Clinical Utility of the Contrast-Enhanced Endoscopic Ultrasound Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Cyst
Abstract
:1. Introduction
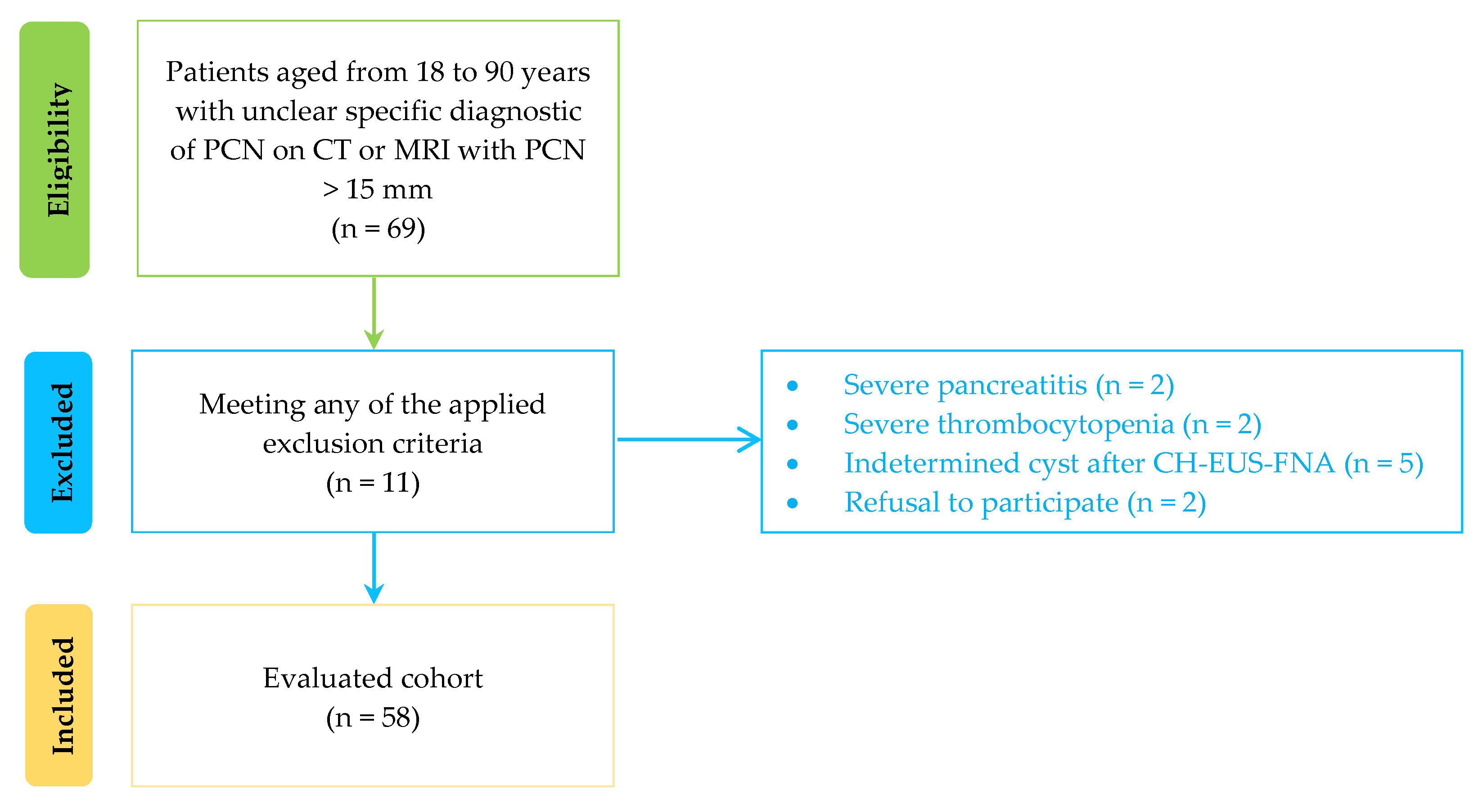
2. Materials and Methods
2.1. Study Design
2.2. Subjects and Data Collection
2.3. Study Outcome and Definitions
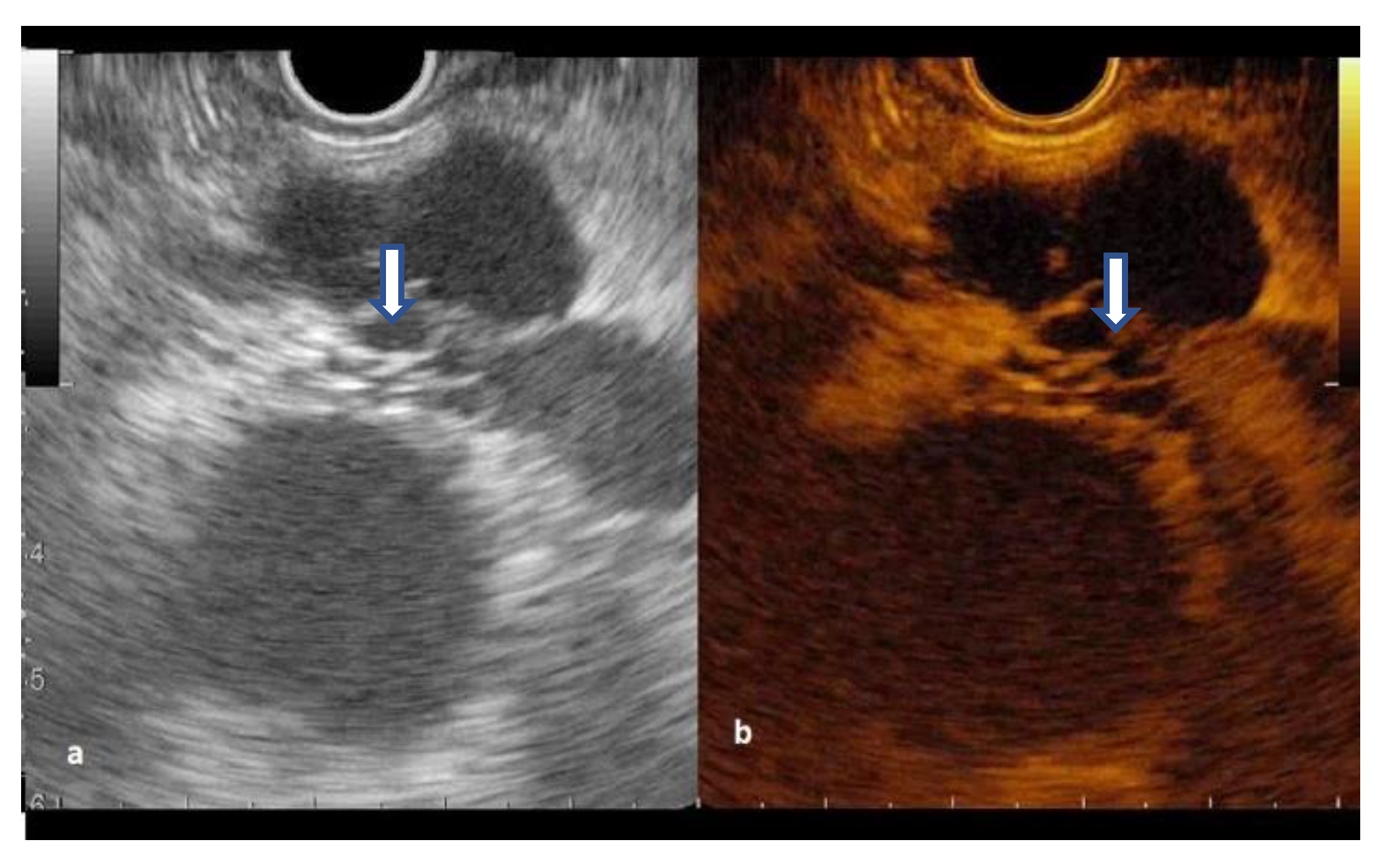
2.4. Procedure
2.5. Preparation of Samples
2.6. Statistical Analysis
3. Results
3.1. Patient Characteristics
3.2. Standard EUS Assessment
3.3. CH-EUS in Diagnosing Pancreatic Cysts
3.4. Contrast- EUS-FNA Assessment of Pancreatic Cysts
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Lee, K.S.; Sekhar, A.; Rofsky, N.M.; Pedrosa, I. Prevalence of incidental pancreatic cysts in the adult population on MR imaging. Am. J. Gastroenterol. 2010, 105, 2079–2084. [Google Scholar] [CrossRef] [PubMed]
- Scheiman, J.M.; Hwang, J.H.; Moayyedi, P. American gastroenterological association technical review on the diagnosis and management of asymptomatic neoplastic pancreatic cysts. Gastroenterology 2015, 148, 824–848.e22. [Google Scholar] [CrossRef] [PubMed]
- Ayoub, F.; Davis, A.M.; Chapman, C.G. Pancreatic Cysts-An Overview and Summary of Society Guidelines, 2021. JAMA 2021, 325, 391–392. [Google Scholar] [CrossRef] [PubMed]
- Balduzzi, A.; Marchegiani, G.; Pollini, T.; Biancotto, M.; Caravati, A.; Stigliani, E.; Burelli, A.; Bassi, C.; Salvia, R. Systematic review and meta-analysis of observational studies on BD-IPMNS progression to malignancy. Pancreatology 2021, 21, 1135–1145. [Google Scholar] [CrossRef]
- Kamata, K.; Kitano, M.; Omoto, S.; Kadosaka, K.; Miyata, T.; Yamao, K.; Imai, H.; Sakamoto, H.; Harwani, Y.; Chikugo, T.; et al. Contrast-enhanced harmonic endoscopic ultrasonography for differential diagnosis of pancreatic cysts. Endoscopy 2016, 48, 35–41. [Google Scholar] [CrossRef]
- Fusaroli, P.; Serrani, M.; De Giorgio, R.; D’Ercole, M.C.; Ceroni, L.; Lisotti, A.; Caletti, G. Contrast Harmonic-Endoscopic Ultrasound Is Useful to Identify Neoplastic Features of Pancreatic Cysts (With Videos). Pancreas 2016, 45, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Hocke, M.; Cui, X.W.; Domagk, D.; Ignee, A.; Dietrich, C.F. Pancreatic cystic lesions: The value of contrast-enhanced endoscopic ultrasound to influence the clinical pathway. Endosc. Ultrasound 2014, 3, 123–130. [Google Scholar] [CrossRef]
- Serrani, M.; Lisotti, A.; Caletti, G.; Fusaroli, P. Role of contrast harmonic-endoscopic ultrasound in pancreatic cystic lesions. Endosc. Ultrasound 2017, 6, 25–30. [Google Scholar] [CrossRef]
- Lisotti, A.; Napoleon, B.; Facciorusso, A.; Cominardi, A.; Crinò, S.F.; Brighi, N.; Gincul, R.; Kitano, M.; Yamashita, Y.; Marchegiani, G.; et al. Contrast-enhanced EUS for the characterization of mural nodules within pancreatic cystic neoplasms: Systematic review and meta-analysis. Gastrointest. Endosc. 2021, 94, 881–889.e5. [Google Scholar] [CrossRef]
- European Study Group on Cystic Tumours of the Pancreas. European evidence-based guidelines on pancreatic cystic neoplasms. Gut 2018, 67, 789–804. [Google Scholar] [CrossRef] [Green Version]
- Polkowski, M.; Jenssen, C.; Kaye, P.; Carrara, S.; Deprez, P.; Gines, A.; Fernández-Esparrach, G.; Eisendrath, P.; Aithal, G.P.; Arcidiacono, P.; et al. Technical aspects of endoscopic ultrasound (EUS)-guided sampling in gastroenterology: European Society of Gastrointestinal Endoscopy (ESGE) Technical Guideline-March 2017. Endoscopy 2017, 49, 989–1006. [Google Scholar] [CrossRef] [PubMed]
- Pitman, M.B.; Layfield, L.J. The Papanicolaou Society of Cytopathology System for Reporting Pancreaticobiliary Cytology, 1st ed.; Springer: Cham, Switzerland, 2015. [Google Scholar]
- Van Huijgevoort, N.C.M.; Del Chiaro, M.; Wolfgang, C.L.; van Hooft, J.E.; Besselink, M.G. Diagnosis and management of pancreatic cystic neoplasms: Current evidence and guidelines. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 676–689. [Google Scholar] [CrossRef] [PubMed]
- Elta, G.H.; Enestvedt, B.K.; Sauer, B.G.; Lennon, A.M. ACG Clinical Guideline: Diagnosis and Management of Pancreatic Cysts. Am. J. Gastroenterol. 2018, 113, 464–479. [Google Scholar] [CrossRef] [PubMed]
- Seicean, A.; Mosteanu, O.; Seicean, R. Maximizing the endosonography: The role of contrast harmonics, elastography and confocal endomicroscopy. World J. Gastroenterol. 2017, 23, 25–41. [Google Scholar] [CrossRef] [PubMed]
- Zhong, N.; Zhang, L.; Takahashi, N.; Shalmiyev, V.; Canto, M.I.; Clain, J.E.; Deutsch, J.C.; DeWitt, J.; Eloubeidi, M.A.; Gleeson, F.C.; et al. Histologic and imaging features of mural nodules in mucinous pancreatic cysts. Clin. Gastroenterol. Hepatol. 2012, 10, 192–198.e2. [Google Scholar] [CrossRef]
- Piscaglia, F.; Nolsøe, C.; Dietrich, C.F.; Cosgrove, D.O.; Gilja, O.H.; Bachmann Nielsen, M.; Albrecht, T.; Barozzi, L.; Bertolotto, M.; Catalano, O.; et al. The EFSUMB Guidelines and Recommendations on the Clinical Practice of Contrast Enhanced Ultrasound (CEUS): Update 2011 on non-hepatic applications. Ultraschall Der Med. 2012, 33, 33–59. [Google Scholar] [CrossRef]
- Muthusamy, V.R.; Chandrasekhara, V.; Acosta, R.D.; Bruining, D.H.; Chathadi, K.V.; Eloubeidi, M.A.; Faulx, A.L.; Fonkalsrud, L.; Gurudu, S.R.; Khashab, M.A.; et al. The role of endoscopy in the diagnosis and treatment of cystic pancreatic neoplasms. Gastrointest. Endosc. 2016, 84, 1–9. [Google Scholar] [CrossRef]
- Mitchell, A.J. Sensitivity×PPV is a recognized test called the clinical utility index (CUI+). Eur. J. Epidemiol. 2011, 26, 251–252. [Google Scholar] [CrossRef]
- Negrao de Figueiredo, G.; Muller-Peltzer, K.; Rubenthaler, J.; Clevert, D.A. CEUS-Diagnostic of malignant liver lesions. Der Radiol. 2018, 58, 528–537. [Google Scholar] [CrossRef]
- Tanaka, M.; Fernandez-Del Castillo, C.; Kamisawa, T.; Jang, J.Y.; Levy, P.; Ohtsuka, T.; Salvia, R.; Shimizu, Y.; Tada, M.; Wolfgang, C.L. Revisions of international consensus Fukuoka guidelines for the management of IPMN of the pancreas. Pancreatology 2017, 17, 738–753. [Google Scholar] [CrossRef]
- Yamashita, Y.; Ueda, K.; Itonaga, M.; Yoshida, T.; Maeda, H.; Maekita, T.; Iguchi, M.; Tamai, H.; Ichinose, M.; Kato, J. Usefulness of contrast-enhanced endoscopic sonography for discriminating mural nodules from mucous clots in intraductal papillary mucinous neoplasms: A single-center prospective study. J. Ultrasound Med. 2013, 32, 61–68. [Google Scholar] [CrossRef] [PubMed]
- Ohno, E.; Hirooka, Y.; Itoh, A.; Ishigami, M.; Katano, Y.; Ohmiya, N.; Niwa, Y.; Goto, H. Intraductal papillary mucinous neoplasms of the pancreas: Differentiation of malignant and benign tumors by endoscopic ultrasound findings of mural nodules. Ann. Surg. 2009, 249, 628–634. [Google Scholar] [CrossRef] [PubMed]
- Harima, H.; Kaino, S.; Shinoda, S.; Kawano, M.; Suenaga, S.; Sakaida, I. Differential diagnosis of benign and malignant branch uct intraductal papillary mucinous neoplasm using contrast-enhanced endoscopic ultrasonography. World J. Gastroenterol. 2015, 21, 6252–6260. [Google Scholar] [CrossRef] [PubMed]
- Thornton, G.D.; McPhail, M.J.; Nayagam, S.; Hewitt, M.J.; Vlavianos, P.; Monahan, K.J. Endoscopic ultrasound guided fine needle aspiration for the diagnosis of pancreatic cystic neoplasms: A meta-analysis. Pancreatology 2013, 13, 48–57. [Google Scholar] [CrossRef]
- Lim, L.G.; Lakhtakia, S.; Ang, T.L.; Vu, C.K.; Dy, F.; Chong, V.H.; Khor, C.J.; Lim, W.C.; Doshi, B.K.; Varadarajulu, S.; et al. Factors determining diagnostic yield of endoscopic ultrasound guided fine-needle aspiration for pancreatic cystic lesions: A multicentre Asian study. Dig. Dis. Sci. 2013, 58, 1751–1757. [Google Scholar] [CrossRef]
- Oppong, K.W.; Dawwas, M.F.; Charnley, R.M.; Wadehra, V.; Elamin, K.; White, S.; Nayar, M. EUS and EUS-FNA diagnosis of suspected pancreatic cystic neoplasms: Is the sum of the parts greater than the CEA? Pancreatology 2015, 15, 531–537. [Google Scholar] [CrossRef]
- Singhi, A.D.; McGrath, K.; Brand, R.E.; Khalid, A.; Zeh, H.J.; Chennat, J.S.; Fasanella, K.E.; Papachristou, G.I.; Slivka, A.; Bartlett, D.L.; et al. Preoperative next-generation sequencing of pancreatic cyst fluid is highly accurate in cyst classification and detection of advanced neoplasia. Gut 2018, 67, 2131–2141. [Google Scholar] [CrossRef]
- Rift, C.V.; Melchior, L.C.; Kovacevic, B.; Klausen, P.; Toxværd, A.; Grossjohann, H.; Karstensen, J.G.; Brink, L.; Hassan, H.; Kalaitzakis, E.; et al. Targeted next generation sequencing of endoscopic ul trasound-guided through-the-needle-biopsies from pancreatic cystic lesions. Gastrointest. Endosc. 2022, 11. [Google Scholar] [CrossRef]
- Tacelli, M.; Celsa, C.; Magro, B.; Barchiesi, M.; Barresi, L.; Capurso, G.; Arcidiacono, P.G.; Cammà, C.; Crinò, S.F. Diagnostic performance of endoscopic ultrasound through-the-needle microforceps biopsy of pancreatic cystic lesions: Systematic review with meta-analysis. Dig. Endosc. 2020, 32, 1018–1030. [Google Scholar] [CrossRef]
- Yamamoto, N.; Kato, H.; Tomoda, T.; Matsumoto, K.; Sakakihara, I.; Noma, Y.; Horiguchi, S.; Harada, R.; Tsutsumi, K.; Hori, K.; et al. Contrast-enhanced harmonic endoscopic ultrasonography with time-intensity curve analysis for intraductal papillary mucinous neoplasms of the pancreas. Endoscopy 2016, 48, 26–34. [Google Scholar] [CrossRef] [Green Version]
- Fusaroli, P.; Kypraios, D.; Mancino, M.G.; Spada, A.; Benini, M.C.; Bianchi, M.; Bocus, P.; De Angelis, C.; De Luca, L.; Fabbri, C.; et al. Interobserver agreement in contrast harmonic endoscopic ultrasound. J. Gastroenterol. Hepatol. 2012, 27, 1063–1069. [Google Scholar] [CrossRef] [PubMed]
- Van Riet, P.A.; Erler, N.S.; Bruno, M.J.; Cahen, D.L. Comparison of fine-needle aspiration and fine- needle biopsy devices for endoscopic ultrasound-guided sampling of solid lesions: A systemic review and meta-analysis. Endoscopy 2021, 53, 411–423. [Google Scholar] [CrossRef] [PubMed]
- Razpotnik, M.; Bota, S.; Kutilek, M.; Essler, G.; Weber-Eibel, J.; Maieron, A.; Peck-Radosavljevic, M. The bleeding risk after endoscopic ultrasound-guided puncture of pancreatic masses. Scand. J. Gastroenterol. 2021, 56, 205–210. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Buccino, V.R.; Sacco, R. A Meta-analysis Confirms that Antibiotic Prophylaxis is Not Needed for Endoscopic Ultrasound-Guided Fine Needle Aspiration of Pancreatic Cysts. Gastroenterology 2021, 160, 969. [Google Scholar] [CrossRef] [PubMed]



| Parameters | Value |
|---|---|
| Age, years (mean ± SD) | 59.8 ± 13.4 |
| Sex female, n (%) | 38 (65.5) |
| BMI, kg/m2 (mean ± SD) | 26.1 ± 3.5 |
| Size, mm Median (Q1 to Q3) | 25 (18.3 to 40.0) |
| Number of lesions, n (%) Unifocal Multifocal | 54 (93.1) 4 (6.9) |
| Location, n (%) Head + isthmus Body Tail Multiple | 33 (56.9) 15 (25.9) 7 (12.1) 3 (5.2) |
| Final diagnosis, n (%) IPMN PK SCN Cystic ductal adk MCN Cystic acinar cell carcinoma | 30 (51.7) 9 (15.5) 8 (13.8) 6 (10.3) 4 (6.9) 1 (1.7) |
| Cyst Feature | Mucinous Cyst | Malignant Cyst | ||||
|---|---|---|---|---|---|---|
| Yes (n = 41) | No (n = 17) | p-Value | Yes (n = 19) | No (n = 39) | p-Value | |
| Wall | ||||||
| Arterial enhancement/n (%) Fast venous wash-out/n (%) | 37/41 (90.2) 20/41 (48.78) | 5/17 (29.41) 1/17 (5.88) | <0.0001 0.0006 | 15/19 (78.94) 11/19 (57.89) | 27/39 (69.23) 10/39 (25.64) | 0.4371 0.0164 |
| Septation * | ||||||
| Arterial enhancement/n (%) Fast venous wash-out/n (%) | 17/29 (58.62) 10/29 (34.48) | 7/10 (70) 2/10 (20) | 0.9839 0.3923 | 8/13 (61.53) 5/13 (38.46) | 16/26 (61.53) 7/26 (26.92) | >0.9999 0.3776 |
| Mural nodule | ||||||
| Arterial enhancement/n (%) Fast venous wash-out/n (%) | 13/13 (100) 8/13 (61.53) | 0/0 0/0 | n.a. n.a. | 10/10 (100) 7/10 (70) | 3/3 (100) 1/3 (30) | n.a. 0.6294 |
| Arterial Enhancement of the Wall for Diagnosing Mucinous Cyst | Arterial Enhancement of the Mural Nodules in Diagnosing Malignant Cyst | Fast Venous Wash Out of the Wall for Diagnosing Mucinous Cysts | Fast Venous Wash Out of the Wall for Diagnosing Malignant Cyst | |
|---|---|---|---|---|
| Se% [95%CI] | 90.2 [81.2 to 99.3] | 100 | 48.8 [33.5 to 64.1] | 58.3 [38.6 to 78.1] |
| Sp% [95%CI] | 70.6 [48.9 to 92.2] | n.a. | 94.1 [82.9 to 100] | 79.4 [65.8 to 93.0] |
| Acc% [95%CI] | 84.5 [75.2 to 93.8] | 76.9 [54.0 to 99.8] | 62.07 [49.6 to 74.6] | 70.7 [59.0 to 82.4] |
| PPV% [95%CI] | 88.1 [78.3 to 97.9] | 76.9 [54.0 to 99.8] | 95.2 [86.1 to 100] | 66.7 [46.5 to 86.8] |
| NPV% [95%CI] | 75.0 [53.8 to 96.2] | n.a. | 43.2 [27.3 to 59.2] | 73.0 [58.7 to 87.3] |
| +LR [95%CI] | 3.07 [1.46 to 6.45] | n.a. | 8.29 [1.21 to 56.8] | 2.83 [1.35 to 5.95] |
| −LR [95%CI] | 0.14 [0.05 to 0.37] | n.a. | 0.54 [0.39 to 0.75] | 0.52 [0.32 to 0.87] |
| +CUI [95%CI] | 0.795 [0.679 to 0.911] | 0.769 [0.543 to 0.995] | 0.465 [0.271 to 0.658] | 0.389 [0.144 to 0.634] |
| −CUI [95%CI] | 0.529 [0.342 to 0.717] | n.a. | 0.407 [0.268 to 0.546] | 0.579 [0.459 to 0.700] |
| Diagnosis of Cysts Assessed with CH- EUS-FNA | Mucinous | Non-Mucinous | Malignant | Non-Malignant |
|---|---|---|---|---|
| Fluid + mural nodules cytology (n = 48) | 34 | 14 | 16 | 25 |
| Conclusive (n = 41) | 27 | 14 | 16 | 18 |
| High dysplasia/carcinoma * Low/moderate dysplasia * No dysplasia * | 16 5 6 | 0 0 14 | 16 0 0 | 0 5 6 |
| Mural nodule cytology (n = 13) | 13 | 0 | 10 | 3 |
| Conclusive (n = 13) | 13 | 0 | 10 | 3 |
| High dysplasia/carcinoma * Low/moderate dysplasia * | 10 3 | 0 0 | 0 0 | 0 0 |
| Diagnostic rate | Mucinous vs. Non-mucinous | Malignant vs. Non-malignant | ||
| Sensitivity (%) Specificity (%) Positive predictive value Negative predictive value +CUI [95%CI] −CUI [95%CI] | 82.4 92.9 96.6 68.4 0.795 [0.66 to 0.92] 0.635 [0.47 to 0.79] | 84.2 100 100 90.6 0.842 [0.68 to 0.997] 0.906 [0.84 to 0.97] | ||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Olar, M.P.; Bolboacă, S.D.; Pojoga, C.; Moșteanu, O.; Gheorghiu, M.; Seicean, R.; Rusu, I.; Sparchez, Z.; Al Hajjar, N.; Seicean, A. Clinical Utility of the Contrast-Enhanced Endoscopic Ultrasound Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Cyst. Diagnostics 2022, 12, 2209. https://doi.org/10.3390/diagnostics12092209
Olar MP, Bolboacă SD, Pojoga C, Moșteanu O, Gheorghiu M, Seicean R, Rusu I, Sparchez Z, Al Hajjar N, Seicean A. Clinical Utility of the Contrast-Enhanced Endoscopic Ultrasound Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Cyst. Diagnostics. 2022; 12(9):2209. https://doi.org/10.3390/diagnostics12092209
Chicago/Turabian StyleOlar, Miruna Patricia, Sorana D. Bolboacă, Cristina Pojoga, Ofelia Moșteanu, Marcel Gheorghiu, Radu Seicean, Ioana Rusu, Zeno Sparchez, Nadim Al Hajjar, and Andrada Seicean. 2022. "Clinical Utility of the Contrast-Enhanced Endoscopic Ultrasound Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Cyst" Diagnostics 12, no. 9: 2209. https://doi.org/10.3390/diagnostics12092209
APA StyleOlar, M. P., Bolboacă, S. D., Pojoga, C., Moșteanu, O., Gheorghiu, M., Seicean, R., Rusu, I., Sparchez, Z., Al Hajjar, N., & Seicean, A. (2022). Clinical Utility of the Contrast-Enhanced Endoscopic Ultrasound Guided Fine Needle Aspiration in the Diagnosis of Pancreatic Cyst. Diagnostics, 12(9), 2209. https://doi.org/10.3390/diagnostics12092209







