Development and Validation of Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) as a Simple and Rapid Diagnostic Tool for SARS-CoV-2 Detection
Abstract
1. Introduction
2. Materials and Methods
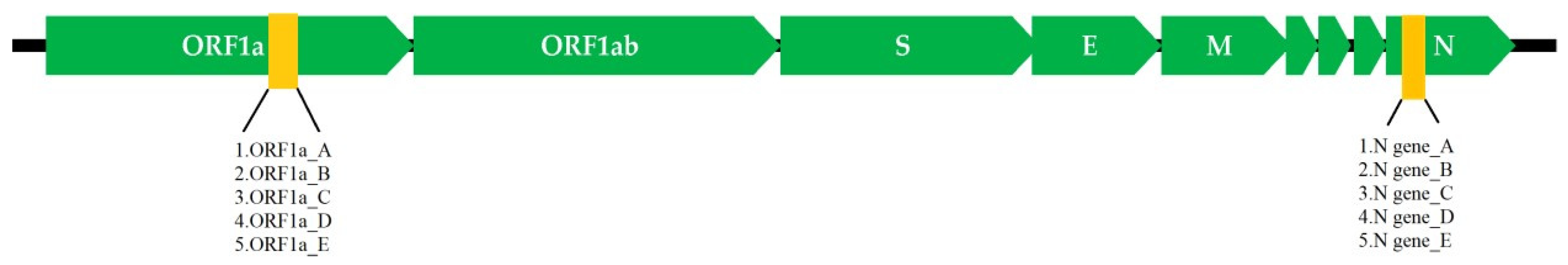
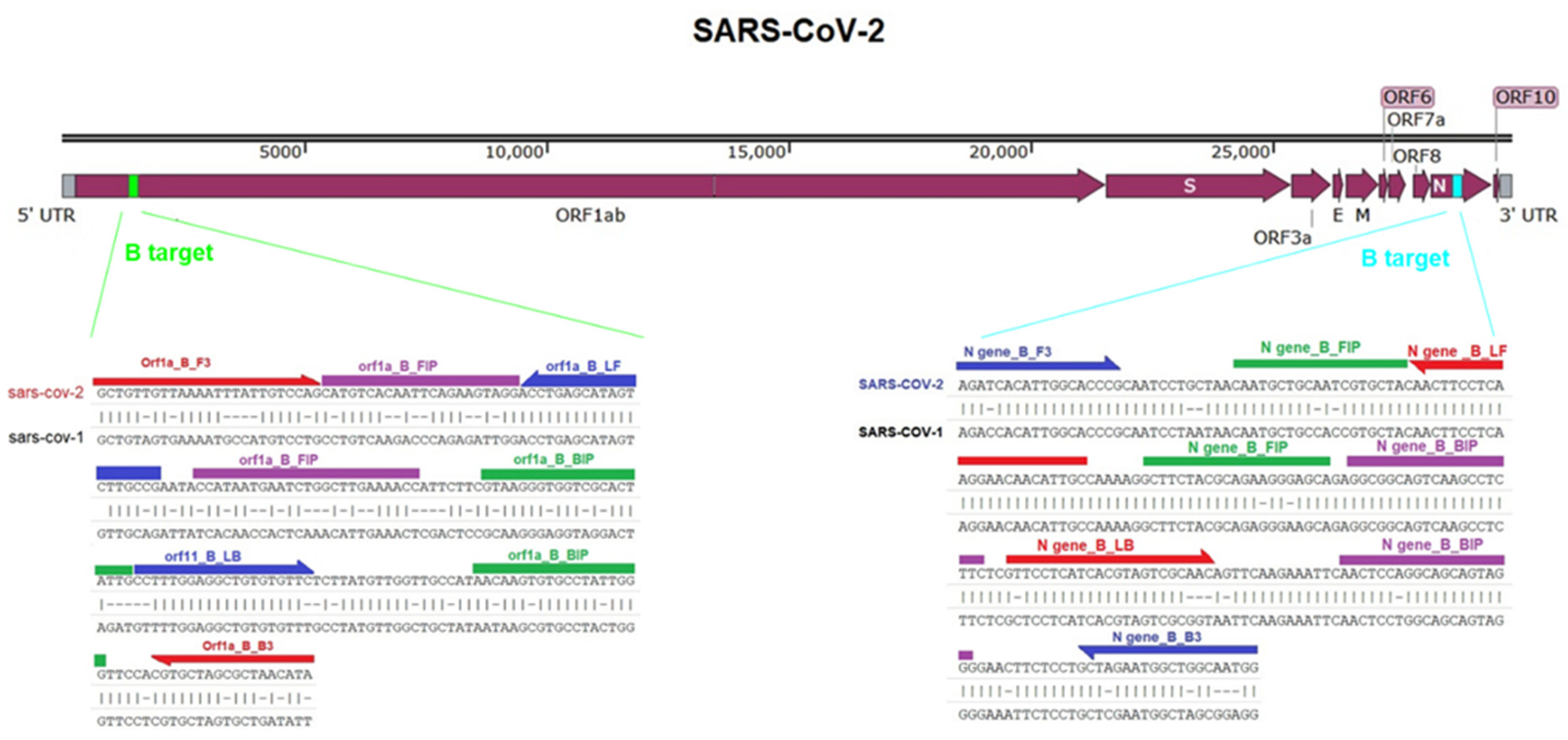
2.1. Target Selection and RT-LAMP Primers Design
2.2. Synthesis of SARS-CoV-2 RNA
2.3. Development of the RT-LAMP Assay
2.4. Validation of the RT-LAMP Assay
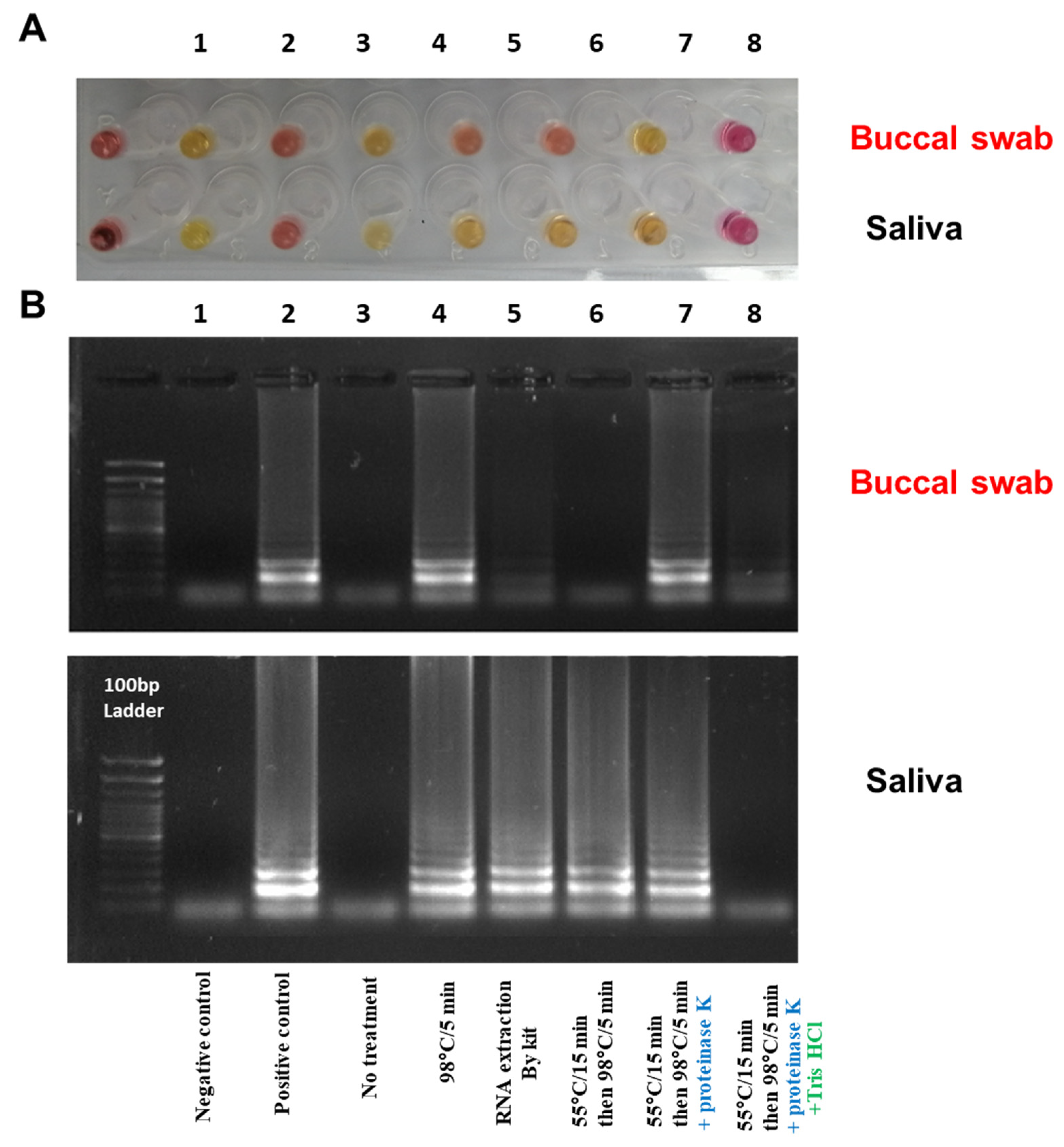
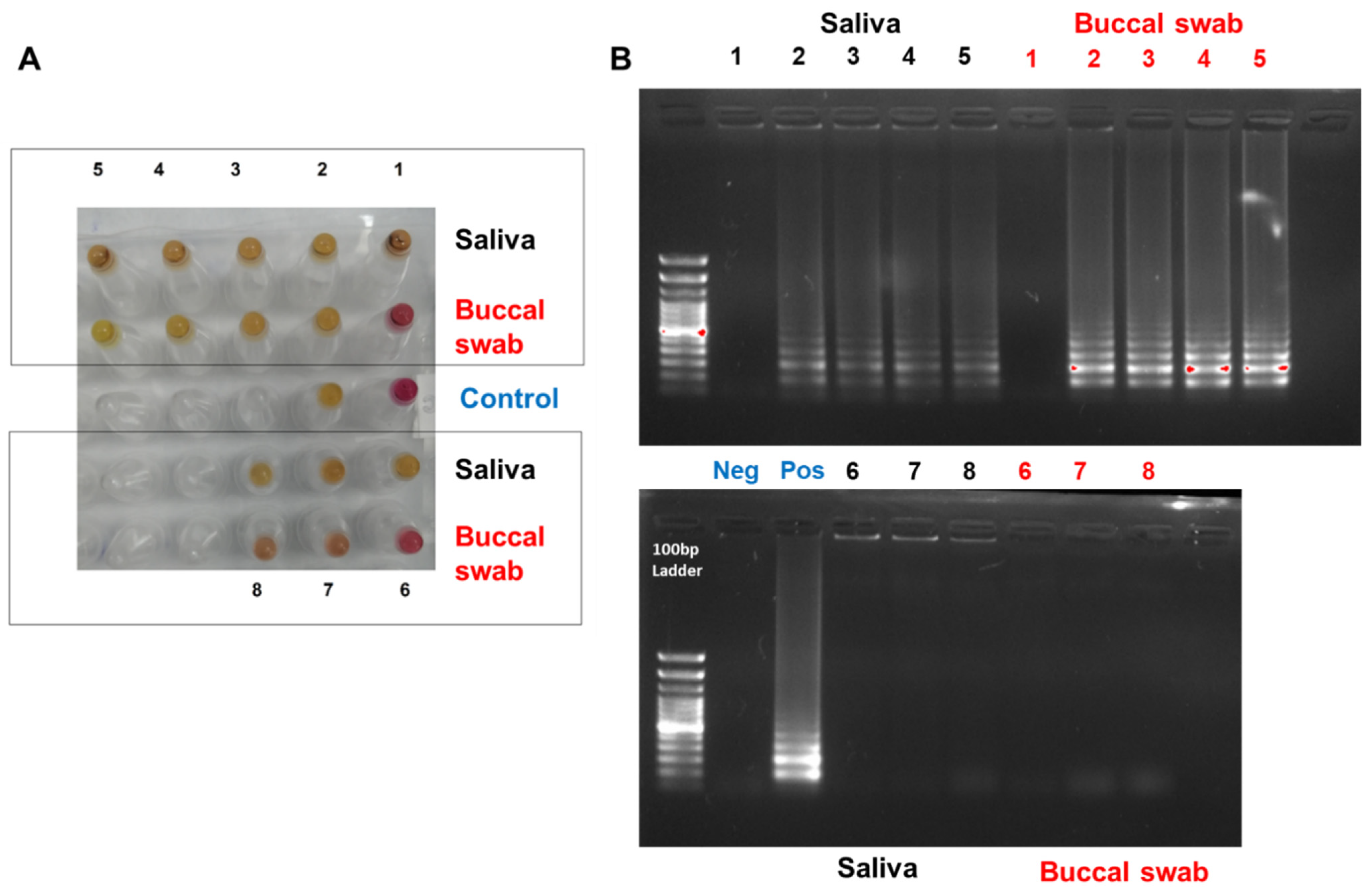
2.5. Optimizing the RNA Extraction from Clinical Samples to the RT-LAMP Assay (Point of Care Testing)
3. Results and Discussion
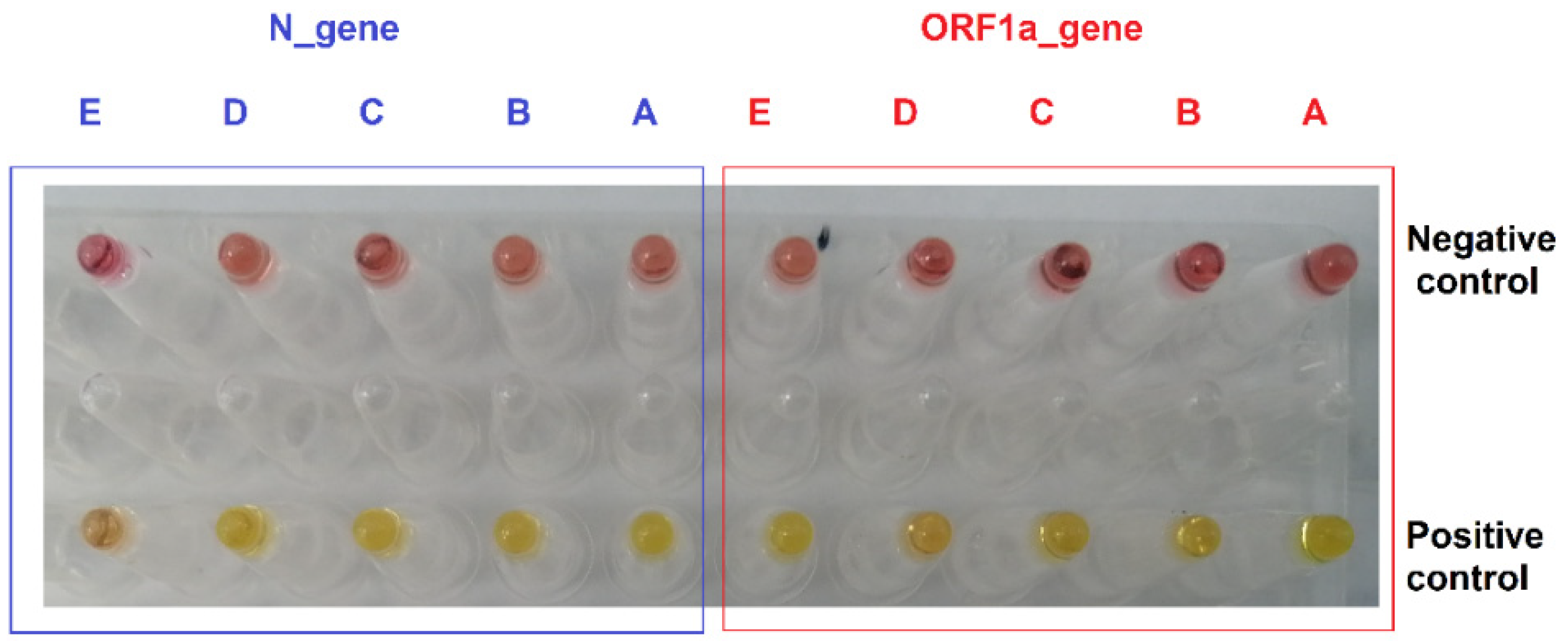
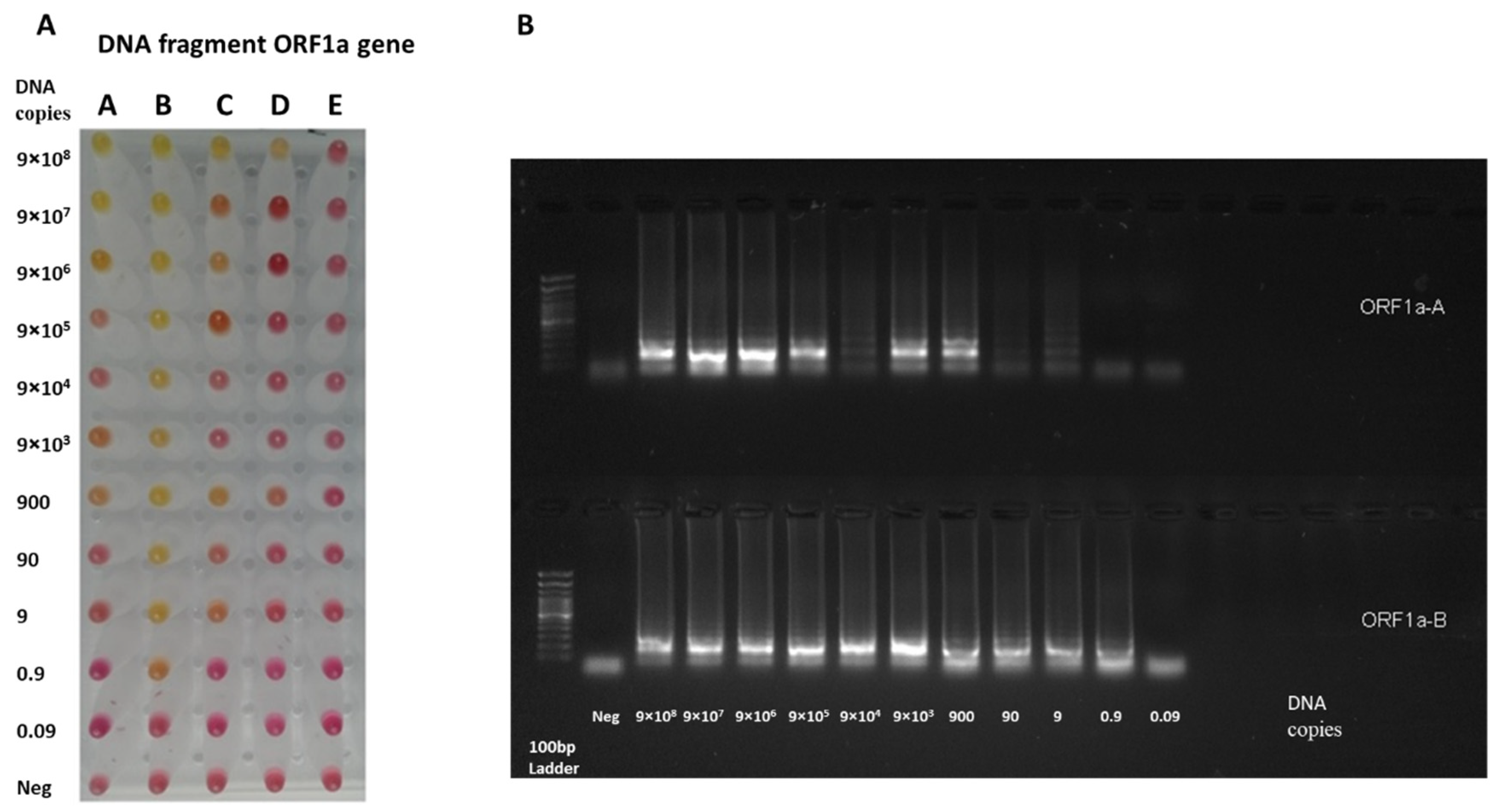
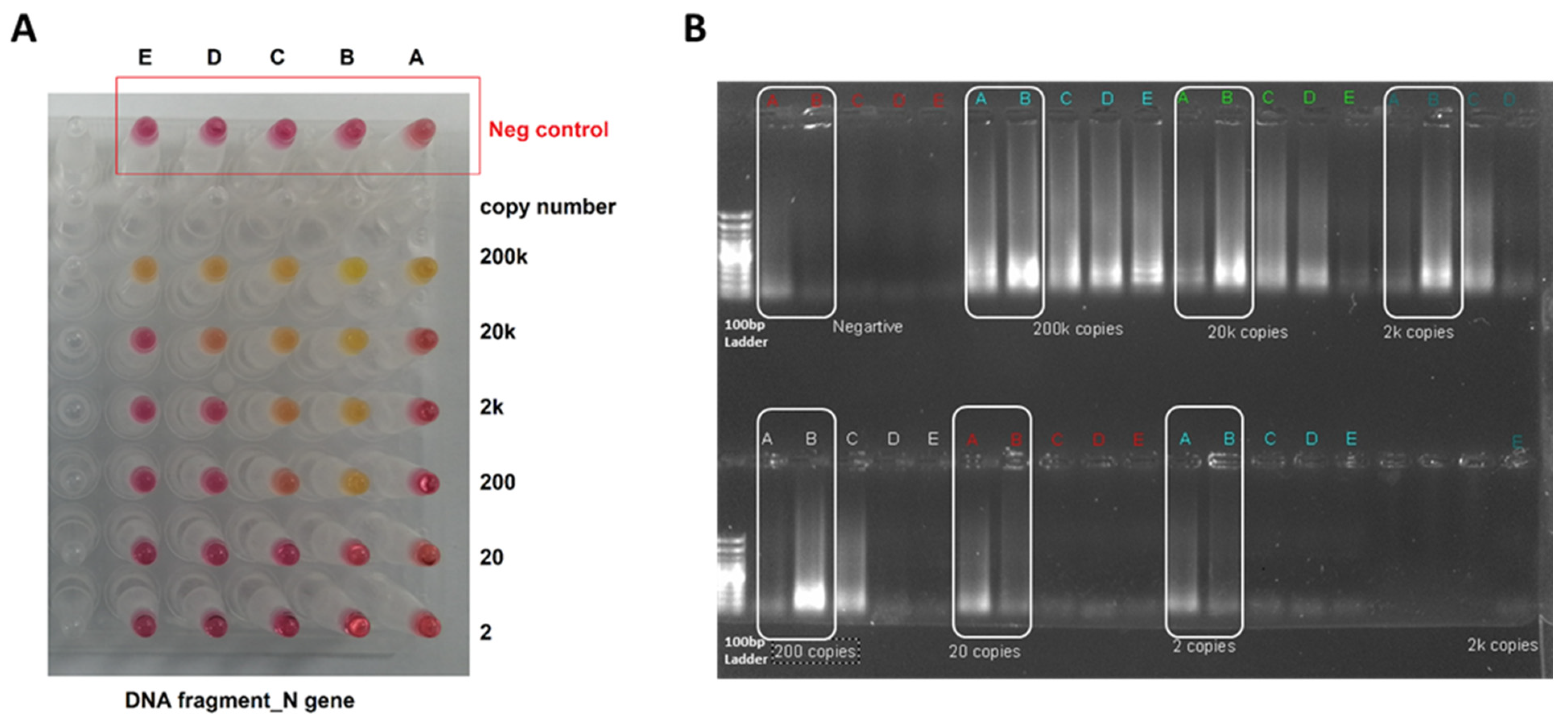
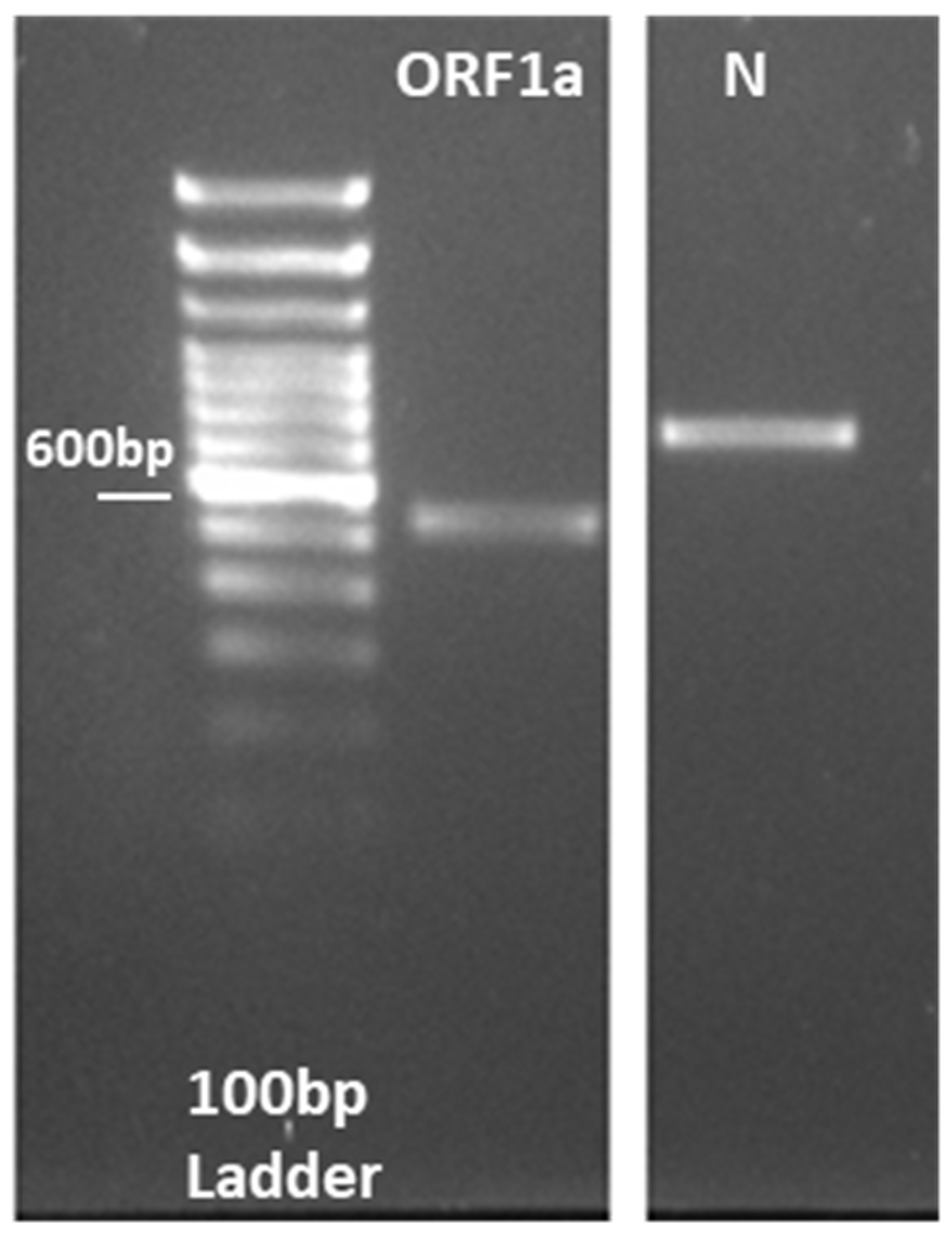
3.1. RT-LAMP Assay Design and Testing against DNA Control Samples
3.2. Testing the RT-LAMP Assay against the Synthesized RNA Samples
3.3. Testing the RT-LAMP Assay against the Clinical RNA Samples
3.4. Optimizing the RNA Extraction from Clinical Samples to the RT-LAMP Assay (Point of Care Testing)
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Andersen, K.G.; Rambaut, A.; Lipkin, W.I.; Holmes, E.C.; Garry, R.F. The proximal origin of SARS-CoV-2. Nat. Med. 2020, 26, 450–452. [Google Scholar] [CrossRef] [PubMed]
- Huang, C.; Wang, Y.; Li, X.; Ren, L.; Zhao, J.; Hu, Y.; Zhang, L.; Fan, G.; Xu, J.; Gu, X.; et al. Clinical features of patients infected with 2019 novel coronavirus in Wuhan, China. Lancet 2020, 395, 497–506. [Google Scholar] [CrossRef]
- Zhou, P.; Yang, X.L.; Wang, X.G.; Hu, B.; Zhang, L.; Zhang, W.; Si, H.R.; Zhu, Y.; Li, B.; Huang, C.L.; et al. A pneumonia outbreak associated with a new coronavirus of probable bat origin. Nature 2020, 579, 270–273. [Google Scholar] [CrossRef] [PubMed]
- Abdelrahman, Z.; Li, M.; Wang, X. Comparative Review of SARS-CoV-2, SARS-CoV, MERS-CoV, and Influenza A Respiratory Viruses. Front. Immunol. 2020, 11, 552909. [Google Scholar] [CrossRef] [PubMed]
- Contini, C.; Di Nuzzo, M.; Barp, N.; Bonazza, A.; De Giorgio, R.; Tognon, M.; Rubino, S. The novel zoonotic COVID-19 pandemic: An expected global health concern. J. Infect. Dev. Ctries. 2020, 14, 254–264. [Google Scholar] [CrossRef]
- WHO Coronavirus (COVID-19) Dashboard. Available online: https://covid19.who.int/ (accessed on 18 June 2022).
- Eftekhari, A.; Alipour, M.; Chodari, L.; Maleki Dizaj, S.; Ardalan, M.; Samiei, M.; Sharifi, S.; Zununi Vahed, S.; Huseynova, I.; Khalilov, R.; et al. A Comprehensive Review of Detection Methods for SARS-CoV-2. Microorganisms 2021, 9, 232. [Google Scholar] [CrossRef]
- Pu, R.; Liu, S.; Ren, X.; Shi, D.; Ba, Y.; Huo, Y.; Zhang, W.; Ma, L.; Liu, Y.; Yang, Y.; et al. The screening value of RT-LAMP and RT-PCR in the diagnosis of COVID-19: Systematic review and meta-analysis. J. Virol. Methods 2022, 300, 114392. [Google Scholar] [CrossRef]
- Vandenberg, O.; Martiny, D.; Rochas, O.; van Belkum, A.; Kozlakidis, Z. Considerations for diagnostic COVID-19 tests. Nat. Rev. Microbiol. 2021, 19, 171–183. [Google Scholar] [CrossRef]
- Mardian, Y.; Kosasih, H.; Karyana, M.; Neal, A.; Lau, C.Y. Review of Current COVID-19 Diagnostics and Opportunities for Further Development. Front. Med. 2021, 8, 615099. [Google Scholar] [CrossRef]
- Notomi, T.; Okayama, H.; Masubuchi, H.; Yonekawa, T.; Watanabe, K.; Amino, N.; Hase, T. Loop-mediated isothermal amplification of DNA. Nucleic Acids Res. 2000, 28, E63. [Google Scholar] [CrossRef]
- Tomita, N.; Mori, Y.; Kanda, H.; Notomi, T. Loop-mediated isothermal amplification (LAMP) of gene sequences and simple visual detection of products. Nat. Protoc. 2008, 3, 877–882. [Google Scholar] [CrossRef] [PubMed]
- Nzelu, C.O.; Gomez, E.A.; Caceres, A.G.; Sakurai, T.; Martini-Robles, L.; Uezato, H.; Mimori, T.; Katakura, K.; Hashiguchi, Y.; Kato, H. Development of a loop-mediated isothermal amplification method for rapid mass-screening of sand flies for Leishmania infection. Acta Trop. 2014, 132, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Lu, R.; Wu, X.; Wan, Z.; Li, Y.; Jin, X.; Zhang, C. A Novel Reverse Transcription Loop-Mediated Isothermal Amplification Method for Rapid Detection of SARS-CoV-2. Int. J. Mol. Sci. 2020, 21, 2826. [Google Scholar] [CrossRef] [PubMed]
- Chow, F.W.; Chan, T.T.; Tam, A.R.; Zhao, S.; Yao, W.; Fung, J.; Cheng, F.K.; Lo, G.C.; Chu, S.; Aw-Yong, K.L.; et al. A Rapid, Simple, Inexpensive, and Mobile Colorimetric Assay COVID-19-LAMP for Mass On-Site Screening of COVID-19. Int. J. Mol. Sci. 2020, 21, 5380. [Google Scholar] [CrossRef] [PubMed]
- Roumani, F.; Azinheiro, S.; Sousa, H.; Sousa, A.; Timóteo, M.; Varandas, T.; Fonseca-Silva, D.; Baldaque, I.; Carvalho, J.; Prado, M.; et al. Optimization and Clinical Evaluation of a Multi-Target Loop-Mediated Isothermal Amplification Assay for the Detection of SARS-CoV-2 in Nasopharyngeal Samples. Viruses 2021, 13, 940. [Google Scholar] [CrossRef] [PubMed]
- Anastasiou, O.E.; Holtkamp, C.; Schäfer, M.; Schön, F.; Eis-Hübinger, A.M.; Krumbholz, A. Fast Detection of SARS-CoV-2 RNA Directly from Respiratory Samples Using a Loop-Mediated Isothermal Amplification (LAMP) Test. Viruses 2021, 13, 801. [Google Scholar] [CrossRef]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Domínguez-Gil, M.; Belhassen-García, M.; Bellido, J.L.M.; Muro, A. A Simple, Affordable, Rapid, Stabilized, Colorimetric, Versatile RT-LAMP Assay to Detect SARS-CoV-2. Diagnostics 2021, 11, 438. [Google Scholar] [CrossRef]
- García-Bernalt Diego, J.; Fernández-Soto, P.; Muñoz-Bellido, J.L.; Febrer-Sendra, B.; Crego-Vicente, B.; Carbonell, C.; López-Bernús, A.; Marcos, M.; Belhassen-García, M.; Muro, A. Detection of SARS-CoV-2 RNA in Urine by RT-LAMP: A Very Rare Finding. J. Clin. Med. 2021, 11, 158. [Google Scholar] [CrossRef]
- Kobayashi, G.S.; Brito, L.A.; Moreira, D.P.; Suzuki, A.M.; Hsia, G.S.P.; Pimentel, L.F.; de Paiva, A.P.B.; Dias, C.R.; Lourenço, N.C.V.; Oliveira, B.A.; et al. A Novel Saliva RT-LAMP Workflow for Rapid Identification of COVID-19 Cases and Restraining Viral Spread. Diagnostics 2021, 11, 1400. [Google Scholar] [CrossRef]
- Rajh, E.; Šket, T.; Praznik, A.; Sušjan, P.; Šmid, A.; Urbančič, D.; Mlinarič-Raščan, I.; Kogovšek, P.; Demšar, T.; Milavec, M.; et al. Robust Saliva-Based RNA Extraction-Free One-Step Nucleic Acid Amplification Test for Mass SARS-CoV-2 Monitoring. Molecules 2021, 26, 6617. [Google Scholar] [CrossRef]
- Londono-Avendano, M.A.; Libreros, G.; Osorio, L.; Parra, B. A Rapid RT-LAMP Assay for SARS-CoV-2 with Colorimetric Detection Assisted by a Mobile Application. Diagnostics 2022, 12, 848. [Google Scholar] [CrossRef] [PubMed]
- Fellner, M.D.; Bonaventura, R.; Basiletti, J.; Avaro, M.; Benedetti, E.; Campos, A.; Dattero, M.E.; Russo, M.; Vladmirsky, S.; Molina, V.; et al. Evaluation of RT-qPCR and Loop-Mediated Isothermal Amplification (LAMP) Assays for the Detection of SARS-CoV-2 in Argentina. Genes 2021, 12, 659. [Google Scholar] [CrossRef] [PubMed]
- El-Kafrawy, S.A.; El-Daly, M.M.; Hassan, A.M.; Harakeh, S.M.; Alandijany, T.A.; Azhar, E.I. Rapid and Reliable Detection of SARS-CoV-2 Using Direct RT-LAMP. Diagnostics 2022, 12, 828. [Google Scholar] [CrossRef] [PubMed]
- Chu, D.K.W.; Pan, Y.; Cheng, S.M.S.; Hui, K.P.Y.; Krishnan, P.; Liu, Y.; Ng, D.Y.M.; Wan, C.K.C.; Yang, P.; Wang, Q.; et al. Molecular Diagnosis of a Novel Coronavirus (2019-nCoV) Causing an Outbreak of Pneumonia. Clin. Chem. 2020, 66, 549–555. [Google Scholar] [CrossRef] [PubMed]
- Tran, D.H.; Hoang, Q.C.; Tran, H.T.; Le, U.P.; Do, H.D.K.; Nguyen, D.H.; Hoang, T.L.; Nguyen, T.T.T.; Nguyen, H.A.; Nguyen, T.H. A comparative study of isothermal nucleic acid amplification methods for SARS-CoV-2 detection at point of care. bioRxiv 2021. [Google Scholar] [CrossRef]
- Park, G.S.; Ku, K.; Baek, S.H.; Kim, S.J.; Kim, S.I.; Kim, B.T.; Maeng, J.S. Development of Reverse Transcription Loop-Mediated Isothermal Amplification Assays Targeting Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2). J. Mol. Diagn. JMD 2020, 22, 729–735. [Google Scholar] [CrossRef]
- Zhang, Y.; Odiwuor, N.; Xiong, J.; Sun, L.; Nyaruaba, R.O.; Wei, H.; Tanner, N.A. Rapid molecular detection of SARS-CoV-2 (COVID-19) virus RNA using colorimetric LAMP. medRxiv 2020. [Google Scholar] [CrossRef]
- Huang, W.E.; Lim, B.; Hsu, C.C.; Xiong, D.; Wu, W.; Yu, Y.; Jia, H.; Wang, Y.; Zeng, Y.; Ji, M.; et al. RT-LAMP for rapid diagnosis of coronavirus SARS-CoV-2. Microb. Biotechnol. 2020, 13, 950–961. [Google Scholar] [CrossRef]
- Jiang, M.; Pan, W.; Arasthfer, A.; Fang, W.; Ling, L.; Fang, H.; Daneshnia, F.; Yu, J.; Liao, W.; Pei, H.; et al. Development and Validation of a Rapid, Single-Step Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) System Potentially to Be Used for Reliable and High-Throughput Screening of COVID-19. Front. Cell. Infect. Microbiol. 2020, 10, 331. [Google Scholar] [CrossRef]
- Shirato, K.; Semba, S.; El-Kafrawy, S.A.; Hassan, A.M.; Tolah, A.M.; Takayama, I.; Kageyama, T.; Notomi, T.; Kamitani, W.; Matsuyama, S.; et al. Development of fluorescent reverse transcription loop-mediated isothermal amplification (RT-LAMP) using quenching probes for the detection of the Middle East respiratory syndrome coronavirus. J. Virol. Methods 2018, 258, 41–48. [Google Scholar] [CrossRef]
- Dao Thi, V.L.; Herbst, K.; Boerner, K.; Meurer, M.; Kremer, L.P.; Kirrmaier, D.; Freistaedter, A.; Papagiannidis, D.; Galmozzi, C.; Stanifer, M.L.; et al. A colorimetric RT-LAMP assay and LAMP-sequencing for detecting SARS-CoV-2 RNA in clinical samples. Sci. Transl. Med. 2020, 12, eabc7075. [Google Scholar] [CrossRef]
- Baba, M.M.; Bitew, M.; Fokam, J.; Lelo, E.A.; Ahidjo, A.; Asmamaw, K.; Beloumou, G.A.; Bulimo, W.D.; Buratti, E.; Chenwi, C.; et al. Diagnostic performance of a colorimetric RT-LAMP for the identification of SARS-CoV-2: A multicenter prospective clinical evaluation in sub-Saharan Africa. EClinicalMedicine 2021, 40, 101101. [Google Scholar] [CrossRef] [PubMed]
- Aoki, M.N.; de Oliveira Coelho, B.; Góes, L.G.B.; Minoprio, P.; Durigon, E.L.; Morello, L.G.; Marchini, F.K.; Riediger, I.N.; do Carmo Debur, M.; Nakaya, H.I.; et al. Colorimetric RT-LAMP SARS-CoV-2 diagnostic sensitivity relies on color interpretation and viral load. Sci. Rep. 2021, 11, 9026. [Google Scholar] [CrossRef] [PubMed]
- Amaral, C.; Antunes, W.; Moe, E.; Duarte, A.G.; Lima, L.M.P.; Santos, C.; Gomes, I.L.; Afonso, G.S.; Vieira, R.; Teles, H.S.S.; et al. A molecular test based on RT-LAMP for rapid, sensitive and inexpensive colorimetric detection of SARS-CoV-2 in clinical samples. Sci. Rep. 2021, 11, 16430. [Google Scholar] [CrossRef]
- Lu, S.; Duplat, D.; Benitez-Bolivar, P.; León, C.; Villota, S.D.; Veloz-Villavicencio, E.; Arévalo, V.; Jaenes, K.; Guo, Y.; Cicek, S.; et al. Multicenter international assessment of a SARS-CoV-2 RT-LAMP test for point of care clinical application. PLoS ONE 2022, 17, e0268340. [Google Scholar] [CrossRef] [PubMed]
- Brown, T.A.; Schaefer, K.S.; Tsang, A.; Yi, H.A.; Grimm, J.B.; Lemire, A.L.; Jradi, F.M.; Kim, C.; McGowan, K.; Ritola, K.; et al. Direct detection of SARS-CoV-2 RNA using high-contrast pH-sensitive dyes. J. Biomol. Tech. JBT 2021, 32, 121–133. [Google Scholar] [CrossRef]
- Nguyen, H.V.; Nguyen, V.D.; Nguyen, H.Q.; Chau, T.H.T.; Lee, E.Y.; Seo, T.S. Nucleic acid diagnostics on the total integrated lab-on-a-disc for point-of-care testing. Biosens. Bioelectron. 2019, 141, 111466. [Google Scholar] [CrossRef]
- Lalli, M.A.; Langmade, J.S.; Chen, X.; Fronick, C.C.; Sawyer, C.S.; Burcea, L.C.; Wilkinson, M.N.; Fulton, R.S.; Heinz, M.; Buchser, W.J.; et al. Rapid and Extraction-Free Detection of SARS-CoV-2 from Saliva by Colorimetric Reverse-Transcription Loop-Mediated Isothermal Amplification. Clin. Chem. 2021, 67, 415–424. [Google Scholar] [CrossRef]
- Wang, X.; Yin, F.; Bi, Y.; Cheng, G.; Li, J.; Hou, L.; Li, Y.; Yang, B.; Liu, W.; Yang, L. Rapid and sensitive detection of Zika virus by reverse transcription loop-mediated isothermal amplification. J. Virol. Methods 2016, 238, 86–93. [Google Scholar] [CrossRef]
- Thompson, D.; Lei, Y. Mini review: Recent progress in RT-LAMP enabled COVID-19 detection. Sens. Actuators Rep. 2020, 2, 100017. [Google Scholar] [CrossRef]
- Esbin, M.N.; Whitney, O.N.; Chong, S.; Maurer, A.; Darzacq, X.; Tjian, R. Overcoming the bottleneck to widespread testing: A rapid review of nucleic acid testing approaches for COVID-19 detection. RNA 2020, 26, 771–783. [Google Scholar] [CrossRef] [PubMed]

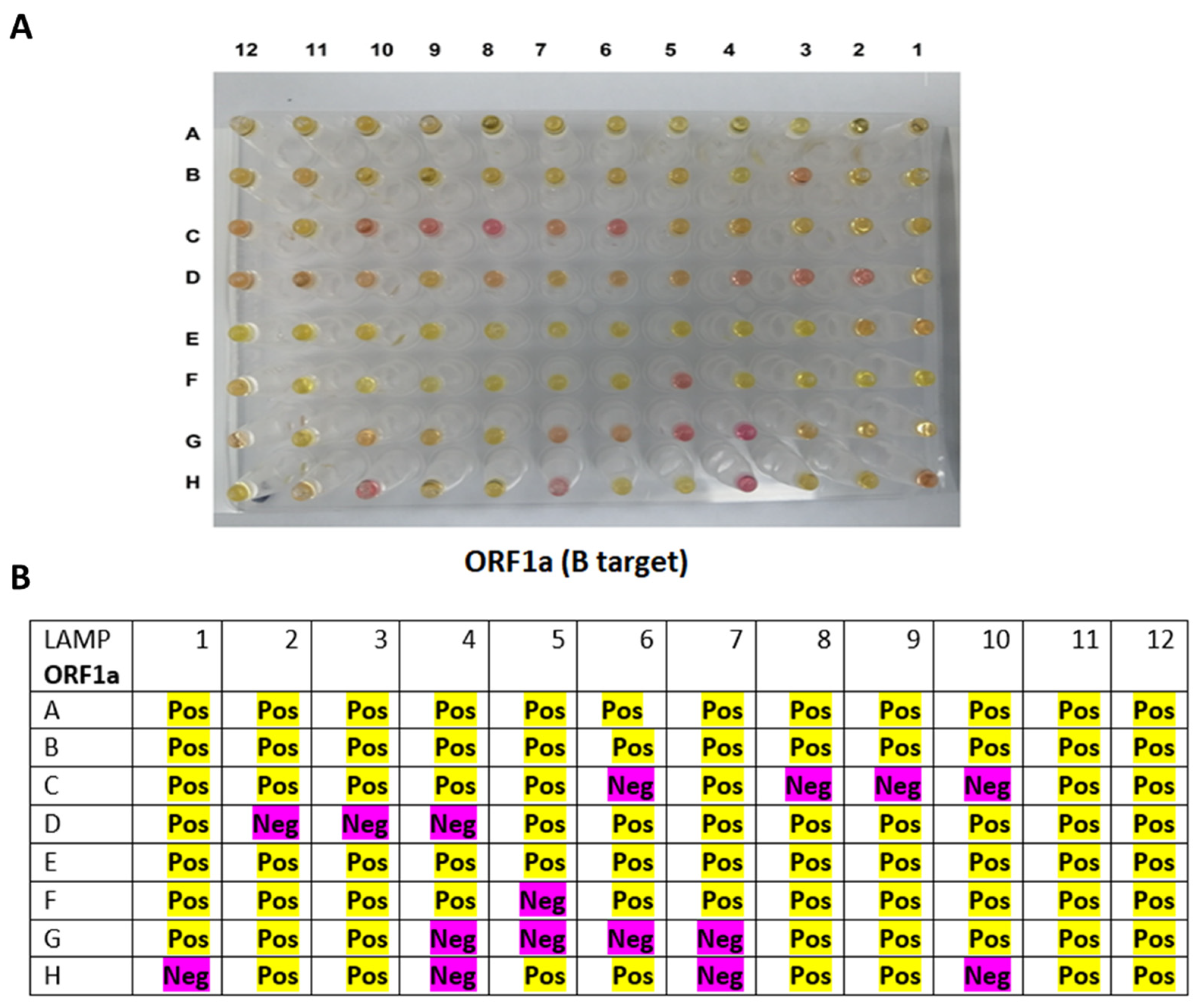
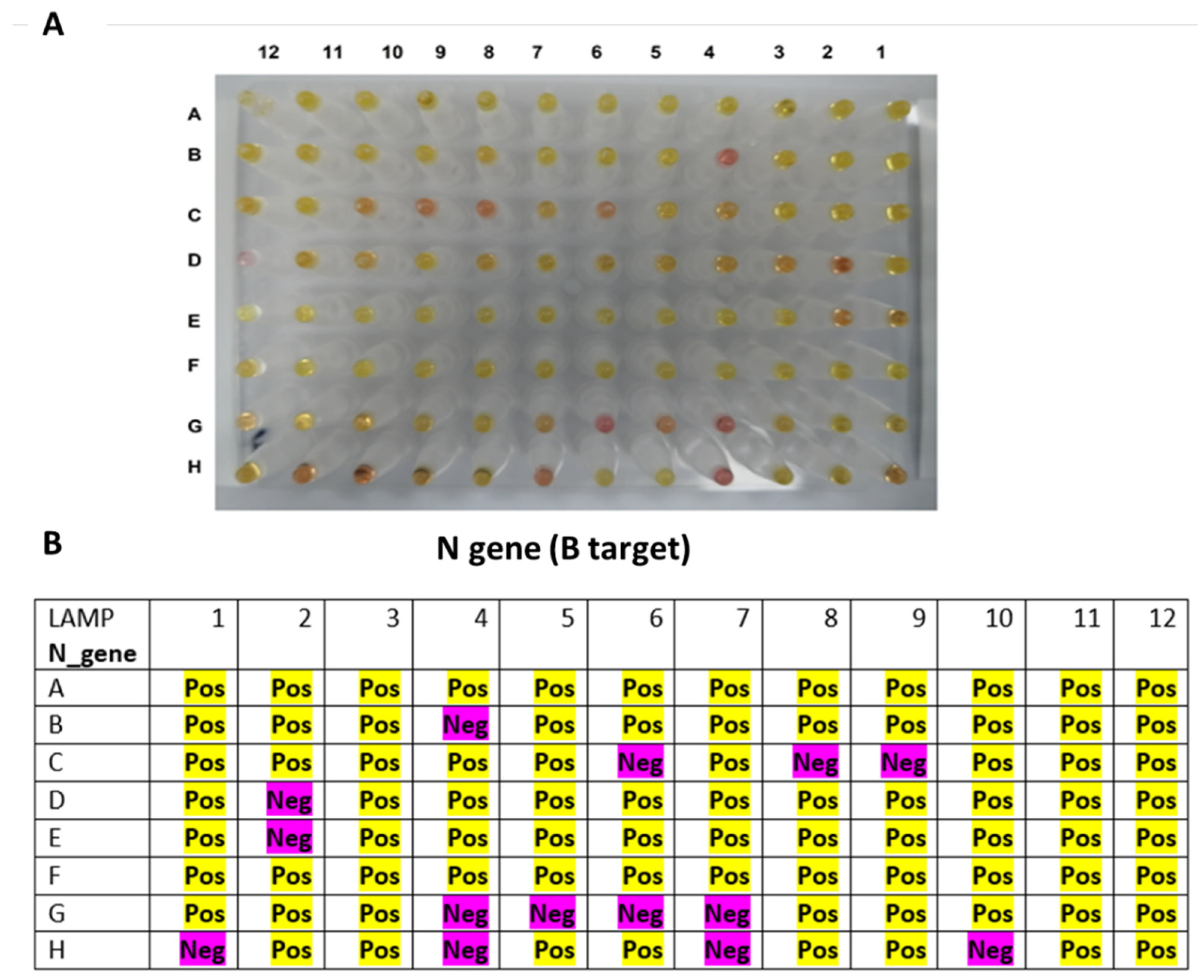
| Cq Value | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | 11 | 12 |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| A | 27 | 22 | 33 | 14 | 21 | 19 | 14 | 18 | 25 | 25 | 21 | 29 |
| B | 15 | 17 | 27 | 21 | 18 | 20 | 18 | 33 | 18 | 27 | 30 | 25 |
| C | 29 | 24 | 18 | 31 | 23 | 29 | 21 | 33 | 25 | 28 | 25 | 24.5 |
| D | 17 | 33 | 33 | 27 | 23 | 22 | 17 | 24 | 14 | 27 | 24 | 32 |
| E | 23 | 32 | 24 | 17 | 26 | 21 | 24 | 31 | 21 | 30 | 16 | 31 |
| F | 28 | 19 | 26 | 17 | 32 | 19 | 20 | 27 | 26 | 17 | 24 | 24 |
| G | 24 | 20 | 30 | 34 | 34 | 33 | 34 | 19 | 27 | 25 | 16 | 34 |
| H | 32 | 21 | 19 | 32 | 18 | 19 | 34 | 15 | 22 | 34 | 29 | 15 |
| Targeted Gene | Cq < 30 (n = 73) | Cq ≥ 30 (n = 23) |
|---|---|---|
| ORF1a | 69 (94.5%) | 11 (47.8%) |
| N | 70 (95.9%) | 12 (52.2%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aldossary, A.M.; Tawfik, E.A.; Altammami, M.A.; Alquait, A.A.; Booq, R.Y.; Sendy, B.K.; Alarawi, M.S.; Gojobori, T.; Altamimi, A.M.; Alaifan, T.A.; et al. Development and Validation of Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) as a Simple and Rapid Diagnostic Tool for SARS-CoV-2 Detection. Diagnostics 2022, 12, 2232. https://doi.org/10.3390/diagnostics12092232
Aldossary AM, Tawfik EA, Altammami MA, Alquait AA, Booq RY, Sendy BK, Alarawi MS, Gojobori T, Altamimi AM, Alaifan TA, et al. Development and Validation of Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) as a Simple and Rapid Diagnostic Tool for SARS-CoV-2 Detection. Diagnostics. 2022; 12(9):2232. https://doi.org/10.3390/diagnostics12092232
Chicago/Turabian StyleAldossary, Ahmad M., Essam A. Tawfik, Musaad A. Altammami, Azzam A. Alquait, Rayan Y. Booq, Bandar K. Sendy, Mohammed S. Alarawi, Takashi Gojobori, Asmaa M. Altamimi, Taghreed A. Alaifan, and et al. 2022. "Development and Validation of Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) as a Simple and Rapid Diagnostic Tool for SARS-CoV-2 Detection" Diagnostics 12, no. 9: 2232. https://doi.org/10.3390/diagnostics12092232
APA StyleAldossary, A. M., Tawfik, E. A., Altammami, M. A., Alquait, A. A., Booq, R. Y., Sendy, B. K., Alarawi, M. S., Gojobori, T., Altamimi, A. M., Alaifan, T. A., Albarrag, A. M., & Alyamani, E. J. (2022). Development and Validation of Reverse Transcriptase Loop-Mediated Isothermal Amplification (RT-LAMP) as a Simple and Rapid Diagnostic Tool for SARS-CoV-2 Detection. Diagnostics, 12(9), 2232. https://doi.org/10.3390/diagnostics12092232





