Application of High-Throughput Sequencing Technology in the Pathogen Identification of Diverse Infectious Diseases in Nephrology Departments
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. Sample Collection and Processing
2.3. Statistical Analysis
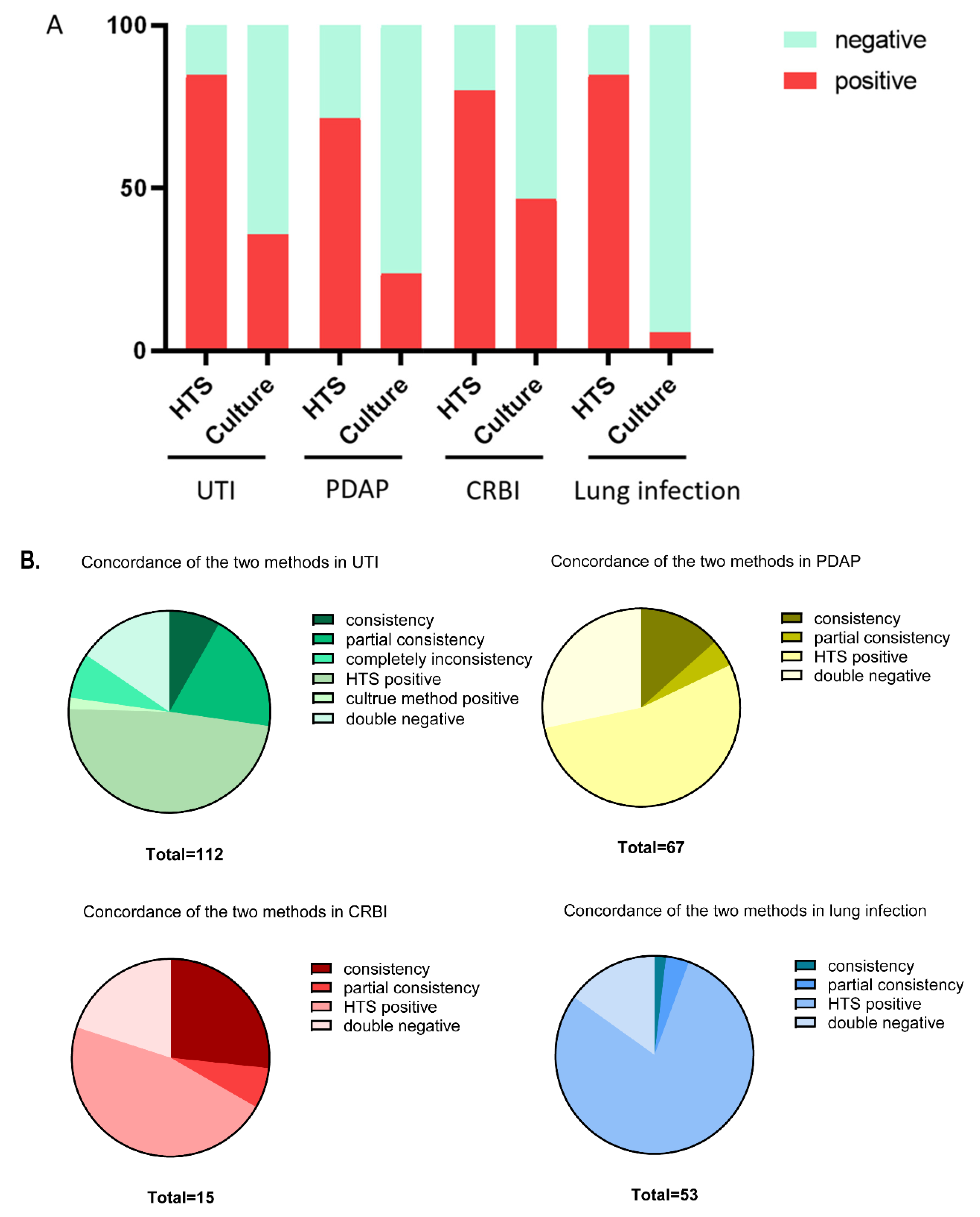
3. Results
3.1. Comparison of the Detection Positive Rate between the Two Methods
3.2. Profile of Identified Pathogens by HTS and Culture Methods on Indicated Diseases
3.3. Comparison of the Detection Effectiveness by Pathogen Type between HTS and Culture Methods
3.4. Different Impacts of Prior Use of Antibiotics on the Detection Efficiency of the Two Methods
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Cohen, G. Immune Dysfunction in Uremia 2020. Toxins 2020, 12, 439. [Google Scholar] [CrossRef] [PubMed]
- Thongprayoon, C.; Cheungpasitporn, W.; Phatharacharukul, P.; Edmonds, P.J.; Kaewpoowat, Q.; Mahaparn, P.; Bruminhent, J.; Erickson, S.B. Chronic kidney disease and end-stage renal disease are risk factors for poor outcomes of Clostridium difficile infection: A systematic review and meta-analysis. Int. J. Clin. Pract. 2015, 69, 998–1006. [Google Scholar] [CrossRef] [PubMed]
- Mermel, L.A.; Allon, M.; Bouza, E.; Craven, D.E.; Flynn, P.; O’Grady, N.P.; Raad, I.I.; Rijnders, B.J.; Sherertz, R.J.; Warren, D.K. Clinical practice guidelines for the diagnosis and management of intravascular catheter-related infection: 2009 Update by the Infectious Diseases Society of America. Clin. Infect. Dis. 2009, 49, 1–45. [Google Scholar] [CrossRef] [PubMed]
- Idelevich, E.A.; Reischl, U.; Becker, K. New Microbiological Techniques in the Diagnosis of Bloodstream Infections. Dtsch. Arztebl. Int. 2018, 115, 822–832. [Google Scholar] [CrossRef]
- Lamy, B.; Sundqvist, M.; Idelevich, E.A. Bloodstream infections—Standard and progress in pathogen diagnostics. Clin. Microbiol. Infect. 2020, 26, 142–150. [Google Scholar] [CrossRef]
- Yu, X.; Jiang, W.; Shi, Y.; Ye, H.; Lin, J. Applications of sequencing technology in clinical microbial infection. J. Cell. Mol. Med. 2019, 23, 7143–7150. [Google Scholar] [CrossRef]
- Zhang, H.C.; Ai, J.W.; Cui, P.; Zhu, Y.M.; Hong-Long, W.; Li, Y.J.; Zhang, W.H. Incremental value of metagenomic next generation sequencing for the diagnosis of suspected focal infection in adults. J. Infect. 2019, 79, 419–425. [Google Scholar] [CrossRef]
- Wang, S.; Chen, Y.; Wang, D.; Wu, Y.; Zhao, D.; Zhang, J.; Xie, H.; Gong, Y.; Sun, R.; Nie, X.; et al. The Feasibility of Metagenomic Next-Generation Sequencing to Identify Pathogens Causing Tuberculous Meningitis in Cerebrospinal Fluid. Front. Microbiol. 2019, 10, 1993. [Google Scholar] [CrossRef]
- Zinter, M.S.; Dvorak, C.C.; Mayday, M.Y.; Iwanaga, K.; Ly, N.P.; McGarry, M.E.; Church, G.D.; Faricy, L.E.; Rowan, C.M.; Hume, J.R.; et al. Pulmonary Metagenomic Sequencing Suggests Missed Infections in Immunocompromised Children. Clin. Infect. Dis. 2019, 68, 1847–1855. [Google Scholar] [CrossRef]
- Cullis, B.; Al-Hwiesh, A.; Kilonzo, K.; McCulloch, M.; Niang, A.; Nourse, P.; Parapiboon, W.; Ponce, D.; Finkelstein, F.O. ISPD guidelines for peritoneal dialysis in acute kidney injury: 2020 update (adults). Perit. Dial. Int. 2021, 41, 15–31. [Google Scholar] [CrossRef]
- Riedel, S.; Carroll, K.C. Blood cultures: Key elements for best practices and future directions. J. Infect. Chemother. 2010, 16, 301–316. [Google Scholar] [CrossRef] [PubMed]
- Fu, Y.; Chen, Q.; Xiong, M.; Zhao, J.; Shen, S.; Chen, L.; Pan, Y.; Li, Z.; Li, Y. Clinical Performance of Nanopore Targeted Sequencing for Diagnosing Infectious Diseases. Microbiol. Spectr. 2022, 10, e27022. [Google Scholar] [CrossRef]
- Jing, C.; Chen, H.; Liang, Y.; Zhong, Y.; Wang, Q.; Li, L.; Sun, S.; Guo, Y.; Wang, R.; Jiang, Z.; et al. Clinical Evaluation of an Improved Metagenomic Next-Generation Sequencing Test for the Diagnosis of Bloodstream Infections. Clin. Chem. 2021, 67, 1133–1143. [Google Scholar] [CrossRef] [PubMed]
- Blauwkamp, T.A.; Thair, S.; Rosen, M.J.; Blair, L.; Lindner, M.S.; Vilfan, I.D.; Kawli, T.; Christians, F.C.; Venkatasubrahmanyam, S.; Wall, G.D.; et al. Analytical and clinical validation of a microbial cell-free DNA sequencing test for infectious disease. Nat. Microbiol. 2019, 4, 663–674. [Google Scholar] [CrossRef]
- Gu, W.; Miller, S.; Chiu, C.Y. Clinical Metagenomic Next-Generation Sequencing for Pathogen Detection. Annu. Rev. Pathol. 2019, 14, 319–338. [Google Scholar] [CrossRef] [PubMed]
- Sabapathypillai, S.L.; James, H.R.; Lyerla, R.R.; Hassman, L. The Next Generation of Ocular Pathogen Detection. Asia. Pac. J. Ophthalmol. 2021, 10, 109–113. [Google Scholar] [CrossRef]
- Miao, Q.; Ma, Y.; Wang, Q.; Pan, J.; Zhang, Y.; Jin, W.; Yao, Y.; Su, Y.; Huang, Y.; Wang, M.; et al. Microbiological Diagnostic Performance of Metagenomic Next-generation Sequencing When Applied to Clinical Practice. Clin. Infect. Dis. 2018, 67 (Suppl. S2), S231–S240. [Google Scholar] [CrossRef]
- Besser, J.; Carleton, H.; Gerner-Smidt, P.; Lindsey, R.; Trees, E. Next-generation sequencing technologies and their application to the study and control of bacterial infections. Clin. Microbiol. Infect. 2018, 24, 335–341. [Google Scholar] [CrossRef]
- Wang, H.; Lu, Z.; Bao, Y.; Yang, Y.; De Groot, R.; Dai, W.; De Jonge, M.I.; Zheng, Y. Clinical diagnostic application of metagenomic next-generation sequencing in children with severe nonresponding pneumonia. PLoS ONE 2020, 15, e232610. [Google Scholar] [CrossRef]
- Wilson, M.R.; O’Donovan, B.D.; Gelfand, J.M.; Sample, H.A.; Chow, F.C.; Betjemann, J.P.; Shah, M.P.; Richie, M.B.; Gorman, M.P.; Hajj-Ali, R.A.; et al. Chronic Meningitis Investigated via Metagenomic Next-Generation Sequencing. JAMA Neurol. 2018, 75, 947–955. [Google Scholar] [CrossRef]
- Li, T.; Mbala-Kingebeni, P.; Naccache, S.N.; Thézé, J.; Bouquet, J.; Federman, S.; Somasekar, S.; Yu, G.; Martin, C.S.-S.; Achari, A.; et al. Metagenomic Next-Generation Sequencing of the 2014 Ebola Virus Disease Outbreak in the Democratic Republic of the Congo. J. Clin. Microbiol. 2019, 57, e00827-19. [Google Scholar] [CrossRef] [PubMed]
- Belete, M.A.; Saravanan, M. A Systematic Review on Drug Resistant Urinary Tract Infection Among Pregnant Women in Developing Countries in Africa and Asia; 2005–2016. Infect. Drug Resist. 2020, 13, 1465–1477. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.A.; Carolisna, Y.I.; Sakura, D.; Yeo, S.T.; Koh, K.H. Demographic characteristics and outcomes of continuous ambulatory peritoneal dialysis related peritonitis in Miri General Hospital, Malaysia. Med. J. Malays. 2019, 74, 270–274. [Google Scholar]
- van Esch, S.; Krediet, R.T.; Struijk, D.G. 32 years’ experience of peritoneal dialysis-related peritonitis in a university hospital. Perit. Dial. Int. 2014, 34, 162–170. [Google Scholar] [CrossRef]
- David, A.; Risitano, D.C.; Mazzeo, G.; Sinardi, L.; Venuti, F.S.; Sinardi, A.U. Central venous catheters and infections. Minerva Anestesiol. 2005, 71, 561–564. [Google Scholar]
- Ruiz-Giardin, J.M.; Ochoa Chamorro, I.; Velázquez Ríos, L.; Jaqueti Aroca, J.; García Arata, M.I.; SanMartín López, J.V.; Guerrero Santillán, M. Blood stream infections associated with central and peripheral venous catheters. BMC Infect. Dis. 2019, 19, 841. [Google Scholar] [CrossRef]
- Sahli, F.; Feidjel, R.; Laalaoui, R. Hemodialysis catheter-related infection: Rates, risk factors and pathogens. J. Infect. Public Health 2017, 10, 403–408. [Google Scholar] [CrossRef]
- Gupta, V.; Yassin, M.H. Infection and hemodialysis access: An updated review. Infect. Disord. Drug Targets 2013, 13, 196–205. [Google Scholar] [CrossRef]
- Pieters, A.; Bakker, M.; Hoek, R.A.S.; Altenburg, J.; van Westreenen, M.; Aerts, J.G.J.V.; van der Eerden, M.M. Predicting factors for chronic colonization of Pseudomonas aeruginosa in bronchiectasis. Eur. J. Clin. Microbiol. Infect. Dis. 2019, 38, 2299–2304. [Google Scholar] [CrossRef]
- Gadsby, N.J.; Russell, C.D.; McHugh, M.P.; Mark, H.; Conway Morris, A.; Laurenson, I.F.; Hill, A.T.; Templeton, K.E. Comprehensive Molecular Testing for Respiratory Pathogens in Community-Acquired Pneumonia. Clin. Infect. Dis. 2016, 62, 817–823. [Google Scholar] [CrossRef]
- Han, D.; Li, Z.; Li, R.; Tan, P.; Zhang, R.; Li, J. mNGS in clinical microbiology laboratories: On the road to maturity. Crit. Rev. Microbiol. 2019, 45, 668–685. [Google Scholar] [CrossRef] [PubMed]
| Diagnostic Criteria | Exclusion Criteria | |
|---|---|---|
| UTI | (1) Patients without urinary catheters: clinical manifestations, urinary leukocyte (WBC) count (≥104 CFU/mL, leukocyte count with Sysmex UF-1000i urinary sediment analyzer), pathogenic bacteria ≤2 kinds, and ≥103 CFU/m (2) Patients with catheterization: clinical manifestations, pathogens ≤2, and ≥105 CFU/mL, regardless of leukocyte count. The clinical manifestations of upper urinary tract infection include renal pain and fever, while the clinical manifestations of lower urinary tract infection include frequent micturition, urgency, and pain | (1) Incomplete clinical data (2) Only one of urine culture or HTS was performed |
| PDAP | Peritonitis can be diagnosed in peritoneal dialysis patients with more than 2 of the following 3 items: (1) Abdominal pain, peritoneal exudate turbidity, with or without fever (2) Leukocyte count in penetrant >10 × 107/L, neutrophil ratio >50% (3) The culture of pathogenic microorganisms in penetrant was positive | (1) Patients with other types of abdominal or pelvic infection (2) Patients with incomplete clinical data (3) The time of starting peritoneal dialysis was less than 1 month (4) Peritoneal dialysis combined with hemodialysis (5) Only one of the tests of peritoneal dialysis effluent culture or HTS was performed |
| CRBI | (1) The patients on in-center hemodialysis using catheters displayed fever, chills, rigors, hypotension before or during the hemodialysis session (2) New unexplained malaise, with concurrent exclusion of catheter–unrelated infectious foci | (1) Incomplete clinical data (2) Only one of culture or HTS was performed |
| Lung infection | (1) Recent cough, expectoration, or aggravation of original respiratory disease symptoms, with or without purulent sputum, chest pain, dyspnea, and hemoptysis (2) Fever (3) Signs of pulmonary consolidation and/or wet rales (4) WBC of peripheral blood leukocytes >10 × 109/L or <4 × 109/L, with or without nuclear left shift (5) Chest imaging examination showed new patchy infiltration, leaf or segment consolidation, ground glass shadow or interstitial changes, with or without pleural effusion. Meet one of item (5) and other items, except pulmonary tuberculosis, pulmonary tumor, non-infectious pulmonary interstitial disease, pulmonary edema, atelectasis, pulmonary embolism, pulmonary eosinophilic infiltration and pulmonary vasculitis | (1) Incomplete clinical data (2) Only one of culture or HTS was performed |
| Category | HTS | Culture Method | p | ||||
|---|---|---|---|---|---|---|---|
| Positive | Negative | Detection Rate | Positive | Negative | Detection Rate | ||
| UTI | 95 | 17 | 84.8% | 40 | 72 | 35.7% | <0.001 |
| PDAP | 48 | 19 | 71.6% | 16 | 51 | 23.9% | <0.001 |
| CRBI | 12 | 3 | 75.0% | 7 | 8 | 46.7% | <0.001 |
| Lung infection | 45 | 8 | 84.9% | 3 | 50 | 5.7% | <0.001 |
| Diseases | Bacterial | Fungi | Atypical Pathogen | Parasite | |||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Pathogens | Positive Rate of HTS | Positive Rate of Culture Method | p | Positive Rate of HTS | Positive Rate of Culture Method | p | Positive Rate of HTS | Positive Rate of Culture Method | p | Positive Rate of HTS | Positive Rate of Culture Method | p | |
| UTI | 97.78% | 36.67% | <0.001 | 97.14% | 25.71% | <0.001 | 100% | 0 | - | - | - | - | |
| PDAP | 93.02% | 30.23% | <0.001 | 100% | 23.10% | <0.001 | 100% | 0 | - | - | - | - | |
| CRBI | 100% | 58.30% | <0.001 | 100% | 0 | - | - | - | - | - | - | - | |
| Lung infection | 100% | 7.32% | <0.001 | 100% | 0 | - | 100% | 0 | - | 100% | 0 | - | |
| Detection Rates | Prior Use of Antibiotics | No Prior Use of Antibiotics | p | |||
|---|---|---|---|---|---|---|
| Diseases | HTS | Culture-Based Method | HTS | Culture-Based Method | ||
| UTI | 81.6% | 27.8% | 91.7% | 39.5% | 0.228 *; 0.165 #; 0.000 △; 0.026 ☆ | |
| PDAP | 68.1% | 14.9% | 80.0% | 45.0% | 0.056 *; 0.322 #; 0.000 △; 0.043 ☆ | |
| CRBI | 72.7% | 36.4% | 100.0% | 50.0% | 0.634 *; 0.243 #; 0.000 △; 0.048 ☆ | |
| Lung infection | 83.3% | 4.2% | 100.0% | 20.0% | 0.145 *; 0.321 #; 0.000 △; 0.048 ☆ | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Hu, X.; Yang, L.; Chen, C.; Cheng, H.; Hu, H.; Liang, W.; Tong, Y.; Wang, M.; Wang, H. Application of High-Throughput Sequencing Technology in the Pathogen Identification of Diverse Infectious Diseases in Nephrology Departments. Diagnostics 2022, 12, 2128. https://doi.org/10.3390/diagnostics12092128
Wang Y, Hu X, Yang L, Chen C, Cheng H, Hu H, Liang W, Tong Y, Wang M, Wang H. Application of High-Throughput Sequencing Technology in the Pathogen Identification of Diverse Infectious Diseases in Nephrology Departments. Diagnostics. 2022; 12(9):2128. https://doi.org/10.3390/diagnostics12092128
Chicago/Turabian StyleWang, Yujuan, Xiaoyi Hu, Lianhua Yang, Cheng Chen, Hui Cheng, Haiyun Hu, Wei Liang, Yongqing Tong, Ming Wang, and Huiming Wang. 2022. "Application of High-Throughput Sequencing Technology in the Pathogen Identification of Diverse Infectious Diseases in Nephrology Departments" Diagnostics 12, no. 9: 2128. https://doi.org/10.3390/diagnostics12092128
APA StyleWang, Y., Hu, X., Yang, L., Chen, C., Cheng, H., Hu, H., Liang, W., Tong, Y., Wang, M., & Wang, H. (2022). Application of High-Throughput Sequencing Technology in the Pathogen Identification of Diverse Infectious Diseases in Nephrology Departments. Diagnostics, 12(9), 2128. https://doi.org/10.3390/diagnostics12092128






