.C.; supervision, E.P.; project administration, I.G., O.A., V.T. and E.P. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
The study was conducted in accordance with the Declaration of Helsinki and approved by the Institutional Review Board of National Medical Research Center for Hematology. Protocol No. 121 dated 24 April 2017.
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study.
Data Availability Statement
Data are not shared due to privacy restrictions.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Abou Dalle, I.; Jabbour, E.; Short, N.J. Evaluation and management of measurable residual disease in acute lymphoblastic leukemia. Ther. Adv. Hematol. 2020, 11, 204062072091002. [Google Scholar] [CrossRef] [PubMed]
- Hein, K.; Short, N.; Jabbour, E.; Yilmaz, M. Clinical value of measurable residual disease in acute lymphoblastic leukemia. Blood Lymphat. Cancer 2022, 12, 7–16. [Google Scholar] [CrossRef] [PubMed]
- Kuiper, R.P.; Hoogeveen, P.G.; Bladergroen, R.; van Dijk, F.; Sonneveld, E.; van Leeuwen, F.N.; Boer, J.; Sergeeva, I.; Feitsma, H.; den Boer, M.L.; et al. Minimal residual disease (MRD) detection in acute lymphoblastic leukaemia based on fusion genes and genomic deletions: Towards MRD for all. Br. J. Haematol. 2021, 194, 888–892. [Google Scholar] [CrossRef] [PubMed]
- Berry, D.A.; Zhou, S.; Higley, H.; Mukundan, L.; Fu, S.; Reaman, G.H.; Wood, B.L.; Kelloff, G.J.; Jessup, J.M.; Radich, J.P. Association of minimal residual disease with clinical outcome in pediatric and adult acute lymphoblastic leukemia: A meta-analysis. JAMA Oncol. 2017, 3, e170580. [Google Scholar] [CrossRef] [PubMed]
- Flohr, T.; Schrauder, A.; Cazzaniga, G.; Panzer-Grümayer, R.; van der Velden, V.; Fischer, S.; Stanulla, M.; Basso, G.; Niggli, F.K.; Schäfer, B.W.; et al. Minimal residual disease-directed risk stratification using real-time quantitative PCR analysis of immunoglobulin and T-cell receptor gene rearrangements in the international multicenter trial AIEOP-BFM ALL 2000 for childhood acute lymphoblastic leukemia. Leukemia 2008, 22, 771–782. [Google Scholar] [CrossRef]
- Basso, G.; Veltroni, M.; Valsecchi, M.G.; Dworzak, M.N.; Ratei, R.; Silvestri, D.; Benetello, A.; Buldini, B.; Maglia, O.; Masera, G.; et al. Risk of relapse of childhood acute lymphoblastic leukemia is predicted by flow cytometric measurement of residual disease on day 15 bone marrow. J. Clin. Oncol. 2009, 27, 5168–5174. [Google Scholar] [CrossRef]
- Borowitz, M.J.; Pullen, D.J.; Shuster, J.J.; Viswanatha, D.; Montgomery, K.; Willman, C.L.; Camitta, B. Minimal residual disease detection in childhood precursor-B-cell acute lymphoblastic leukemia: Relation to other risk factors. A Children’s Oncology Group study. Leukemia 2003, 17, 1566–1572. [Google Scholar] [CrossRef]
- Björklund, E.; Mazur, J.; Söderhäll, S.; Porwit-MacDonald, A. Flow cytometric follow-up of minimal residual disease in bone marrow gives prognostic information in children with acute lymphoblastic leukemia. Leukemia 2003, 17, 138–148. [Google Scholar] [CrossRef]
- Ribera, J.-M.; Oriol, A.; Morgades, M.; Montesinos, P.; Sarrà, J.; González-Campos, J.; Brunet, S.; Tormo, M.; Fernández-Abellán, P.; Guàrdia, R.; et al. Treatment of high-risk Philadelphia chromosome-negative acute lymphoblastic leukemia in adolescents and adults according to early cytologic response and minimal residual disease after consolidation assessed by flow cytometry: Final results of the PETHEMA. J. Clin. Oncol. 2014, 32, 1595–1604. [Google Scholar] [CrossRef]
- Gökbuget, N.; Kneba, M.; Raff, T.; Trautmann, H.; Bartram, C.-R.; Arnold, R.; Fietkau, R.; Freund, M.; Ganser, A.; Ludwig, W.-D.; et al. Adult patients with acute lymphoblastic leukemia and molecular failure display a poor prognosis and are candidates for stem cell transplantation and targeted therapies. Blood 2012, 120, 1868–1876. [Google Scholar] [CrossRef]
- Mullighan, C.G.; Su, X.; Zhang, J.; Radtke, I.; Phillips, L.A.A.; Miller, C.B.; Ma, J.; Liu, W.; Cheng, C.; Schulman, B.A.; et al. Deletion of IKZF1 and prognosis in acute lymphoblastic leukemia. N. Engl. J. Med. 2009, 360, 470–480. [Google Scholar] [CrossRef] [PubMed]
- Borowitz, M.J.; Wood, B.L.; Devidas, M.; Loh, M.L.; Raetz, E.A.; Salzer, W.L.; Nachman, J.B.; Carroll, A.J.; Heerema, N.A.; Gastier-Foster, J.M.; et al. Prognostic significance of minimal residual disease in high risk B-ALL: A report from Children’s Oncology Group study AALL0232. Blood 2015, 126, 964–971. [Google Scholar] [CrossRef] [PubMed]
- Brüggemann, M.; Kotrova, M. Minimal residual disease in adult ALL: Technical aspects and implications for correct clinical interpretation. Hematol. Am. Soc. Hematol. Educ. Program 2017, 2017, 13–21. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Beldjord, K.; Chevret, S.; Asnafi, V.; Huguet, F.; Boulland, M.-L.; Leguay, T.; Thomas, X.; Cayuela, J.-M.; Grardel, N.; Chalandon, Y.; et al. Oncogenetics and minimal residual disease are independent outcome predictors in adult patients with acute lymphoblastic leukemia. Blood 2014, 123, 3739–3749. [Google Scholar] [CrossRef]
- Bassan, R.; Spinelli, O.; Oldani, E.; Intermesoli, T.; Tosi, M.; Peruta, B.; Rossi, G.; Borlenghi, E.; Pogliani, E.M.; Terruzzi, E.; et al. Improved risk classification for risk-specific therapy based on the molecular study of minimal residual disease (MRD) in adult acute lymphoblastic leukemia (ALL). Blood 2009, 113, 4153–4162. [Google Scholar] [CrossRef]
- Patel, B.; Rai, L.; Buck, G.; Richards, S.M.; Mortuza, Y.; Mitchell, W.; Gerrard, G.; Moorman, A.V.; Duke, V.; Hoffbrand, A.V.; et al. Minimal residual disease is a significant predictor of treatment failure in non T-lineage adult acute lymphoblastic leukaemia: Final results of the international trial UKALL XII/ECOG2993. Br. J. Haematol. 2010, 148, 80–89. [Google Scholar] [CrossRef]
- Van der Velden, V.H.J.; Cazzaniga, G.; Schrauder, A.; Hancock, J.; Bader, P.; Panzer-Grumayer, E.R.; Flohr, T.; Sutton, R.; Cave, H.; Madsen, H.O.; et al. Analysis of minimal residual disease by Ig/TCR gene rearrangements: Guidelines for interpretation of real-time quantitative PCR data. Leukemia 2007, 21, 604–611. [Google Scholar] [CrossRef]
- Pieters, R.; De Groot-Kruseman, H.; Van Der Velden, V.; Fiocco, M.; Van Den Berg, H.; De Bont, E.; Egeler, R.M.; Hoogerbrugge, P.; Kaspers, G.; Van Der Schoot, E.; et al. Successful therapy reduction and intensification for childhood acute lymphoblastic leukemia based on minimal residual disease monitoring: Study ALL10 from the Dutch Childhood Oncology Group. J. Clin. Oncol. 2016, 34, 2591–2601. [Google Scholar] [CrossRef]
- Szczepański, T.; Willemse, M.J.; Brinkhof, B.; Van Wering, E.R.; Van Der Burg, M.; Van Dongen, J.J.M. Comparative analysis of Ig and TCR gene rearrangements at diagnosis and at relapse of childhood precursor-B–ALL provides improved strategies for selection of stable PCR targets for monitoring of minimal residual disease. Blood 2002, 99, 2315–2323. [Google Scholar] [CrossRef]
- Van der Velden, V.H.J.; Brüggemann, M.; Hoogeveen, P.G.; de Bie, M.; Hart, P.G.; Raff, T.; Pfeifer, H.; Lüschen, S.; Szczepański, T.; van Wering, E.R.; et al. TCRB gene rearrangements in childhood and adult precursor-B-ALL: Frequency, applicability as MRD-PCR target, and stability between diagnosis and relapse. Leukemia 2004, 18, 1971–1980. [Google Scholar] [CrossRef]
- Wood, B.L. Principles of minimal residual disease detection for hematopoietic neoplasms by flow cytometry. Cytom. B Clin. Cytom. 2016, 90, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Dworzak, M.N.; Gaipa, G.; Ratei, R.; Veltroni, M.; Schumich, A.; Maglia, O.; Karawajew, L.; Benetello, A.; Pötschger, U.; Husak, Z.; et al. Standardization of flow cytometric minimal residual disease evaluation in acute lymphoblastic leukemia: Multicentric assessment is feasible. Cytom. Part B Clin. Cytom. 2008, 74, 331–340. [Google Scholar] [CrossRef] [PubMed]
- Bene, M.C.; Castoldi, G.; Knapp, W.; Ludwig, W.D.; Matutes, E.; Orfao, A.; Van’t Veer, M.B. Proposals for the immunological classification of acute leukemias. European Group for the Immunological Characterization of Leukemias (EGIL). Leukemia 1995, 9, 1783–1786. [Google Scholar] [PubMed]
- Galtseva, I.V.; Davydova, Y.O.; Parovichnikova, E.N.; Gavrilina, O.A.; Troitskaya, V.V.; Kapranov, N.M.; Nikiforova, K.A.; Isinova, G.A.; Zarubina, K.I.; Sokolov, A.N.; et al. Minimal residual disease and b-cell subpopulation monitoring in acute B-Iymphoblastic leukaemia patients treated on RALL-2016 protocol. Gematol. Transfusiologiya 2021, 66, 192–205. (In Russian) [Google Scholar] [CrossRef]
- Dworzak, M.N.; Buldini, B.; Gaipa, G.; Ratei, R.; Hrusak, O.; Luria, D.; Rosenthal, E.; Bourquin, J.P.; Sartor, M.; Schumich, A.; et al. AIEOP-BFM consensus guidelines 2016 for flow cytometric immunophenotyping of Pediatric acute lymphoblastic leukemia. Cytom. Part B Clin. Cytom. 2018, 94, 82–93. [Google Scholar] [CrossRef]
- Modvig, S.; Hallböök, H.; Madsen, H.O.; Siitonen, S.; Rosthøj, S.; Tierens, A.; Juvonen, V.; Osnes, L.T.N.; Vålerhaugen, H.; Hultdin, M.; et al. Value of flow cytometry for MRD-based relapse prediction in B-cell precursor ALL in a multicenter setting. Leukemia 2020, 35, 1894–1906. [Google Scholar] [CrossRef]
- Kulis, J.; Wawrowski, Ł.; Sędek, Ł.; Wróbel, Ł.; Słota, Ł.; van der Velden, V.H.J.; Szczepański, T.; Sikora, M. Machine learning based analysis of relations between antigen expression and genetic aberrations in childhood B-cell precursor acute lymphoblastic leukaemia. J. Clin. Med. 2022, 11, 2281. [Google Scholar] [CrossRef]
- Kulis, J.; Sędek, Ł.; Słota, Ł.; Perkowski, B.; Szczepański, T. Commonly assessed markers in childhood BCP-ALL diagnostic panels and their association with genetic aberrations and outcome prediction. Genes 2022, 13, 1374. [Google Scholar] [CrossRef]
- Chulián, S.; Martínez-Rubio, Á.; Pérez-García, V.M.; Rosa, M.; Goñi, C.B.; Gutiérrez, J.F.R.; Hermosín-Ramos, L.; Quintana, Á.M.; Caballero-Velázquez, T.; Ramírez-Orellana, M.; et al. High-dimensional analysis of single-cell flow cytometry data predicts relapse in childhood acute lymphoblastic leukaemia. Cancers 2021, 13, 17. [Google Scholar] [CrossRef]
- Li, X.M.; Zhang, L.P.; Wang, Y.Z.; Lu, A.D.; Chang, Y.; Zhu, H.H.; Qin, Y.Z.; Lai, Y.Y.; Kong, Y.; Huang, X.J.; et al. CD38+ CD58− is an independent adverse prognostic factor in paediatric Philadelphia chromosome negative B cell acute lymphoblastic leukaemia patients. Leuk. Res. 2016, 43, 33–38. [Google Scholar] [CrossRef]
- Theunissen, P.M.J.; Sedek, L.; De Haas, V.; Szczepanski, T.; Van Der Sluijs, A.; Mejstrikova, E.; Nováková, M.; Kalina, T.; Lecrevisse, Q.; Orfao, A.; et al. Detailed immunophenotyping of B-cell precursors in regenerating bone marrow of acute lymphoblastic leukaemia patients: Implications for minimal residual disease detection. Br. J. Haematol. 2017, 178, 257–266. [Google Scholar] [CrossRef]
- Chung, J.-W.; Park, C.-J.; Cha, C.-H.; Cho, Y.-U.; Jang, S.; Chi, H.-S.; Seo, E.-J.; Lee, J.-H.; Lee, J.-H.; Lee, K.-H.; et al. A combination of CD15/CD10, CD64/CD33, CD16/CD13 or CD11b flow cytometric granulocyte panels is sensitive and specific for diagnosis of myelodysplastic syndrome. Ann. Clin. Lab. Sci. 2012, 42, 271–280. [Google Scholar]
- Schwartz, S.; Rieder, H.; Schläger, B.; Burmeister, T.; Fischer, L.; Thiel, E. Expression of the human homologue of rat NG2 in adult acute lymphoblastic leukemia: Close association with MLL rearrangement and a CD10−/CD24−/CD65s+/CD15+ B-cell phenotype. Leukemia 2003, 17, 1589–1595. [Google Scholar] [CrossRef]
- Gleissner, B.; Goekbuget, N.; Rieder, H.; Arnold, R.; Schwartz, S.; Diedrich, H.; Schoch, C.; Heinze, B.; Fonatsch, C.; Bartram, C.R.; et al. CD10− pre-B acute lymphoblastic leukemia (ALL) is a distinct high-risk subgroup of adult ALL associated with a high frequency of MLL aberrations: Results of the German Multicenter Trials for Adult ALL (GMALL). Blood 2005, 106, 4054–4056. [Google Scholar] [CrossRef]
- Ali Shah, M.; Ahmad, U.; Tariq Mahmood, M.; Ahmad, A.H.; Abu Bakar, M. Frequency of CD34 and CD10 expression in adolescent and young adult patients having precursor B-cell acute lymphoblastic leukemia and its correlation with clinical outcomes: A single-center study. Cureus 2022, 14, e21261. [Google Scholar] [CrossRef]
- Hogan, K.A.; Chini, C.C.S.; Chini, E.N. The multi-faceted ecto-enzyme CD38: Roles in immunomodulation, cancer, aging, and metabolic diseases. Front. Immunol. 2019, 10, 1187. [Google Scholar] [CrossRef] [PubMed]
- Calabretta, E.; Carlo-Stella, C. The many facets of CD38 in lymphoma: From tumor-microenvironment cell interactions to acquired resistance to immunotherapy. Cells 2020, 9, 802. [Google Scholar] [CrossRef]
- Karawajew, L.; Dworzak, M.; Ratei, R.; Rhein, P.; Gaipa, G.; Buldini, B.; Basso, G.; Hrusak, O.; Ludwig, W.D.; Henze, G.; et al. Minimal residual disease analysis by eight-color flow cytometry in relapsed childhood acute lymphoblastic leukemia. Haematologica 2015, 100, 935–944. [Google Scholar] [CrossRef]
- Ibrahim, S.; Keating, M.; Do, K.A.; O’Brien, S.; Huh, Y.O.; Jilani, I.; Lerner, S.; Kantarjian, H.M.; Albitar, M. CD38 expression as an important prognostic factor in B-cell chronic lymphocytic leukemia. Blood 2001, 98, 181–186. [Google Scholar] [CrossRef]
- Del Poeta, G.; Maurillo, L.; Venditti, A.; Buccisano, F.; Epiceno, A.M.; Capelli, G.; Tamburini, A.; Suppo, G.; Battaglia, A.; Del Principe, M.I.; et al. Clinical significance of CD38 expression in chronic lymphocytic leukemia. Blood 2001, 98, 2633–2639. [Google Scholar] [CrossRef]
- Chang, C.C.; Cleveland, R.P. Conversion of CD38 and/or myeloid-associated marker expression status during the course of B-CLL: Association with a change to an aggressive clinical course. Blood 2002, 100, 1106. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Lee, R.V.; Braylan, R.C.; Rimsza, L.M. CD58 expression decreases as nonmalignant B cells mature in bone marrow and is frequently overexpressed in adult and pediatric precursor B-cell acute lymphoblastic leukemia. Am. J. Clin. Pathol. 2005, 123, 119–124. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.S.; Coustan-Smith, E.; Suzuki, T.; Neale, G.A.; Mihara, K.; Pui, C.H.; Campana, D. Identification of novel markers for monitoring minimal residual disease in acute lymphoblastic leukemia. Blood 2001, 97, 2115–2120. [Google Scholar] [CrossRef] [PubMed]
- De Waele, M.; Renmans, W.; Jochmans, K.; Schots, R.; Lacor, P.; Trullemans, F.; Otten, J.; Balduck, N.; Vander Gucht, K.; Van Camp, B.; et al. Different expression of adhesion molecules on CD34+ cells in AML and B-lineage ALL and their normal bone marrow counterparts. Eur. J. Haematol. 1999, 63, 192–201. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Q.; Liu, J.; Liao, Q. Upregulated CD58 is associated with clinicopathological characteristics and poor prognosis of patients with pancreatic ductal adenocarcinoma. Cancer Cell Int. 2021, 21, 327. [Google Scholar] [CrossRef]
- Dworzak, M.N.; Gaipa, G.; Schumich, A.; Maglia, O.; Ratei, R.; Veltroni, M.; Husak, Z.; Basso, G.; Karawajew, L.; Gadner, H.; et al. Modulation of antigen expression in B-cell precursor acute lymphoblastic leukemia during induction therapy is partly transient: Evidence for a drug-induced regulatory phenomenon. Results of the AIEOP-BFM-ALL-FLOW-MRD-Study Group. Cytom. B Clin. Cytom. 2010, 78, 147–153. [Google Scholar] [CrossRef]
- Burnusuzov, H.A.; Spasova, M.I.; Murdjeva, M.A.; Stoyanova, A.A.; Mumdziev, I.N.; Kaleva, V.I.; Belcheva, M.I.; Bosheva, M.N. Immunophenotypic modulation of the blast cells in childhood acute lymphoblastic leukemia minimal residual disease detection. Folia Med. 2016, 58, 28–35. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).