Coronary Computed Tomography vs. Cardiac Magnetic Resonance Imaging in the Evaluation of Coronary Artery Disease
Abstract
1. Introduction
2. Diagnostic Performance of cCTA vs. CMR
3. Advances in cCTA
4. Advances in CMR
5. Safety Profile of cCTA vs. CMR
6. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| ASA | Acetylsalicylic acid |
| CAD | Coronary artery disease |
| cCTA | Coronary computed tomography angiography |
| CMR | Cardiac magnetic resonance imaging |
| CT | Computed tomography |
| CTP | CT perfusion |
| DECT | Dual-energy computed tomography |
| ECG | Electrocardiogram |
| FFR | Fractional Flow Reserve |
| GTN | Glyceryl Trinitrate |
| LAD | Left anterior descending artery |
| LGE | Late Gadolinium Enhancement |
| MACE | Major adverse cardiac event |
| MINOCA | Myocardial infarction with nonobstructive coronary arteries |
| NICE | National Institute for Health and Care Excellence |
References
- Roth, G.A.; Mensah, G.A.; Johnson, C.O.; Addolorato, G.; Ammirati, E.; Baddour, L.M.; Barengo, N.C.; Beaton, A.Z.; Benjamin, E.J.; Benziger, C.P.; et al. Global burden of cardiovascular diseases and risk factors, 1990–2019: Update from the GBD 2019 study. J. Am. Coll. Cardiol. 2020, 76, 2982–3021. [Google Scholar] [CrossRef] [PubMed]
- Knuuti, J.; Wijns, W.; Saraste, A.; Capodanno, D.; Barbato, E.; Funck-Brentano, C.; Prescott, E.; Storey, R.F.; Deaton, C.; Cuisset, T.; et al. 2019 Esc Guidelines for the Diagnosis and Management of Chronic Coronary Syndromes. Eur. Heart J. 2020, 41, 407–477. [Google Scholar] [CrossRef] [PubMed]
- Writing Committee Members; Gulati, M.; Levy, P.D.; Mukherjee, D.; Amsterdam, E.; Bhatt, D.L.; Birtcher, K.K.; Blankstein, R.; Boyd, J.; Bullock-Palmer, R.P.; et al. 2021 AHA/ACC/ASE/CHEST/SAEM/SCCT/SCMR guideline for the evaluation and diagnosis of chest pain: A report of the American College of Cardiology/American Heart Association Joint Committee on Clinical Practice Guidelines. J. Am. Coll. Cardiol. 2021, 78, e187–e285. [Google Scholar] [PubMed]
- National Institute for Health and Clinical Excellence (NICE). Chest Pain of Recent Onset: Assessment and Diagnosis of Recent Onset Chest Pain or Discomfort of Suspected Cardiac Origin (Update); NICE: London, UK, 2016. [Google Scholar]
- Baessato, F.; Guglielmo, M.; Muscogiuri, G.; Baggiano, A.; Fusini, L.; Scafuri, S.; Babbaro, M.; Mollace, R.; Collevecchio, A.; Guaricci, A.I.; et al. Stress CMR in Known or Suspected CAD: Diagnostic and Prognostic Role. BioMed. Res. Int. 2021, 2021, 6678029. [Google Scholar] [CrossRef]
- Reeves, R.A.; Halpern, E.J.; Rao, V.M. Cardiac Imaging Trends from 2010 to 2019 in the Medicare Population. Radiol. Cardiothorac. Imaging 2021, 3, e210156. [Google Scholar] [CrossRef]
- Foldyna, B.; Uhlig, J.; Gohmann, R.; Lücke, C.; Mayrhofer, T.; Lehmkuhl, L.; Natale, L.; Vliegenthart, R.; Lotz, J.; Salgado, R.; et al. Quality and safety of coronary computed tomography angiography at academic and non-academic sites: Insights from a large European registry (ESCR MR/CT Registry). Eur. Radiol. 2022, 32, 5246–5255. [Google Scholar] [CrossRef]
- Menadas, J.V.M.; Gonzalez, M.P.G.; Lopez-Lereu, M.P.; Ortega, L.H.; Gonzalez, A.M.M. Safety and tolerability of regadenoson in comparison with adenosine stress cardiovascular magnetic resonance: Data from a multicentre prospective registry. Int. J. Cardiovasc. Imaging 2021, 38, 195–209. [Google Scholar] [CrossRef]
- Knuuti, J.; Ballo, H.; Juarez-Orozco, L.E.; Saraste, A.; Kolh, P.; Rutjes, A.W.S.; Jüni, P.; Windecker, S.; Bax, J.J.; Wijns, W. The performance of non-invasive tests to rule-in and rule-out significant coronary artery stenosis in patients with stable angina: A meta-analysis focused on post-test disease probability. Eur. Heart J. 2018, 39, 3322–3330. [Google Scholar] [CrossRef]
- Mancini, G.J.; Leipsic, J.; Budoff, M.J.; Hague, C.J.; Min, J.K.; Stevens, S.R.; Reynolds, H.R.; O’Brien, S.M.; Shaw, L.J.; Manjunath, C.N.; et al. CT Angiography Followed by Invasive Angiography in Patients With Moderate or Severe Ischemia-Insights From the ISCHEMIA Trial. JACC Cardiovasc. Imaging 2021, 14, 1384–1393. [Google Scholar] [CrossRef]
- Vanhecke, T.E.; Madder, R.; Weber, J.E.; Bielak, L.F.; Peyser, P.A.; Chinnaiyan, K.M. Development and Validation of a Predictive Screening Tool for Uninterpretable Coronary CT Angiography Results. Circ. Cardiovasc. Imaging 2011, 4, 490–497. [Google Scholar] [CrossRef]
- Virmani, R.; Burke, A.P.; Farb, A.; Kolodgie, F.D. Pathology of the vulnerable plaque. J. Am. Coll. Cardiol. 2006, 47, C13–C18. [Google Scholar] [CrossRef]
- A Ferraro, R.; Van Rosendael, A.R.; Lu, Y.; Andreini, D.; Al-Mallah, M.H.; Cademartiri, F.; Chinnaiyan, K.; Chow, B.J.W.; Conte, E.; Cury, R.C.; et al. Non-obstructive high-risk plaques increase the risk of future culprit lesions comparable to obstructive plaques without high-risk features: The ICONIC study. Eur. Heart J. Cardiovasc. Imaging 2020, 21, 973–980. [Google Scholar] [CrossRef]
- von Knebel Doeberitz, P.L.; De Cecco, C.N.; Schoepf, U.J.; Albrecht, M.H.; van Assen, M.; De Santis, D.; Gaskins, J.; Martin, S.; Bauer, M.J.; Ebersberger, U.; et al. Impact of Coronary Computerized Tomography Angiography-Derived Plaque Quantification and Machine-Learning Computerized Tomography Fractional Flow Reserve on Adverse Cardiac Outcome. Am. J. Cardiol. 2019, 124, 1340–1348. [Google Scholar] [CrossRef]
- Williams, M.C.; Moss, A.J.; Dweck, M.; Adamson, P.D.; Alam, S.; Hunter, A.; Shah, A.S.; Pawade, T.; Weir-McCall, J.R.; Roditi, G.; et al. Coronary Artery Plaque Characteristics Associated With Adverse Outcomes in the SCOT-HEART Study. J. Am. Coll. Cardiol. 2019, 73, 291–301. [Google Scholar] [CrossRef]
- Chow, B.J.; Small, G.; Yam, Y.; Chen, L.; McPherson, R.; Achenbach, S.; Al-Mallah, M.; Berman, D.S.; Budoff, M.J.; Cademartiri, F. Prognostic and Therapeutic Implications of Statin and Aspirin Therapy in Individuals with Nonobstructive Coronary Artery Disease: Results from the Confirm (Coronary Ct Angiography Evaluation for Clinical Outcomes: An International Multicenter Registry) Registry. Arter. Thromb. Vasc. Biol. 2015, 35, 981–989. [Google Scholar]
- Hoffmann, U.; Ferencik, M.; Udelson, J.E.; Picard, M.H.; Truong, Q.A.; Patel, M.R.; Huang, M.; Pencina, M.; Mark, D.B.; Heitner, J.F. Prognostic Value of Noninvasive Cardiovascular Testing in Patients with Stable Chest Pain: Insights from the Promise Trial (Prospective Multicenter Imaging Study for Evaluation of Chest Pain). Circulation 2017, 135, 2320–2332. [Google Scholar] [CrossRef]
- Scot-Heart investigators. Ct Coronary Angiography in Patients with Suspected Angina Due to Coronary Heart Disease (Scot-Heart): An Open-Label, Parallel-Group, Multicentre Trial. Lancet 2015, 385, 2383–2391. [Google Scholar] [CrossRef]
- Williams, M.C.; Hunter, A.; Shah, A.S.V.; Assi, V.; Lewis, S.; Smith, J.; Berry, C.; Boon, N.A.; Clark, E.; Flather, M.; et al. Use of Coronary Computed Tomographic Angiography to Guide Management of Patients With Coronary Disease. J. Am. Coll. Cardiol. 2016, 67, 1759–1768. [Google Scholar] [CrossRef]
- Douglas, P.S.; Hoffmann, U.; Patel, M.R.; Mark, D.B.; Al-Khalidi, H.R.; Cavanaugh, B.; Cole, J.; Dolor, R.J.; Fordyce, C.B.; Huang, M.; et al. Outcomes of Anatomical versus Functional Testing for Coronary Artery Disease. New Engl. J. Med. 2015, 372, 1291–1300. [Google Scholar] [CrossRef]
- Øvrehus, K.A.; Diederichsen, A.; Grove, E.L.; Steffensen, F.H.; Mortensen, M.B.; Jensen, J.M.; Mickley, H.; Nielsen, L.H.; Busk, M.; Sand, N.P.R.; et al. Reduction of Myocardial Infarction and All-Cause Mortality Associated to Statins in Patients Without Obstructive CAD. JACC Cardiovasc. Imaging 2021, 14, 2400–2410. [Google Scholar] [CrossRef]
- Mortensen, M.B.; Steffensen, F.H.; Bøtker, H.E.; Jensen, J.M.; Sand, N.P.R.; Kragholm, K.H.; Kanstrup, H.; Sørensen, H.T.; Leipsic, J.; Blaha, M.J.; et al. CAD Severity on Cardiac CTA Identifies Patients With Most Benefit of Treating LDL-Cholesterol to ACC/AHA and ESC/EAS Targets. JACC Cardiovasc. Imaging 2020, 13, 1961–1972. [Google Scholar] [CrossRef] [PubMed]
- Jørgensen, M.E.; Andersson, C.; Nørgaard, B.; Abdulla, J.; Shreibati, J.B.; Torp-Pedersen, C.; Gislason, G.; Shaw, R.E.; Hlatky, M. Functional Testing or Coronary Computed Tomography Angiography in Patients With Stable Coronary Artery Disease. J. Am. Coll. Cardiol. 2017, 69, 1761–1770. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.K.; Desai, M.Y.; Marwan, M.; Kotanidis, C.P.; Antonopoulos, A.S.; Schottlander, D.; Channon, K.M.; Neubauer, S.; Achenbach, S.; Antoniades, C. Perivascular Fat Attenuation Index Stratifies Cardiac Risk Associated With High-Risk Plaques in the CRISP-CT Study. J. Am. Coll. Cardiol. 2020, 76, 755–757. [Google Scholar] [CrossRef] [PubMed]
- Oikonomou, E.; Marwan, M.; Desai, M.Y.; Mancio, J.; Alashi, A.; Centeno, E.H.; Thomas, S.; Herdman, L.; Kotanidis, C.; Thomas, K.E.; et al. Non-invasive detection of coronary inflammation using computed tomography and prediction of residual cardiovascular risk (the CRISP CT study): A post-hoc analysis of prospective outcome data. Lancet 2018, 392, 929–939. [Google Scholar] [CrossRef] [PubMed]
- The DISCHARGE Trial Group; Maurovich-Horvat, P.; Bosserdt, M.; Kofoed, K.F.; Rieckmann, N.; Benedek, T.; Donnelly, P.; Rodriguez-Palomares, J.; Erglis, A.; Štěchovský, C.; et al. CT or Invasive Coronary Angiography in Stable Chest Pain. New Engl. J. Med. 2022, 386, 1591–1602. [Google Scholar] [CrossRef]
- Stillman, A.E.; Oudkerk, M.; Bluemke, D.A.; de Boer, M.J.; Bremerich, J.; Garcia, E.V.; Gutberlet, M.; van der Harst, P.; Hundley, W.G.; Jerosch-Herold, M.; et al. Imaging the myocardial ischemic cascade. Int. J. Cardiovasc. Imaging 2018, 34, 1249–1263. [Google Scholar] [CrossRef]
- Belardinelli, L.; Shryock, J.C.; Snowdy, S.; Zhang, Y.; Monopoli, A.; Lozza, G.; Ongini, E.; A Olsson, R.; Dennis, D.M. The A2A adenosine receptor mediates coronary vasodilation. J. Pharmacol. Exp. Ther. 1998, 284, 1066–1073. [Google Scholar]
- Nagel, E.; Greenwood, J.P.; McCann, G.P.; Bettencourt, N.; Shah, A.M.; Hussain, S.T.; Perera, D.; Plein, S.; Bucciarelli-Ducci, C.; Paul, M.; et al. Magnetic Resonance Perfusion or Fractional Flow Reserve in Coronary Disease. New Engl. J. Med. 2019, 380, 2418–2428. [Google Scholar] [CrossRef]
- Greenwood, J.P.; Ripley, D.P.; Berry, C.; McCann, G.P.; Plein, S.; Bucciarelli-Ducci, C.; Dall, E.; Prasad, A.; Bijsterveld, P.; Foley, J.R.; et al. Effect of Care Guided by Cardiovascular Magnetic Resonance, Myocardial Perfusion Scintigraphy, or Nice Guidelines on Subsequent Unnecessary Angiography Rates: The Ce-Marc 2 Randomized Clinical Trial. JAMA 2016, 316, 1051–1060. [Google Scholar] [CrossRef]
- Kwong, R.Y.; Ge, Y.; Steel, K.; Bingham, S.; Abdullah, S.; Fujikura, K.; Wang, W.; Pandya, A.; Chen, Y.-Y.; Mikolich, J.R.; et al. Cardiac Magnetic Resonance Stress Perfusion Imaging for Evaluation of Patients With Chest Pain. J. Am. Coll. Cardiol. 2019, 74, 1741–1755. [Google Scholar] [CrossRef]
- Lipinski, M.J.; McVey, C.M.; Berger, J.S.; Kramer, C.M.; Salerno, M. Prognostic Value of Stress Cardiac Magnetic Resonance Imaging in Patients With Known or Suspected Coronary Artery Disease: A Systematic Review and Meta-Analysis. J. Am. Coll. Cardiol. 2013, 62, 826–838. [Google Scholar] [CrossRef]
- Gu, H.; Bing, R.; Chin, C.; Fang, L.; White, A.C.; Everett, R.; Spath, N.; Park, E.; Chambers, J.B.; Newby, D.E.; et al. First-phase ejection fraction by cardiovascular magnetic resonance predicts outcomes in aortic stenosis. J. Cardiovasc. Magn. Reson. 2021, 23, 73. [Google Scholar] [CrossRef]
- Soto-Iglesias, D.; Penela, D.; Jáuregui, B.; Acosta, J.; Fernández-Armenta, J.; Linhart, M.; Zucchelli, G.; Syrovnev, V.; Zaraket, F.; Terés, C.; et al. Cardiac Magnetic Resonance-Guided Ventricular Tachycardia Substrate Ablation. JACC Clin. Electrophysiol. 2020, 6, 436–447. [Google Scholar] [CrossRef]
- Bisbal, F.; Guiu, E.; Cabanas-Grandío, P.; Berruezo, A.; Prat-Gonzalez, S.; Vidal, B.; Garrido, C.; Andreu, D.; Fernandez-Armenta, J.; Tolosana, J.M.; et al. CMR-Guided Approach to Localize and Ablate Gaps in Repeat AF Ablation Procedure. JACC Cardiovasc. Imaging 2014, 7, 653–663. [Google Scholar] [CrossRef]
- Pegg, T.J.; Selvanayagam, J.B.; Jennifer, J.; Francis, J.M.; Karamitsos, T.D.; Dall, E.; Smith, K.L.; Taggart, D.P.; Neubauer, S. Prediction of Global Left Ventricular Functional Recovery in Patients with Heart Failure Undergoing Surgical Revascularisation, Based on Late Gadolinium Enhancement Cardiovascular Magnetic Resonance. J. Cardiovasc. Magn. Reson. 2010, 12, 56. [Google Scholar] [CrossRef]
- Klug, G.; Mayr, A.; Schenk, S.; Esterhammer, R.; Schocke, M.; Nocker, M.; Jaschke, W.; Pachinger, O.; Metzler, B. Prognostic value at 5 years of microvascular obstruction after acute myocardial infarction assessed by cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2012, 14, 46. [Google Scholar] [CrossRef]
- Wu, K.C.; Weiss, R.G.; Thiemann, D.R.; Kitagawa, K.; Schmidt, A.; Dalal, D.; Lai, S.; Bluemke, D.A.; Gerstenblith, G.; Marbán, E.; et al. Late Gadolinium Enhancement by Cardiovascular Magnetic Resonance Heralds an Adverse Prognosis in Nonischemic Cardiomyopathy. J. Am. Coll. Cardiol. 2008, 51, 2414–2421. [Google Scholar] [CrossRef]
- Solomon, S.D.; Anavekar, N.; Skali, H.; McMurray, J.J.; Swedberg, K.; Yusuf, S.; Granger, C.B.; Michelson, E.L.; Wang, D.; Pocock, S.; et al. Influence of Ejection Fraction on Cardiovascular Outcomes in a Broad Spectrum of Heart Failure Patients. Circulation 2005, 112, 3738–3744. [Google Scholar] [CrossRef]
- Scott, P.A.; Rosengarten, J.A.; Murday, D.C.; Peebles, C.R.; Harden, S.P.; Curzen, N.P.; Morgan, J.M. Left Ventricular Scar Burden Specifies the Potential for Ventricular Arrhythmogenesis: An LGE-CMR Study. J. Cardiovasc. Electrophysiol. 2012, 24, 430–436. [Google Scholar] [CrossRef]
- Reynolds, H.R.; Maehara, A.; Kwong, R.Y.; Sedlak, T.; Saw, J.; Smilowitz, N.R.; Mahmud, E.; Wei, J.; Marzo, K.; Matsumura, M.; et al. Coronary Optical Coherence Tomography and Cardiac Magnetic Resonance Imaging to Determine Underlying Causes of Myocardial Infarction With Nonobstructive Coronary Arteries in Women. Circulation 2021, 143, 624–640. [Google Scholar] [CrossRef]
- Ferreira, V.M.; Schulz-Menger, J.; Holmvang, G.; Kramer, C.M.; Carbone, I.; Sechtem, U.; Kindermann, I.; Gutberlet, M.; Cooper, L.T.; Liu, P.; et al. Cardiovascular Magnetic Resonance in Nonischemic Myocardial Inflammation: Expert Recommendations. J. Am. Coll. Cardiol. 2018, 72, 3158–3176. [Google Scholar] [CrossRef] [PubMed]
- Patel, A.R.; Kramer, C.M. Role of Cardiac Magnetic Resonance in the Diagnosis and Prognosis of Nonischemic Cardiomyopathy. JACC Cardiovasc. Imaging 2017, 10, 1180–1193. [Google Scholar] [CrossRef] [PubMed]
- Tsurusaki, M.; Sofue, K.; Hori, M.; Sasaki, K.; Ishii, K.; Murakami, T.; Kudo, M. Dual-Energy Computed Tomography of the Liver: Uses in Clinical Practices and Applications. Diagnostics 2021, 11, 161. [Google Scholar] [CrossRef] [PubMed]
- Siemens Healthcare GmbH. Somatom Force Product Brochure; Siemens Healthcare GmbH: Erlangen, Germany, 2020. [Google Scholar]
- Vingiani, V.; Abadia, A.F.; Schoepf, U.J.; Fischer, A.M.; Varga-Szemes, A.; Sahbaee, P.; Allmendinger, T.; Tesche, C.; Griffith, L.P.; Marano, R.; et al. Low-Kv Coronary Artery Calcium Scoring with Tin Filtration Using a Kv-Independent Reconstruction Algorithm. J. Cardiovasc. Comput. Tomogr. 2020, 14, 246–250. [Google Scholar] [CrossRef]
- Si-Mohamed, S.A.; Boccalini, S.; Lacombe, H.; Diaw, A.; Varasteh, M.; Rodesch, P.-A.; Dessouky, R.; Villien, M.; Tatard-Leitman, V.; Bochaton, T.; et al. Coronary CT Angiography with Photon-counting CT: First-In-Human Results. Radiology 2022, 303, 303–313. [Google Scholar] [CrossRef]
- André, F.; Fortner, P.; Vembar, M.; Mueller, D.; Stiller, W.; Buss, S.J.; Kauczor, H.-U.; Katus, H.A.; Korosoglou, G. Improved image quality with simultaneously reduced radiation exposure: Knowledge-based iterative model reconstruction algorithms for coronary CT angiography in a clinical setting. J. Cardiovasc. Comput. Tomogr. 2017, 11, 213–220. [Google Scholar] [CrossRef]
- Andreini, D.; Lin, F.Y.; Rizvi, A.; Cho, I.; Heo, R.; Pontone, G.; Bartorelli, A.L.; Mushtaq, S.; Villines, T.C.; Carrascosa, P.; et al. Diagnostic Performance of a Novel Coronary CT Angiography Algorithm: Prospective Multicenter Validation of an Intracycle CT Motion Correction Algorithm for Diagnostic Accuracy. Am. J. Roentgenol. 2018, 210, 1208–1215. [Google Scholar] [CrossRef]
- Deseive, S.; Chen, M.Y.; Korosoglou, G.; Leipsic, J.; Martuscelli, E.; Carrascosa, P.; Mirsadraee, S.; White, C.; Hadamitzky, M.; Martinoff, S.; et al. Prospective Randomized Trial on Radiation Dose Estimates of Ct Angiography Applying Iterative Image Reconstruction: The Protection V Study. JACC Cardiovasc. Imaging 2015, 8, 888–896. [Google Scholar] [CrossRef]
- Lossnitzer, D.; Klenantz, S.; Andre, F.; Goerich, J.; Schoepf, U.J.; Pazzo, K.L.; Sommer, A.; Brado, M.; Gückel, F.; Sokiranski, R.; et al. Stable patients with suspected myocardial ischemia: Comparison of machine-learning computed tomography-based fractional flow reserve and stress perfusion cardiovascular magnetic resonance imaging to detect myocardial ischemia. BMC Cardiovasc. Disord. 2022, 22, 34. [Google Scholar] [CrossRef]
- Coenen, A.; Kim, Y.H.; Kruk, M.; Tesche, C.; De Geer, J.; Kurata, A.; Lubbers, M.L.; Daemen, J.; Itu, L.; Rapaka, S.; et al. Diagnostic Accuracy of a Machine-Learning Approach to Coronary Computed Tomographic Angiography-Based Fractional Flow Reserve: Result from the Machine Consortium. Circ Cardiovasc. Imaging 2018, 11, e007217. [Google Scholar] [CrossRef]
- Tesche, C.; Gray, H.N. Machine Learning and Deep Neural Networks Applications in Coronary Flow Assessment: The Case of Computed Tomography Fractional Flow Reserve. J. Thorac. Imaging 2020, 35, S66–S71. [Google Scholar] [CrossRef]
- Koo, B.-K.; Erglis, A.; Doh, J.-H.; Daniels, D.V.; Jegere, S.; Kim, H.-S.; Dunning, A.; DeFrance, T.; Lansky, A.; Leipsic, J.; et al. Diagnosis of Ischemia-Causing Coronary Stenoses by Noninvasive Fractional Flow Reserve Computed From Coronary Computed Tomographic Angiograms: Results From the Prospective Multicenter DISCOVER-FLOW (Diagnosis of Ischemia-Causing Stenoses Obtained Via Noninvasive Fractional Flow Reserve) Study. J. Am. Coll. Cardiol. 2011, 58, 1989–1997. [Google Scholar] [CrossRef]
- Norgaard, B.L.; Leipsic, J.; Gaur, S.; Seneviratne, S.; Ko, B.S.; Ito, H.; Jensen, J.M.; Mauri, L.; De Bruyne, B.; Bezerra, H.; et al. Diagnostic Performance of Noninvasive Fractional Flow Reserve Derived from Coronary Computed Tomography Angiography in Suspected Coronary Artery Disease: The Nxt Trial (Analysis of Coronary Blood Flow Using Ct Angiography: Next Steps). J. Am. Coll. Cardiol. 2014, 63, 1145–1155. [Google Scholar] [CrossRef]
- Nakazato, R.; Park, H.B.; Berman, D.S.; Gransar, H.; Koo, B.K.; Erglis, A.; Lin, F.Y.; Dunning, A.M.; Budoff, M.J.; Malpeso, J.; et al. Noninvasive Fractional Flow Reserve Derived from Computed Tomography Angiography for Coronary Lesions of Intermediate Stenosis Severity: Results from the Defacto Study. Circ Cardiovasc. Imaging 2013, 6, 881–889. [Google Scholar] [CrossRef]
- Douglas, P.S.; Pontone, G.; Hlatky, M.A.; Patel, M.R.; Norgaard, B.L.; Byrne, R.A.; Curzen, N.; Purcell, I.; Gutberlet, M.; Rioufol, G.; et al. Clinical Outcomes of Fractional Flow Reserve by Computed Tomographic Angiography-Guided Diagnostic Strategies Vs. Usual Care in Patients with Suspected Coronary Artery Disease: The Prospective Longitudinal Trial of Ffr(Ct): Outcome and Resource Impacts Study. Eur. Heart J. 2015, 36, 3359–3367. [Google Scholar] [PubMed]
- Dey, D.; Gaur, S.; Ovrehus, K.A.; Slomka, P.J.; Betancur, J.; Goeller, M.; Hell, M.M.; Gransar, H.; Berman, D.S.; Achenbach, S.; et al. Integrated prediction of lesion-specific ischaemia from quantitative coronary CT angiography using machine learning: A multicentre study. Eur. Radiol. 2018, 28, 2655–2664. [Google Scholar] [CrossRef] [PubMed]
- Collet, C.; Onuma, Y.; Andreini, D.; Sonck, J.; Pompilio, G.; Mushtaq, S.; La Meir, M.; Miyazaki, Y.; De Mey, J.; Gaemperli, O.; et al. Coronary computed tomography angiography for heart team decision-making in multivessel coronary artery disease. Eur. Heart J. 2018, 39, 3689–3698. [Google Scholar] [CrossRef]
- Lee, J.M.; Choi, K.H.; Koo, B.-K.; Park, J.; Kim, J.; Hwang, D.; Rhee, T.-M.; Kim, H.Y.; Jung, H.W.; Kim, K.-J.; et al. Prognostic Implications of Plaque Characteristics and Stenosis Severity in Patients With Coronary Artery Disease. J. Am. Coll. Cardiol. 2019, 73, 2413–2424. [Google Scholar] [CrossRef]
- Balla, S.; Nieman, K. From Inception to 2020: A Review of Dynamic Myocardial CT Perfusion Imaging. Curr. Cardiovasc. Imaging Rep. 2021, 14, 1. [Google Scholar] [CrossRef]
- Baumann, S.; Rutsch, M.; Becher, T.; Kryeziu, P.; Haubenreisser, H.; Vogler, N.; Schoenike, A.C.; Borggrefe, M.; Schoenberg, O.S.; Akin, I.; et al. Clinical Impact of Rest Dual–energy Computed Tomography Myocardial Perfusion in Patients with Coronary Artery Disease. Vivo 2017, 31, 1153–1157. [Google Scholar] [CrossRef][Green Version]
- Andreini, D.; Mushtaq, S.; Pontone, G.; Conte, E.; Collet, C.; Sonck, J.; D’Errico, A.; Di Odoardo, L.A.F.; Guglielmo, M.; Baggiano, A.; et al. CT Perfusion Versus Coronary CT Angiography in Patients With Suspected In-Stent Restenosis or CAD Progression. JACC Cardiovasc. Imaging 2020, 13, 732–742. [Google Scholar] [CrossRef] [PubMed]
- Takx, R.A.; Blomberg, B.A.; El Aidi, H.; Habets, J.; de Jong, P.A.; Nagel, E.; Hoffmann, U.; Leiner, T. Diagnostic Accuracy of Stress Myocardial Perfusion Imaging Compared to Invasive Coronary Angiography With Fractional Flow Reserve Meta-Analysis. Circ. Cardiovasc. Imaging 2015, 8, e002666. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Wang, S.; Sirajuddin, A.; Arai, A.E.; Zhao, S. Dynamic stress computed tomography myocardial perfusion for detecting myocardial ischemia: A systematic review and meta-analysis. Int. J. Cardiol. 2018, 258, 325–331. [Google Scholar] [CrossRef]
- van Assen, M.; De Cecco, C.N.; Eid, M.; von Knebel Doeberitz, P.; Scarabello, M.; Lavra, F.; Bauer, M.J.; Mastrodicasa, D.; Duguay, T.M.; Zaki, B.; et al. Prognostic value of CT myocardial perfusion imaging and CT-derived fractional flow reserve for major adverse cardiac events in patients with coronary artery disease. J. Cardiovasc. Comput. Tomogr. 2019, 13, 26–33. [Google Scholar] [CrossRef]
- Rossi, A.; Wragg, A.; Klotz, E.; Pirro, F.; Moon, J.C.; Nieman, K.; Pugliese, F. Dynamic Computed Tomography Myocardial Perfusion Imaging: Comparison of Clinical Analysis Methods for the Detection of Vessel-Specific Ischemia. Circ. Cardiovasc. Imaging 2017, 10, e005505. [Google Scholar] [CrossRef]
- Kido, T.; Kido, T.; Nakamura, M.; Watanabe, K.; Schmidt, M.; Forman, C.; Mochizuki, T. Compressed sensing real-time cine cardiovascular magnetic resonance: Accurate assessment of left ventricular function in a single-breath-hold. J. Cardiovasc. Magn. Reson. 2016, 18, 50. [Google Scholar] [CrossRef]
- Hirschberg, K.; Braun, S.M.; Paul, O.; Ochs, M.; Riffel, J.; Andre, F.; Salatzki, J.; Lebel, J.; Luu, J.; Hillier, E.; et al. The diagnostic accuracy of truncated cardiovascular MR protocols for detecting non-ischemic cardiomyopathies. Int. J. Cardiovasc. Imaging 2021, 38, 841–852. [Google Scholar] [CrossRef]
- Haaf, P.; Garg, P.; Messroghli, D.R.; Broadbent, D.A.; Greenwood, J.P.; Plein, S. Cardiac T1 Mapping and Extracellular Volume (ECV) in clinical practice: A comprehensive review. J. Cardiovasc. Magn. Reson. 2016, 18, 89. [Google Scholar] [CrossRef]
- Karur, G.R.; Hanneman, K. Cardiac MRI T1, T2, and T2* mapping in clinical practice. Adv. Clin. Radiol. 2019, 1, 27–41. [Google Scholar] [CrossRef]
- Mao, X.; Lee, H.L.; Hu, Z.; Cao, T.; Han, F.; Ma, S.; Serry, F.M.; Fan, Z.; Xie, Y.; Li, D.; et al. Simultaneous Multi-Slice Cardiac Mr Multitasking for Motion-Resolved, Non-Ecg, Free-Breathing T1-T2 Mapping. Front. Cardiovasc. Med. 2022, 9, 833257. [Google Scholar] [CrossRef]
- Morales, M.A.; Assana, S.; Cai, X.; Chow, K.; Haji-Valizadeh, H.; Sai, E.; Tsao, C.; Matos, J.; Rodriguez, J.; Berg, S.; et al. An inline deep learning based free-breathing ECG-free cine for exercise cardiovascular magnetic resonance. J. Cardiovasc. Magn. Reson. 2022, 24, 47. [Google Scholar] [CrossRef] [PubMed]
- Amzulescu, M.S.; De Craene, M.; Langet, H.; Pasquet, A.; Vancraeynest, D.; Pouleur, A.C.; Vanoverschelde, J.L.; Gerber, B.L. Myocardial Strain Imaging: Review of General Principles, Validation, and Sources of Discrepancies. Eur. Heart J. Cardiovasc. Imaging 2019, 20, 605–619. [Google Scholar] [CrossRef]
- Romano, S.; Judd, R.M.; Kim, R.J.; Kim, H.W.; Klem, I.; Heitner, J.F.; Shah, D.J.; Jue, J.; White, B.E.; Indorkar, R.; et al. Feature-Tracking Global Longitudinal Strain Predicts Death in a Multicenter Population of Patients With Ischemic and Nonischemic Dilated Cardiomyopathy Incremental to Ejection Fraction and Late Gadolinium Enhancement. JACC Cardiovasc. Imaging 2018, 11, 1419–1429. [Google Scholar] [CrossRef]
- Giusca, S.; Korosoglou, G.; Montenbruck, M.; Geršak, B.; Schwarz, A.K.; Esch, S.; Kelle, S.; Wülfing, P.; Dent, S.; Lenihan, D.; et al. Multiparametric Early Detection and Prediction of Cardiotoxicity Using Myocardial Strain, T1 and T2 Mapping, and Biochemical Markers: A Longitudinal Cardiac Resonance Imaging Study During 2 Years of Follow-Up. Circ. Cardiovasc. Imaging 2021, 14, e012459. [Google Scholar] [CrossRef]
- Korosoglou, G.; Giusca, S.; Montenbruck, M.; Patel, A.R.; Lapinskas, T.; Götze, C.; Zieschang, V.; Al-Tabatabaee, S.; Pieske, B.; Florian, A.; et al. Fast Strain-Encoded Cardiac Magnetic Resonance for Diagnostic Classification and Risk Stratification of Heart Failure Patients. JACC Cardiovasc. Imaging 2021, 14, 1177–1188. [Google Scholar] [CrossRef]
- Grove, G.L.; Pedersen, S.; Olsen, F.J.; Skaarup, K.G.; Jørgensen, P.G.; Shah, A.M.; Biering-Sørensen, T. Layer-specific global longitudinal strain obtained by speckle tracking echocardiography for predicting heart failure and cardiovascular death following STEMI treated with primary PCI. Int. J. Cardiovasc. Imaging 2021, 37, 2207–2215. [Google Scholar] [CrossRef]
- Giusca, S.; Korosoglou, G.; Zieschang, V.; Stoiber, L.; Schnackenburg, B.; Stehning, C.; Gebker, R.; Pieske, B.; Schuster, A.; Backhaus, S.; et al. Reproducibility study on myocardial strain assessment using fast-SENC cardiac magnetic resonance imaging. Sci. Rep. 2018, 8, 14100. [Google Scholar] [CrossRef]
- Korosoglou, G.; Youssef, A.A.; Bilchick, K.C.; Ibrahim el, S.; Lardo, A.C.; Lai, S.; Osman, N.F. Real-Time Fast Strain-Encoded Magnetic Resonance Imaging to Evaluate Regional Myocardial Function at 3.0 Tesla: Comparison to Conventional Tagging. J. Magn. Reson. Imaging 2008, 27, 1012–1018. [Google Scholar] [CrossRef]
- Nakamura, S.; Ishida, M.; Nakata, K.; Ichikawa, Y.; Takase, S.; Takafuji, M.; Ito, H.; Nakamori, S.; Kurita, T.; Dohi, K.; et al. Long-term prognostic value of whole-heart coronary magnetic resonance angiography. J. Cardiovasc. Magn. Reson. 2021, 23, 56. [Google Scholar] [CrossRef]
- Bustin, A.; Rashid, I.; Cruz, G.; Hajhosseiny, R.; Correia, T.; Neji, R.; Rajani, R.; Ismail, T.F.; Botnar, R.M.; Prieto, C. 3D whole-heart isotropic sub-millimeter resolution coronary magnetic resonance angiography with non-rigid motion-compensated PROST. J. Cardiovasc. Magn. Reson. 2020, 22, 24. [Google Scholar] [CrossRef]
- Sharrack, N.; Chiribiri, A.; Schwitter, J.; Plein, S. How to do quantitative myocardial perfusion cardiovascular magnetic resonance. Eur. Heart J. Cardiovasc. Imaging 2021, 23, 315–318. [Google Scholar] [CrossRef]
- Ochs, A.; Nippes, M.; Salatzki, J.; Weberling, L.D.; Riffel, J.; Müller-Hennessen, M.; Giannitsis, E.; Osman, N.; Stehning, C.; André, F.; et al. Dynamic Handgrip Exercise: Feasibility and Physiologic Stress Response of a Potential Needle-Free Cardiac Magnetic Resonance Stress Test. Front. Cardiovasc. Med. 2021, 8, 755759. [Google Scholar] [CrossRef] [PubMed]
- Ochs, M.M.; Kajzar, I.; Salatzki, J.; Ochs, A.T.; Riffel, J.; Osman, N.; Katus, H.A.; Friedrich, M.G. Hyperventilation/Breath-Hold Maneuver to Detect Myocardial Ischemia by Strain-Encoded Cmr: Diagnostic Accuracy of a Needle-Free Stress Protocol. JACC Cardiovasc. Imaging 2021, 14, 1932–1944. [Google Scholar] [CrossRef] [PubMed]
- Siry, D.; Riffel, J.H.; Salatzki, J.; Andre, F.; Ochs, M.; Weberling, L.D.; Giannitsis, E.; Katus, H.A.; Friedrich, M.G. Hypverventilation Strain Cmr Imaging in Patients with Acute Chest Pain. Sci. Rep. 2022, 12, 13584. [Google Scholar] [CrossRef] [PubMed]
- Weberling, L.D.; Friedrich, M.G. Oxygenation-Sensitive Cardiac Magnetic Resonance Imaging; Radiologie: Berlin/Heidelberg, Germany, 2022. [Google Scholar]
- Fischer, K.; Yamaji, K.; Luescher, S.; Ueki, Y.; Jung, B.; von Tengg-Kobligk, H.; Windecker, S.; Friedrich, M.G.; Eberle, B.; Guensch, D.P. Feasibility of cardiovascular magnetic resonance to detect oxygenation deficits in patients with multi-vessel coronary artery disease triggered by breathing maneuvers. J. Cardiovasc. Magn. Reson. 2018, 20, 31. [Google Scholar] [CrossRef]
- Weintraub, M.I.; Khoury, A.; Cole, S.P. Biologic Effects of 3 Tesla (T) Mr Imaging Comparing Traditional 1.5 T and 0.6 T in 1023 Consecutive Outpatients. J. Neuroimaging 2007, 17, 241–245. [Google Scholar] [CrossRef]
- Ocazionez, D.; Dicks, D.L.; Favinger, J.L.; Shroff, G.S.; Damani, S.; Kicska, G.A.; Reddy, G.P. Magnetic Resonance Imaging Safety in Cardiothoracic Imaging. J. Thorac. Imaging 2014, 29, 262–269. [Google Scholar] [CrossRef]
- Sáfrány, G.; Lumniczky, K.; Manti, L. New Discoveries in Radiation Science. Cancers 2021, 13, 1034. [Google Scholar] [CrossRef]
- Stocker, T.J.; Deseive, S.; Leipsic, J.; Hadamitzky, M.; Chen, M.Y.; Rubinshtein, R.; Heckner, M.; Bax, J.J.; Fang, X.-M.; Grove, E.L.; et al. Reduction in radiation exposure in cardiovascular computed tomography imaging: Results from the PROspective multicenter registry on radiaTion dose Estimates of cardiac CT angIOgraphy iN daily practice in 2017 (PROTECTION VI). Eur. Heart J. 2018, 39, 3715–3723. [Google Scholar] [CrossRef]
- Lin, E.C. Radiation Risk From Medical Imaging. Mayo Clin. Proc. 2010, 85, 1142–1146. [Google Scholar] [CrossRef]
- Cao, C.-F.; Ma, K.-L.; Shan, H.; Liu, T.-F.; Zhao, S.-Q.; Wan, Y.; Zhang, J.; Wang, H.-Q. CT Scans and Cancer Risks: A Systematic Review and Dose-response Meta-analysis. BMC Cancer 2022, 22, 1238. [Google Scholar] [CrossRef]
- Aran, S.; Shaqdan, K.; Abujudeh, H. Adverse allergic reactions to linear ionic gadolinium-based contrast agents: Experience with 194, 400 injections. Clin. Radiol. 2015, 70, 466–475. [Google Scholar] [CrossRef]
- Fakhran, S.; Alhilali, L.; Kale, H.; Kanal, E. Assessment of Rates of Acute Adverse Reactions to Gadobenate Dimeglumine: Review of More Than 130,000 Administrations in 7.5 Years. AJR Am. J. Roentgenol. 2015, 204, 703–706. [Google Scholar] [CrossRef]
- Andre, F.; Fortner, P.; Emami, M.; Seitz, S.; Brado, M.; Gückel, F.; Sokiranski, R.; Sommer, A.; Frey, N.; Görich, J.; et al. Factors influencing the safety of outpatient coronary CT angiography: A clinical registry study. BMJ Open 2022, 12, e058304. [Google Scholar] [CrossRef]
- Nijssen, E.C.; Rennenberg, R.J.; Nelemans, P.J.; Essers, B.A.; Janssen, M.M.; Vermeeren, M.A.; Ommen, V.V.; Wildberger, J.E. Prophylactic Hydration to Protect Renal Function from Intravascular Iodinated Contrast Material in Patients at High Risk of Contrast-Induced Nephropathy (Amacing): A Prospective, Randomised, Phase 3, Controlled, Open-Label, Non-Inferiority Trial. Lancet 2017, 389, 1312–1322. [Google Scholar] [CrossRef]
- McDonald, R.J.; McDonald, J.S.; Kallmes, D.F.; Jentoft, M.E.; Murray, D.L.; Thielen, K.R.; Williamson, E.E.; Eckel, L.J. Intracranial Gadolinium Deposition after Contrast-enhanced MR Imaging. Radiology 2015, 275, 772–782. [Google Scholar] [CrossRef]
- Lange, S.; Mędrzycka-Dąbrowska, W.; Zorena, K.; Dąbrowski, S.; Ślęzak, D.; Malecka-Dubiela, A.; Rutkowski, P. Nephrogenic Systemic Fibrosis as a Complication after Gadolinium-Containing Contrast Agents: A Rapid Review. Int. J. Environ. Res. Public Health 2021, 18, 3000. [Google Scholar] [CrossRef]
- Chen, J.W. Does Brain Gadolinium Deposition Have Clinical Consequence? Lessons from Animal Studies. Radiology 2021, 301, 417–419. [Google Scholar] [CrossRef]
- Radbruch, A.; Haase, R.; Kieslich, P.J.; Weberling, L.D.; Kickingereder, P.; Wick, W.; Schlemmer, H.-P.; Bendszus, M. No Signal Intensity Increase in the Dentate Nucleus on Unenhanced T1-weighted MR Images after More than 20 Serial Injections of Macrocyclic Gadolinium-based Contrast Agents. Radiology 2017, 282, 699–707. [Google Scholar] [CrossRef]
- Radbruch, A.; Weberling, L.D.; Kieslich, P.J.; Eidel, O.; Burth, S.; Kickingereder, P.; Heiland, S.; Wick, W.; Schlemmer, H.P.; Bendszus, M. Gadolinium Retention in the Dentate Nucleus and Globus Pallidus Is Dependent on the Class of Contrast Agent. Radiology 2015, 275, 783–791. [Google Scholar] [CrossRef]
- Weberling, L.D.; Kieslich, P.J.; Kickingereder, P.V.; Wick, W.; Bendszus, M.; Schlemmer, H.-P.; Radbruch, A. Increased Signal Intensity in the Dentate Nucleus on Unenhanced T1-Weighted Images After Gadobenate Dimeglumine Administration. Investig. Radiol. 2015, 50, 743–748. [Google Scholar] [CrossRef] [PubMed]
- Woolen, S.A.; Shankar, P.R.; Gagnier, J.J.; MacEachern, M.P.; Singer, L.; Davenport, M.S. Risk of Nephrogenic Systemic Fibrosis in Patients with Stage 4 or 5 Chronic Kidney Disease Receiving a Group Ii Gadolinium-Based Contrast Agent: A Systematic Review and Meta-Analysis. JAMA Intern. Med. 2020, 180, 223–230. [Google Scholar] [CrossRef] [PubMed]
- Karamitsos, T.D.; Arnold, J.R.; Pegg, T.J.; Cheng, A.S.H.; Van Gaal, W.J.; Francis, J.M.; Banning, A.P.; Neubauer, S.; Selvanayagam, J.B. Tolerance and safety of adenosine stress perfusion cardiovascular magnetic resonance imaging in patients with severe coronary artery disease. Int. J. Cardiovasc. Imaging 2008, 25, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Wahl, A.; Paetsch, I.; Gollesch, A.; Roethemeyer, S.; Foell, D.; Gebker, R.; Langreck, H.; Klein, C.; Fleck, E.; Nagel, E. Safety and feasibility of high-dose dobutamine-atropine stress cardiovascular magnetic resonance for diagnosis of myocardial ischaemia: Experience in 1000 consecutive cases. Eur. Heart J. 2004, 25, 1230–1236. [Google Scholar] [CrossRef] [PubMed]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Mileva, N.; Nagumo, S.; Mizukami, T.; Sonck, J.; Berry, C.; Gallinoro, E.; Monizzi, G.; Candreva, A.; Munhoz, D.; Vassilev, D.; et al. Prevalence of Coronary Microvascular Disease and Coronary Vasospasm in Patients With Nonobstructive Coronary Artery Disease: Systematic Review and Meta-Analysis. J. Am. Heart Assoc. 2022, 11, e023207. [Google Scholar] [CrossRef]
- Michallek, F.; Nakamura, S.; Ota, H.; Ogawa, R.; Shizuka, T.; Nakashima, H.; Wang, Y.-N.; Ito, T.; Sakuma, H.; Dewey, M.; et al. Fractal analysis of 4D dynamic myocardial stress-CT perfusion imaging differentiates micro- and macrovascular ischemia in a multi-center proof-of-concept study. Sci. Rep. 2022, 12, 5085. [Google Scholar] [CrossRef]
- Perera, D.; Clayton, T.; O’Kane, P.D.; Greenwood, J.P.; Weerackody, R.; Ryan, M.; Morgan, H.P.; Dodd, M.; Evans, R.; Canter, R.; et al. Percutaneous Revascularization for Ischemic Left Ventricular Dysfunction. N. Engl. J. Med. 2022, 387, 1351–1360. [Google Scholar] [CrossRef]
- Maron, D.J.; Hochman, J.S.; Reynolds, H.R.; Bangalore, S.; O’Brien, S.M.; Boden, W.E.; Chaitman, B.R.; Senior, R.; López-Sendón, J.; Alexander, K.P.; et al. Initial Invasive or Conservative Strategy for Stable Coronary Disease. N. Engl. J. Med. 2020, 382, 1395–1407. [Google Scholar] [CrossRef]
- Arnold, J.R.; Karamitsos, T.D.; Bhamra-Ariza, P.; Francis, J.M.; Searle, N.; Robson, M.D.; Howells, R.K.; Choudhury, R.P.; Rimoldi, O.E.; Camici, P.G.; et al. Myocardial Oxygenation in Coronary Artery Disease: Insights from Blood Oxygen Level-Dependent Magnetic Resonance Imaging at 3 Tesla. J. Am. Coll. Cardiol. 2012, 59, 1954–1964. [Google Scholar] [CrossRef]
- Karamitsos, T.D.; Leccisotti, L.; Arnold, J.R.; Recio-Mayoral, A.; Bhamra-Ariza, P.; Howells, R.K.; Searle, N.; Robson, M.D.; Rimoldi, O.E.; Camici, P.G.; et al. Relationship between Regional Myocardial Oxygenation and Perfusion in Patients with Coronary Artery Disease: Insights from Cardiovascular Magnetic Resonance and Positron Emission Tomography. Circ. Cardiovasc. Imaging 2010, 3, 32–40. [Google Scholar] [CrossRef]
- Hertz, J.T.; Fu, T.; Vissoci, J.R.; Rocha, T.A.H.; Carvalho, E.; Flanagan, B.; De Andrade, L.; Limkakeng, A.T.; Staton, C.A. The distribution of cardiac diagnostic testing for acute coronary syndrome in the Brazilian healthcare system: A national geospatial evaluation of health access. PLoS ONE 2019, 14, e0210502. [Google Scholar] [CrossRef]
- Petersen, S.E.; Friebel, R.; Ferrari, V.; Han, Y.; Aung, N.; Kenawy, A.; Albert, T.S.E.; Naci, H. Recent Trends and Potential Drivers of Non-invasive Cardiovascular Imaging Use in the United States of America and England. Front. Cardiovasc. Med. 2021, 7, 617771. [Google Scholar] [CrossRef]
- Pandya, A.; Yu, Y.-J.; Ge, Y.; Nagel, E.; Kwong, R.Y.; Abu Bakar, R.; Grizzard, J.D.; Merkler, A.E.; Ntusi, N.; Petersen, S.E.; et al. Evidence-based cardiovascular magnetic resonance cost-effectiveness calculator for the detection of significant coronary artery disease. J. Cardiovasc. Magn. Reson. 2022, 24, 1. [Google Scholar] [CrossRef]
- Stone, G.W.; Maehara, A.; Lansky, A.J.; de Bruyne, B.; Cristea, E.; Mintz, G.S.; Mehran, R.; McPherson, J.; Farhat, N.; Marso, S.P.; et al. A Prospective Natural-History Study of Coronary Atherosclerosis. N. Engl. J. Med. 2011, 364, 226–235. [Google Scholar] [CrossRef]
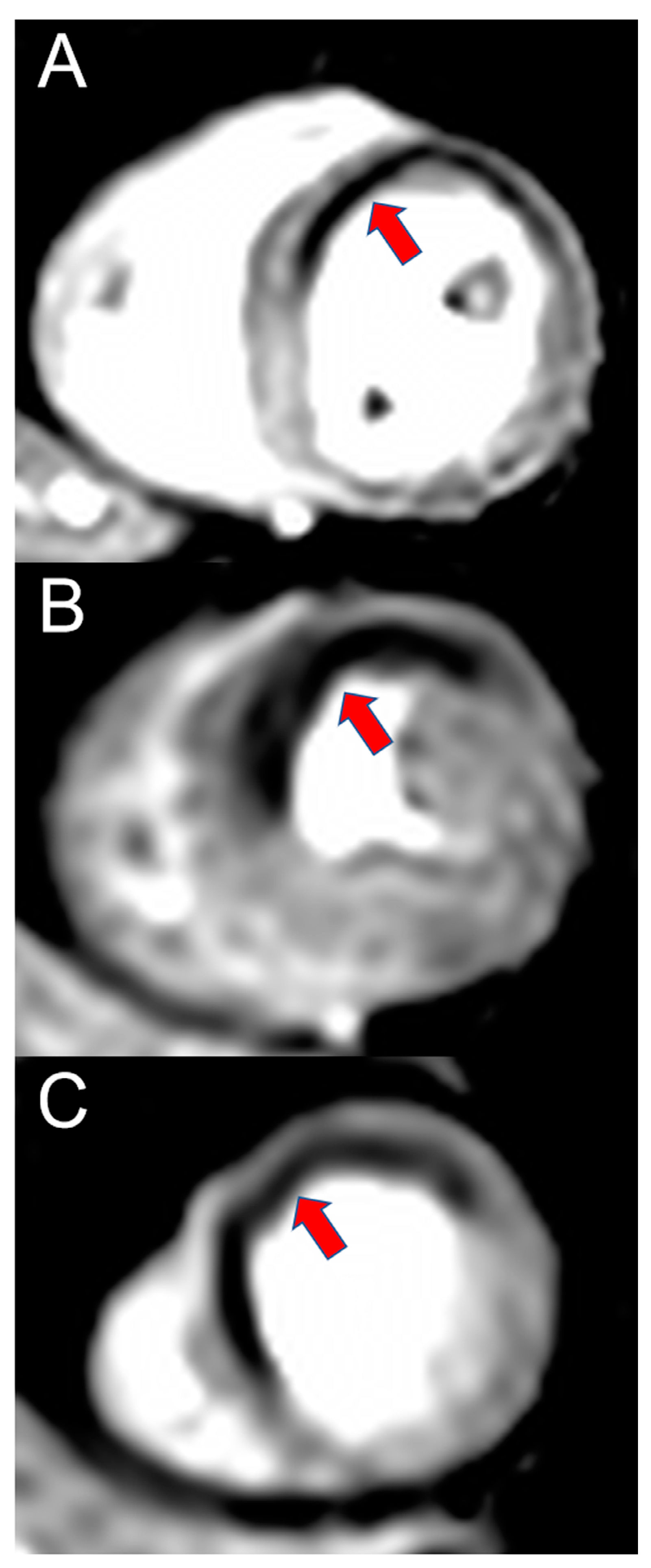
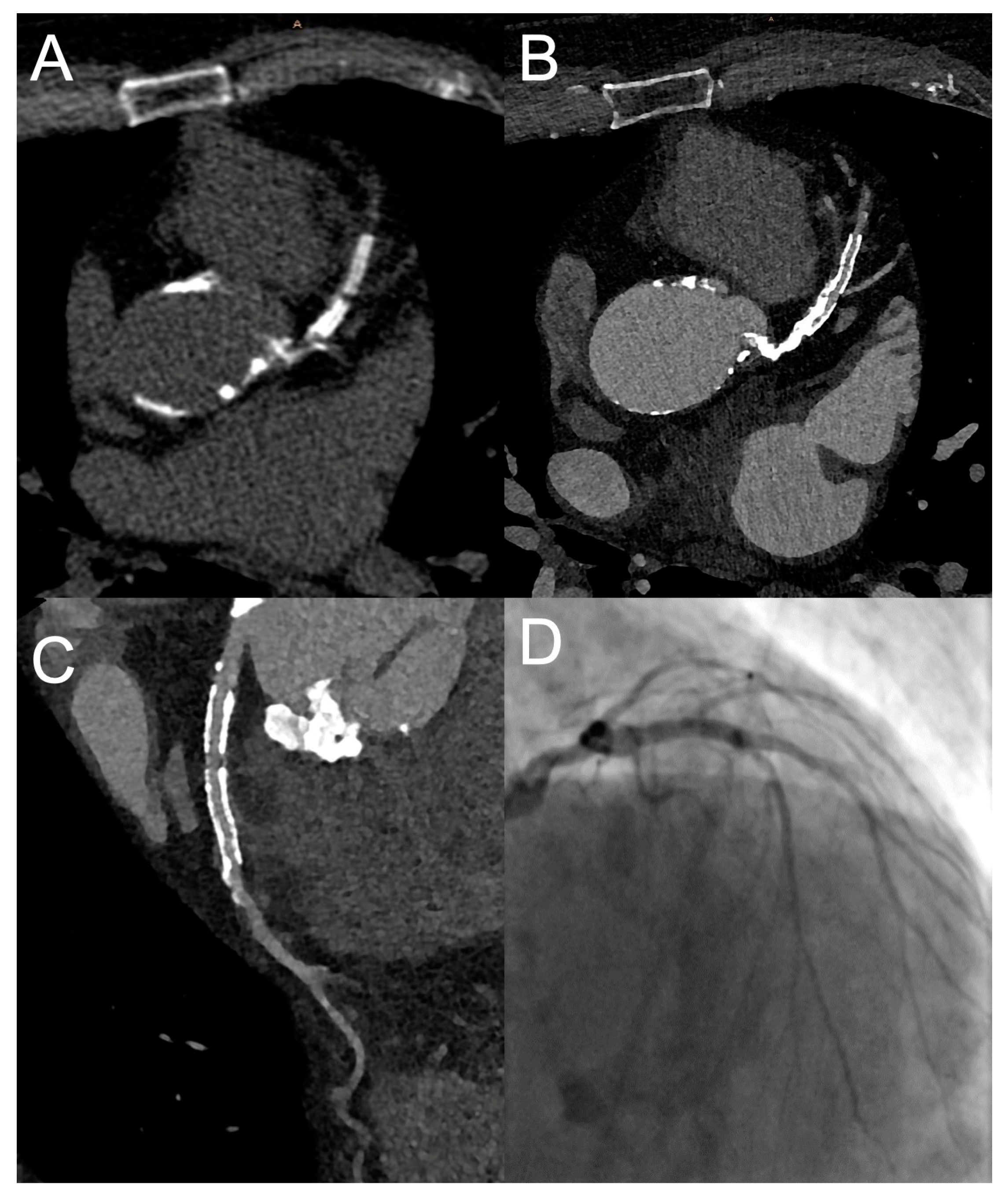
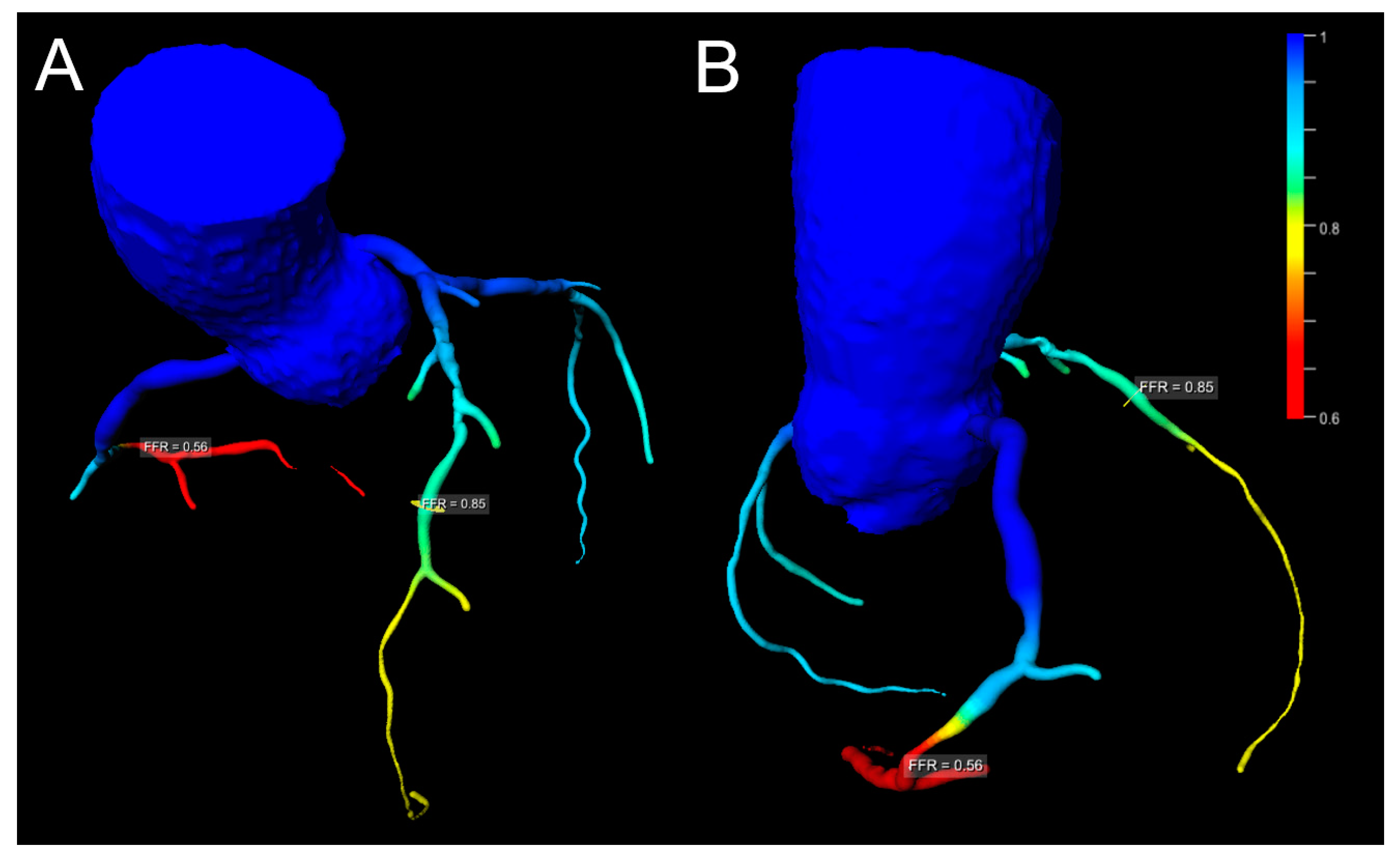
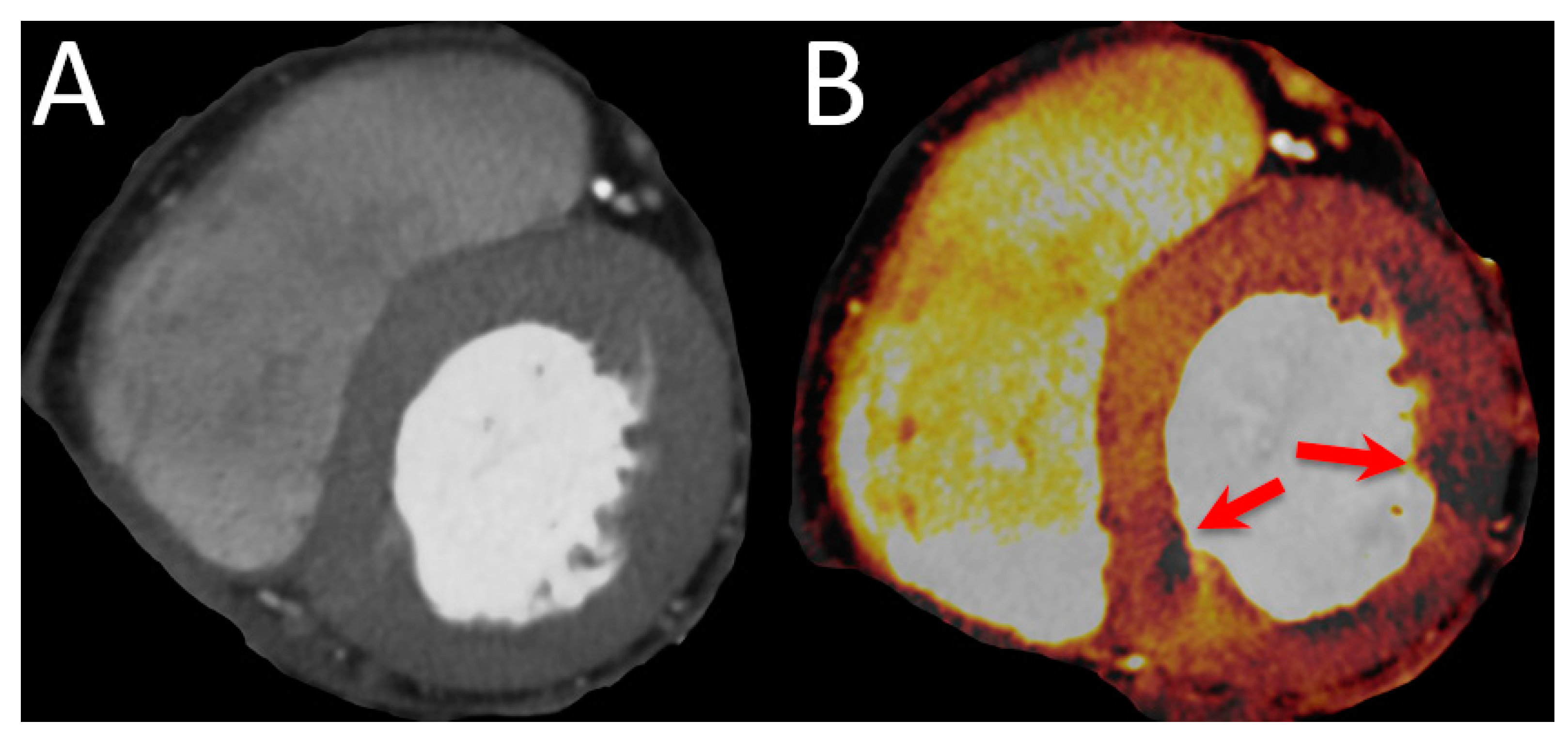
| Favor cCTA | Favor Stress CMR | |
|---|---|---|
| CAD | Unknown | Known |
| Previous coronary stent | No | Yes |
| Preventive medical therapy | None | ASA, statin |
| Patient age | Middle-aged | Advanced, Young with known coronary anomalies |
| Pretest probability of CAD | Low/Intermediate | High |
| Regular follow-up needed | No | Yes |
| Previous diagnostic work-up | Inconclusive stress test | cCTA with non-diagnostic image quality or stenosis of indetermined hemodynamic significance |
| Metallic implants | Non-removable metallic implants without or with unknown MR safety, Non-removable metallic implants which may impair CMR image quality severely | - |
| Comorbidities | COPD/Asthma Severe claustrophobia | Hyperthyroidism, moderately/ severely impaired kidney function, high heart rate |
| Potential differential diagnosis other than CAD | Pulmonary or aortic pathology | Myocarditis, pericarditis, thrombembolism–MINOCA assessment |
| Viability assessment required | No | Yes |
| Assessment of myocardial edema, function, scar tissue or fibrosis required | No | Yes |
| Severe or extensive coronary calcifications expected or proven | No | Yes |
| Allergies | Allergy to Gadolinium-based contrast agents | Allergy to Iodine-based contrast agents |
| Claustrophobic | Yes | No |
| Modern CT scanners available (≥128 slice detectors) | Yes | No |
| Additional factors to be considered | Local expertise Timely availability (if necessary) Patient preference | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Weberling, L.D.; Lossnitzer, D.; Frey, N.; André, F. Coronary Computed Tomography vs. Cardiac Magnetic Resonance Imaging in the Evaluation of Coronary Artery Disease. Diagnostics 2023, 13, 125. https://doi.org/10.3390/diagnostics13010125
Weberling LD, Lossnitzer D, Frey N, André F. Coronary Computed Tomography vs. Cardiac Magnetic Resonance Imaging in the Evaluation of Coronary Artery Disease. Diagnostics. 2023; 13(1):125. https://doi.org/10.3390/diagnostics13010125
Chicago/Turabian StyleWeberling, Lukas D., Dirk Lossnitzer, Norbert Frey, and Florian André. 2023. "Coronary Computed Tomography vs. Cardiac Magnetic Resonance Imaging in the Evaluation of Coronary Artery Disease" Diagnostics 13, no. 1: 125. https://doi.org/10.3390/diagnostics13010125
APA StyleWeberling, L. D., Lossnitzer, D., Frey, N., & André, F. (2023). Coronary Computed Tomography vs. Cardiac Magnetic Resonance Imaging in the Evaluation of Coronary Artery Disease. Diagnostics, 13(1), 125. https://doi.org/10.3390/diagnostics13010125





