The Use of Optical Coherence Tomography for Gross Examination and Sampling of Fixed Breast Specimens: A Pilot Study
Abstract
:1. Introduction
2. Methods
3. Results
3.1. Histopathologic Review of TS
3.2. OCT Interpretation
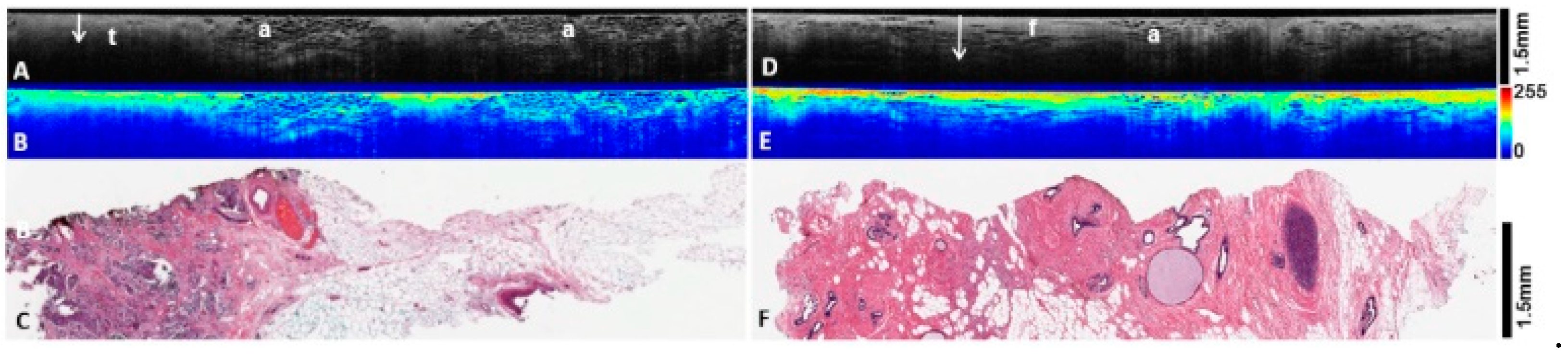
3.3. Heatmap Light Scatter Analysis
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matsen, C.B.; Neumayer, L.A. Breast cancer: A review for the general surgeon. JAMA Surg. 2013, 148, 971–979. [Google Scholar] [CrossRef] [PubMed]
- ACS. Key Statistics for Breast Cancer. Available online: https://www.cancer.org/cancer/breast-cancer/about/how-common-is-breast-cancer.html (accessed on 7 April 2022).
- Cleary, A.S.; Lester, S.C. The Critical Role of Breast Specimen Gross Evaluation for Optimal Personalized Cancer Care. Surg. Pathol. Clin. 2022, 15, 121–132. [Google Scholar] [CrossRef] [PubMed]
- Han, R.; Regpala, S.; Slodkowska, E.; Nofech-Mozes, S.; Hanna, W.; Parra-Herran, C.; Lu, F.I. Lack of Standardization in the Processing and Reporting of Post-Neoadjuvant Breast Cancer Specimens. Arch. Pathol. Lab. Med. 2020, 144, 1262–1270. [Google Scholar] [CrossRef] [PubMed]
- Wiley, E.L.; Keh, P. Diagnostic discrepancies in breast specimens subjected to gross reexamination. Am. J. Surg. Pathol. 1999, 23, 876–879. [Google Scholar] [CrossRef]
- Lester, S.C.; French, C.A.; Curtis, S.G. Manual of Surgical Pathology, 3rd ed.; Saunders; Elsevier: Phiadelphia, PA, USA, 2010; p. 266. [Google Scholar]
- Drexler, W.; Fujimoto, J.G. Optical Coherence Tomography: Technology and Applications, 2nd ed.; Springer: Berlin, Germany, 2015; pp. 3–64. [Google Scholar]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical coherence tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef]
- Wang, J.; Xu, Y.; Boppart, S.A. Review of optical coherence tomography in oncology. J. Biomed. Opt. 2017, 22, 1–23. [Google Scholar] [CrossRef]
- Schmidt, H.; Connolly, C.; Jaffer, S.; Oza, T.; Weltz, C.R.; Port, E.R.; Corben, A. Evaluation of surgically excised breast tissue microstructure using wide-field optical coherence tomography. Breast J. 2020, 26, 917–923. [Google Scholar] [CrossRef]
- Wilson, B.C.; Jermyn, M.; Leblond, F. Challenges and opportunities in clinical translation of biomedical optical spectroscopy and imaging. J. Biomed. Opt. 2018, 23, 1–13. [Google Scholar] [CrossRef]
- Wells, W.A.; Thrall, M.; Sorokina, A.; Fine, J.; Krishnamurthy, S.; Haroon, A.; Rao, B.; Shevchuk, M.M.; Wolfsen, H.C.; Tearney, G.J.; et al. In Vivo and Ex Vivo Microscopy: Moving Toward the Integration of Optical Imaging Technologies into Pathology Practice. Arch. Pathol. Lab. Med. 2019, 143, 288–298. [Google Scholar] [CrossRef]
- Nguyen, F.T.; Zysk, A.M.; Chaney, E.J.; Kotynek, J.G.; Oliphant, U.J.; Bellafiore, F.J.; Rowland, K.M.; Johnson, P.A.; Boppart, S.A. Intraoperative evaluation of breast tumor margins with optical coherence tomography. Cancer Res. 2009, 69, 8790–8796. [Google Scholar] [CrossRef] [Green Version]
- Yemul, K.S.; Zysk, A.M.; Richardson, A.L.; Tangella, K.V.; Jacobs, L.K. Interpretation of Optical Coherence Tomography Images for Breast Tissue Assessment. Surg. Innov. 2019, 26, 50–56. [Google Scholar] [CrossRef]
- Yao, X.; Gan, Y.; Chang, E.; Hibshoosh, H.; Feldman, S.; Hendon, C. Visualization and tissue classification of human breast cancer images using ultrahigh-resolution OCT. Lasers Surg. Med. 2017, 49, 258–269. [Google Scholar] [CrossRef]
- Zhou, C.; Cohen, D.W.; Wang, Y.; Lee, H.C.; Mondelblatt, A.E.; Tsai, T.H.; Aguirre, A.D.; Fujimoto, J.G.; Connolly, J.L. Integrated optical coherence tomography and microscopy for ex vivo multiscale evaluation of human breast tissues. Cancer Res. 2010, 70, 10071–10079. [Google Scholar] [CrossRef] [PubMed]
- Assayag, O.; Antoine, M.; Sigal-Zafrani, B.; Riben, M.; Harms, F.; Burcheri, A.; Grieve, K.; Dalimier, E.; Le Conte de Poly, B.; Boccara, C. Large field, high resolution full-field optical coherence tomography: A pre-clinical study of human breast tissue and cancer assessment. Technol. Cancer Res. Treat. 2014, 13, 455–468. [Google Scholar] [CrossRef] [PubMed]
- Hammond, M.E.; Hayes, D.F.; Dowsett, M.; Allred, D.C.; Hagerty, K.L.; Badve, S.; Fitzgibbons, P.L.; Francis, G.; Goldstein, N.S.; Hayes, M.; et al. American Society of Clinical Oncology/College Of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. J. Clin. Oncol. 2010, 28, 2784–2795. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.N.; Ritter, J.; Dornbluth, N.C.; Mais, D.D.; Nazarullah, A.N. Grossing Breast Cancer Specimens: A Comprehensive Review. AJSP Rev. Rep. 2020, 25, 148–155. [Google Scholar] [CrossRef]
- Shea, B.; Boyan, W.P., Jr.; Kamrani, K.; Lepis, G.; Dupree, D.; Chang, S.; Goldfarb, M.; Kohli, M. Let us cut to the core: Is core biopsy enough for subcentimeter breast cancer? J. Surg. Res. 2017, 216, 30–34. [Google Scholar] [CrossRef]
- Kerlikowske, K. Epidemiology of ductal carcinoma in situ. J. Natl. Cancer Inst. Monogr. 2010, 2010, 139–141. [Google Scholar] [CrossRef]
- Lester, S.C.; Bose, S.; Chen, Y.Y.; Connolly, J.L.; de Baca, M.E.; Fitzgibbons, P.L.; Hayes, D.F.; Kleer, C.; O’Malley, F.P.; Page, D.L.; et al. Protocol for the examination of specimens from patients with ductal carcinoma in situ of the breast. Arch. Pathol. Lab. Med. 2009, 133, 15–25. [Google Scholar] [CrossRef]
- Johnson, S.M.; Vanleer, J.P.; O’Connor, S.M.; Maygarden, S.J. Current Procedural Terminology Coding in an Academic Breast Pathology Service: An Illustration of the Undervaluation of Breast Pathology. Am. J. Surg. Pathol. 2019, 43, 1510–1517. [Google Scholar] [CrossRef]
- Arudra, S.K.C.; Garvey, L.C.; Hagemann, I.S. In-laboratory breast specimen radiography reduces tissue block utilization and improves turnaround time of pathologic examination. BMC Med. Imaging 2021, 21, 59. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.Q.; Que, J.; Chen, L.; Dingee, C.K.; Warburton, R.; McKevitt, E.C.; Kuusk, U.; Pao, J.S.; Bazzarelli, A. Measurements using mammography and ultrasonography underestimate the size of high-volume ductal carcinoma in situ. Am. J. Surg. 2021, 221, 1167–1171. [Google Scholar] [CrossRef] [PubMed]
- Tromberg, B.J.; Shah, N.; Lanning, R.; Cerussi, A.; Espinoza, J.; Pham, T.; Svaasand, L.; Butler, J. Non-invasive in vivo characterization of breast tumors using photon migration spectroscopy. Neoplasia 2000, 2, 26–40. [Google Scholar] [CrossRef] [PubMed] [Green Version]

| Lumpectomy | Mastectomy | Prophylactic Mastectomy | Re-Excision | Reduction Mammoplasty | |
|---|---|---|---|---|---|
| Number of specimens | 26 | 3 a | 4 | 2 b | 5 |
| Average patient age | 56 | 51 | 42 | 69 | 43 |
| Fixation time (d) | 149 | 84 | 136 | 172 | 172 |
| Invasive + DCIS c | 15 | 3 | - | 1 | - |
| Invasive only | 5 | - | - | - | - |
| Atypical | 2 | - | 1 | - | 1 |
| Benign | 4 | - | 3 | 1 | 4 |
| Histopathologic Finding | OCT Appearance |
|---|---|
| Benign: | |
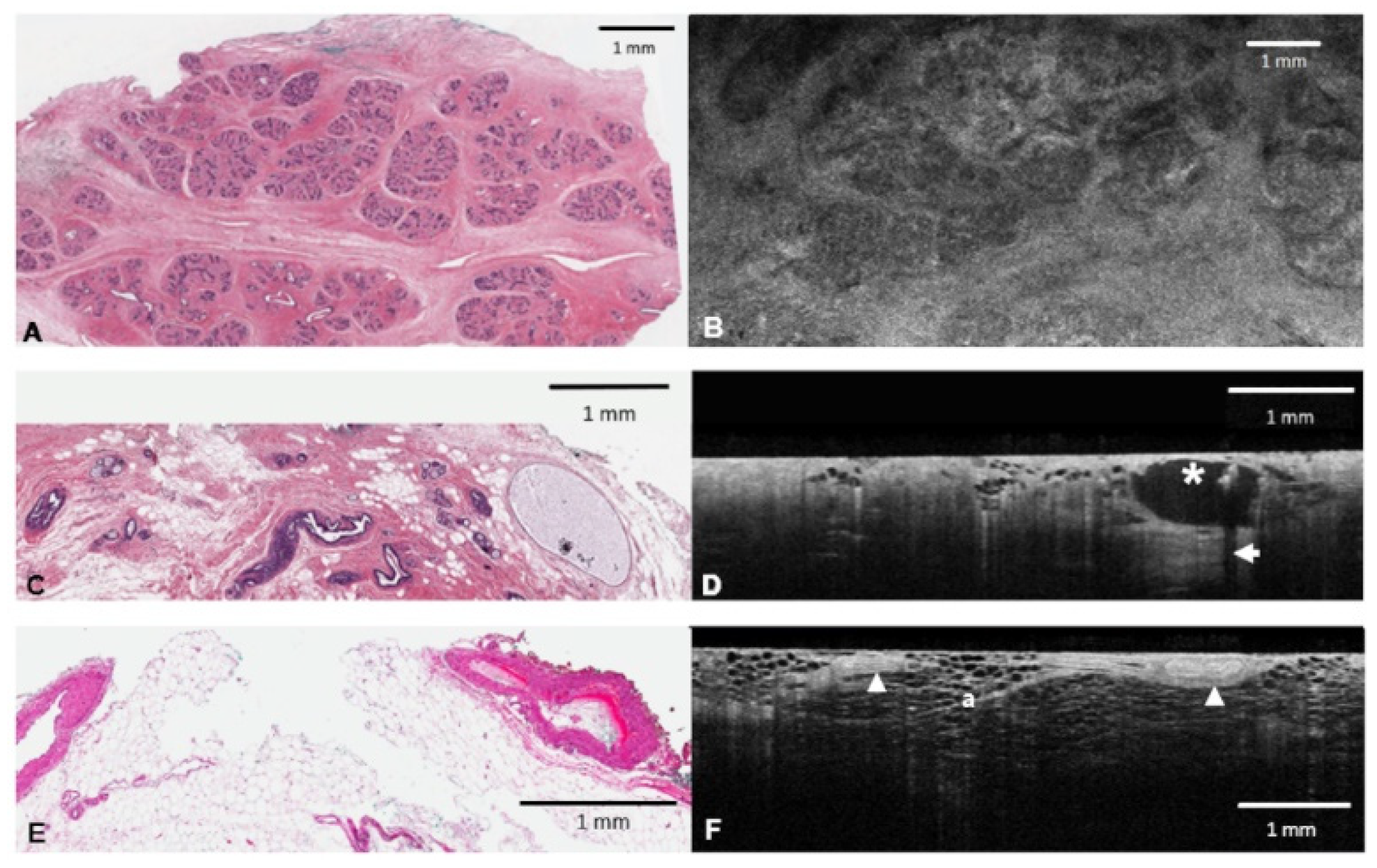
| Fibroglandular tissue | Terminal duct lobular units (TDLUs) are subtle, delicate clusters of rounded ducts, interdigitated by gray fibrous tissue (Figure 2B). The boundary with fat is sharp and well delineated. |
| Adipose tissue (fat) | Honeycomb pattern of dark adipocytes and bright supporting stroma (Figure 1F). |
| Cyst | Spherical shape with a dark lumen. Subtle gray epithelial lining may be appreciated (Figure 1D). |
| Vessel | Elongated tubal structures with a bright smooth muscle and slightly darker adventitia. Where present, clotted blood is strongly hyper-reflective (Figure 1F). |
| Calcification | Strongly hyper-reflective with posterior shadowing seen on 2D depth profiles (B scans) (Figure 1D). |
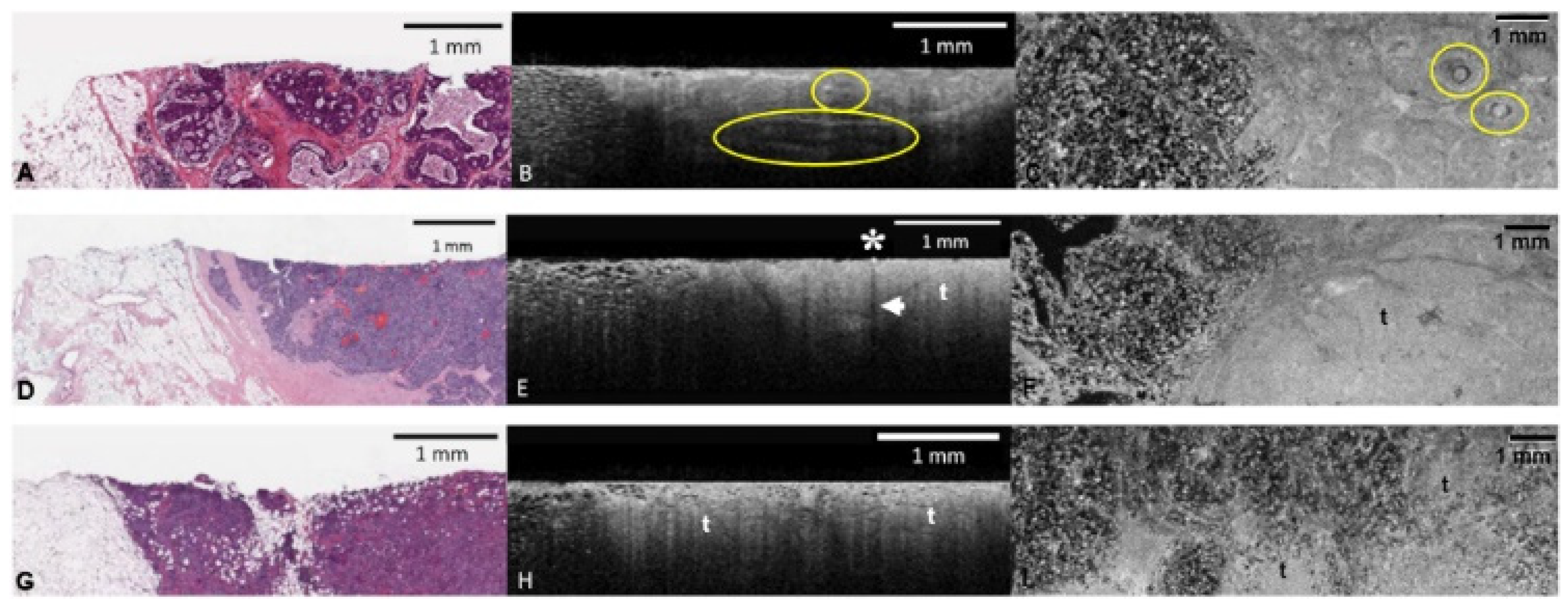
| Malignant: | |
| Invasive tumor | Invasive carcinoma has varied histomorphology, and this is reflected in the OCT findings. Generally, tumor intensity is duller and more heterogenous than uninvolved fibrous tissue. The boundary with adjacent fat or fibroglandular tissue is ill defined. It also exhibits reduced PD on 2D depth profiles (B scans) (Figure 2E,F). |
| DCIS | DCIS without comedo necrosis or calcification can be difficult to recognize on OCT. Look for groups of distending ducts which do not represent a cyst (see above). When present, comedo necrosis and calcification are both hyper-reflective, and both exhibit posterior shadowing. Cribriform architecture may be appreciated within the epithelial proliferation. The immediate stroma surrounding DCIS will appear brighter (Figure 2B,C). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Faragalla, H.; Davoudi, B.; Nofech-Moses, N.; Yucel, Y.; Jakate, K. The Use of Optical Coherence Tomography for Gross Examination and Sampling of Fixed Breast Specimens: A Pilot Study. Diagnostics 2022, 12, 2191. https://doi.org/10.3390/diagnostics12092191
Faragalla H, Davoudi B, Nofech-Moses N, Yucel Y, Jakate K. The Use of Optical Coherence Tomography for Gross Examination and Sampling of Fixed Breast Specimens: A Pilot Study. Diagnostics. 2022; 12(9):2191. https://doi.org/10.3390/diagnostics12092191
Chicago/Turabian StyleFaragalla, Hala, Bahar Davoudi, Naama Nofech-Moses, Yeni Yucel, and Kiran Jakate. 2022. "The Use of Optical Coherence Tomography for Gross Examination and Sampling of Fixed Breast Specimens: A Pilot Study" Diagnostics 12, no. 9: 2191. https://doi.org/10.3390/diagnostics12092191
APA StyleFaragalla, H., Davoudi, B., Nofech-Moses, N., Yucel, Y., & Jakate, K. (2022). The Use of Optical Coherence Tomography for Gross Examination and Sampling of Fixed Breast Specimens: A Pilot Study. Diagnostics, 12(9), 2191. https://doi.org/10.3390/diagnostics12092191





