Bioinformatics Identification of TUBB as Potential Prognostic Biomarker for Worse Prognosis in ERα-Positive and Better Prognosis in ERα-Negative Breast Cancer
Abstract
1. Introduction
2. Materials and Methods
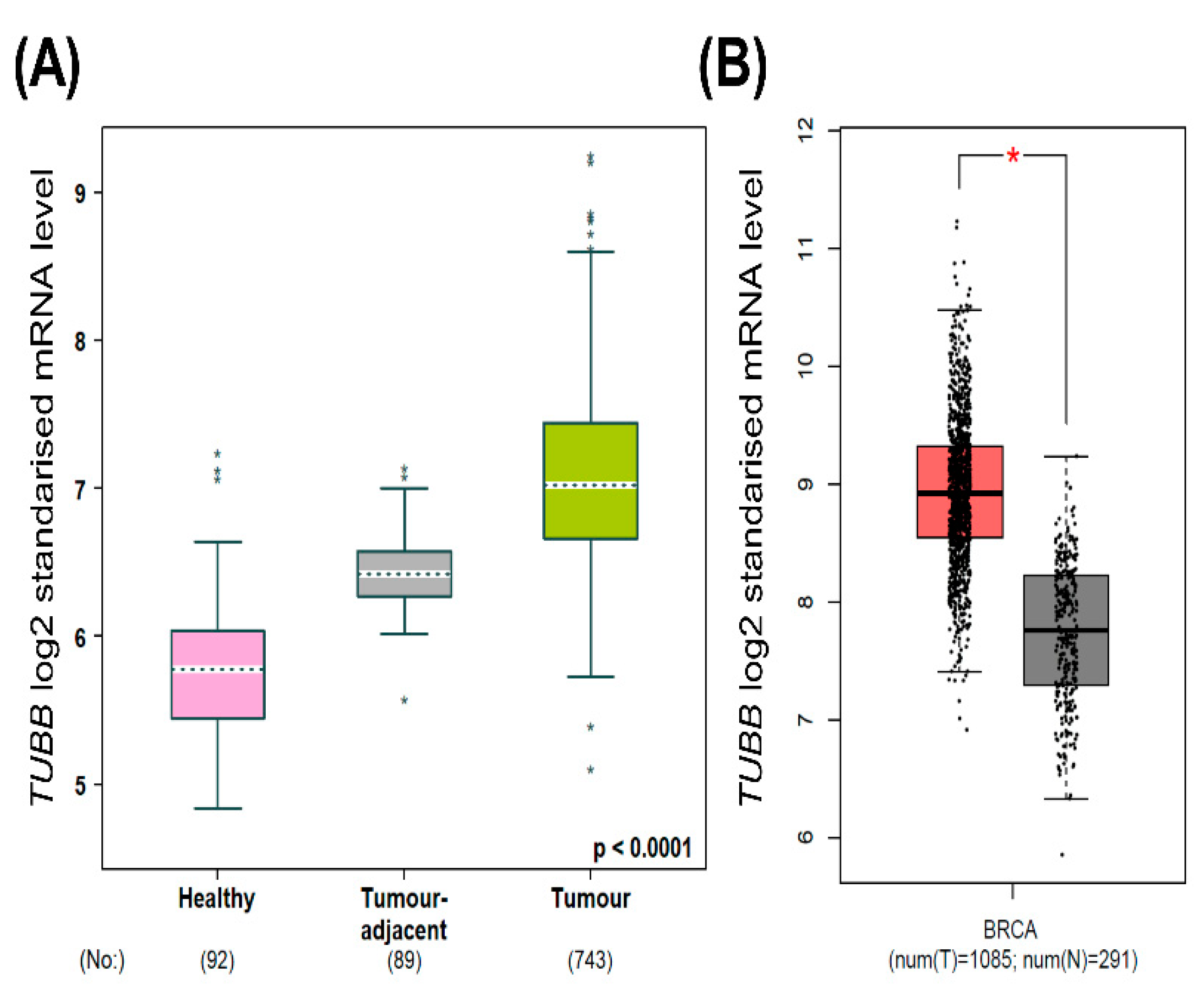
2.1. TUBB mRNA Expression in Normal Breast Tissue versus Breast Cancer Tissue
2.2. Kaplan–Meier Overall Survival Analysis
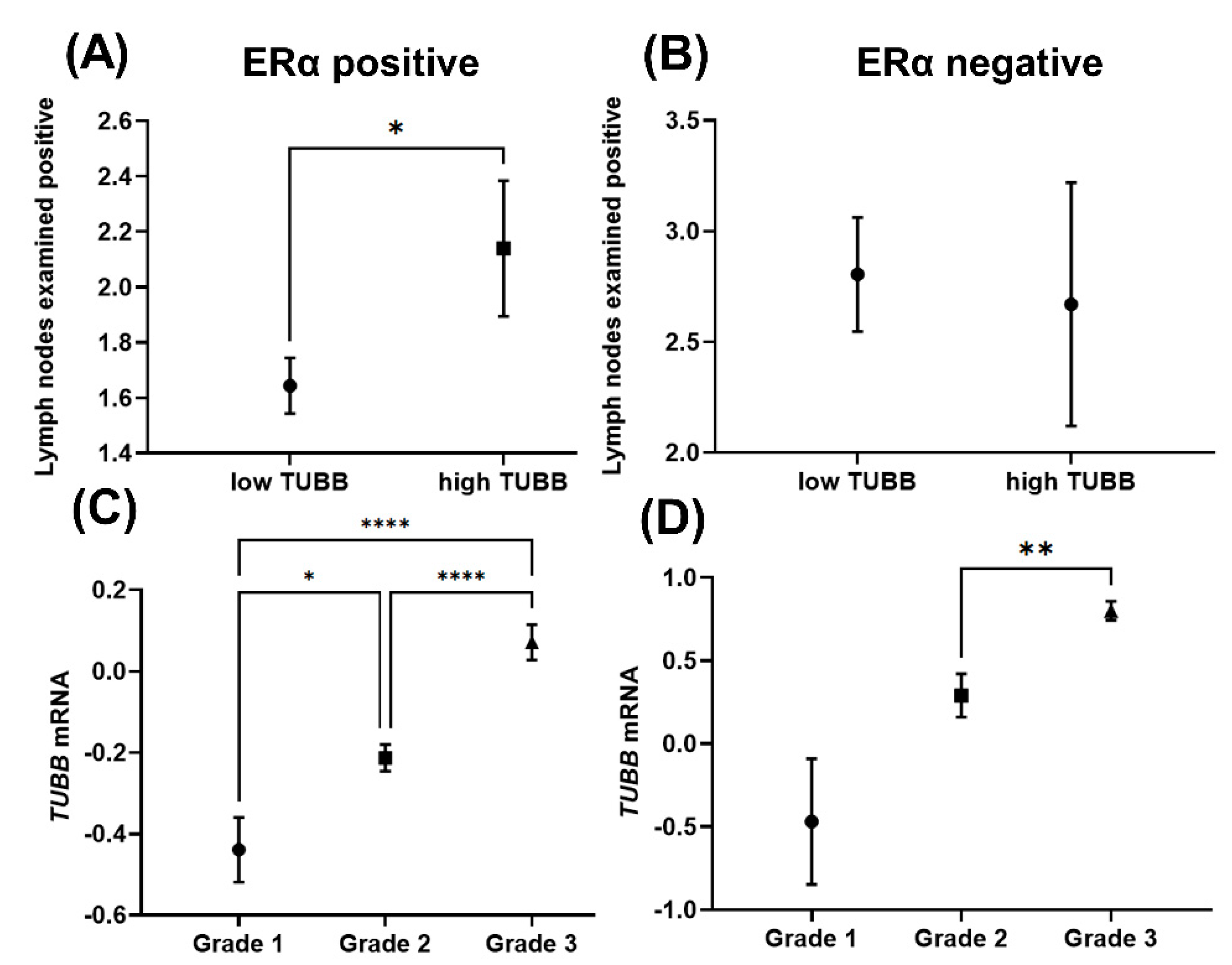
2.3. TUBB Correlation with Neoplasm Histologic Grades and Lymph Nodes
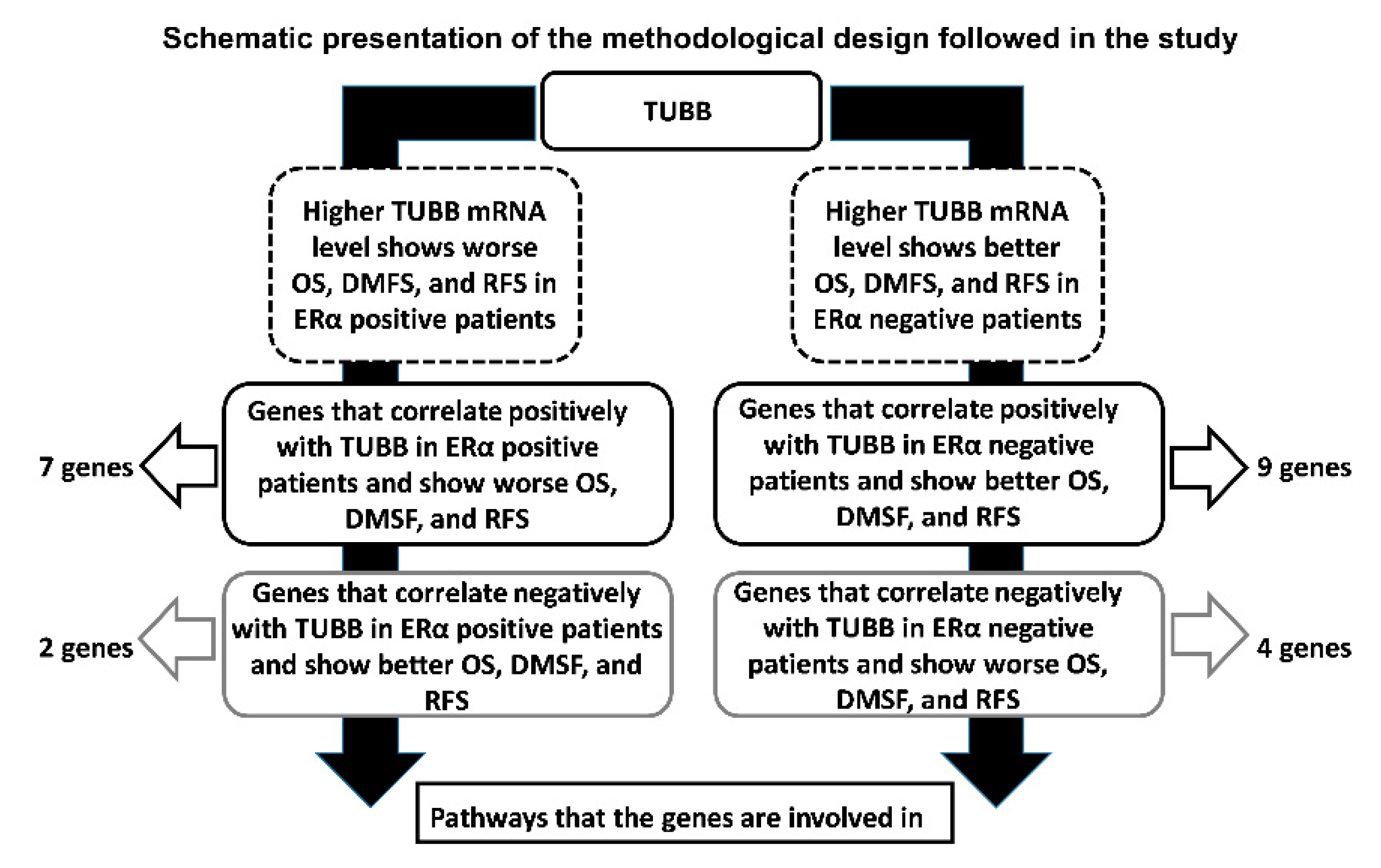
2.4. Identification of Genes That Correlate with TUBB in ERα-Positive and ERα-Negative Breast Cancer and Show Similar KM Plots
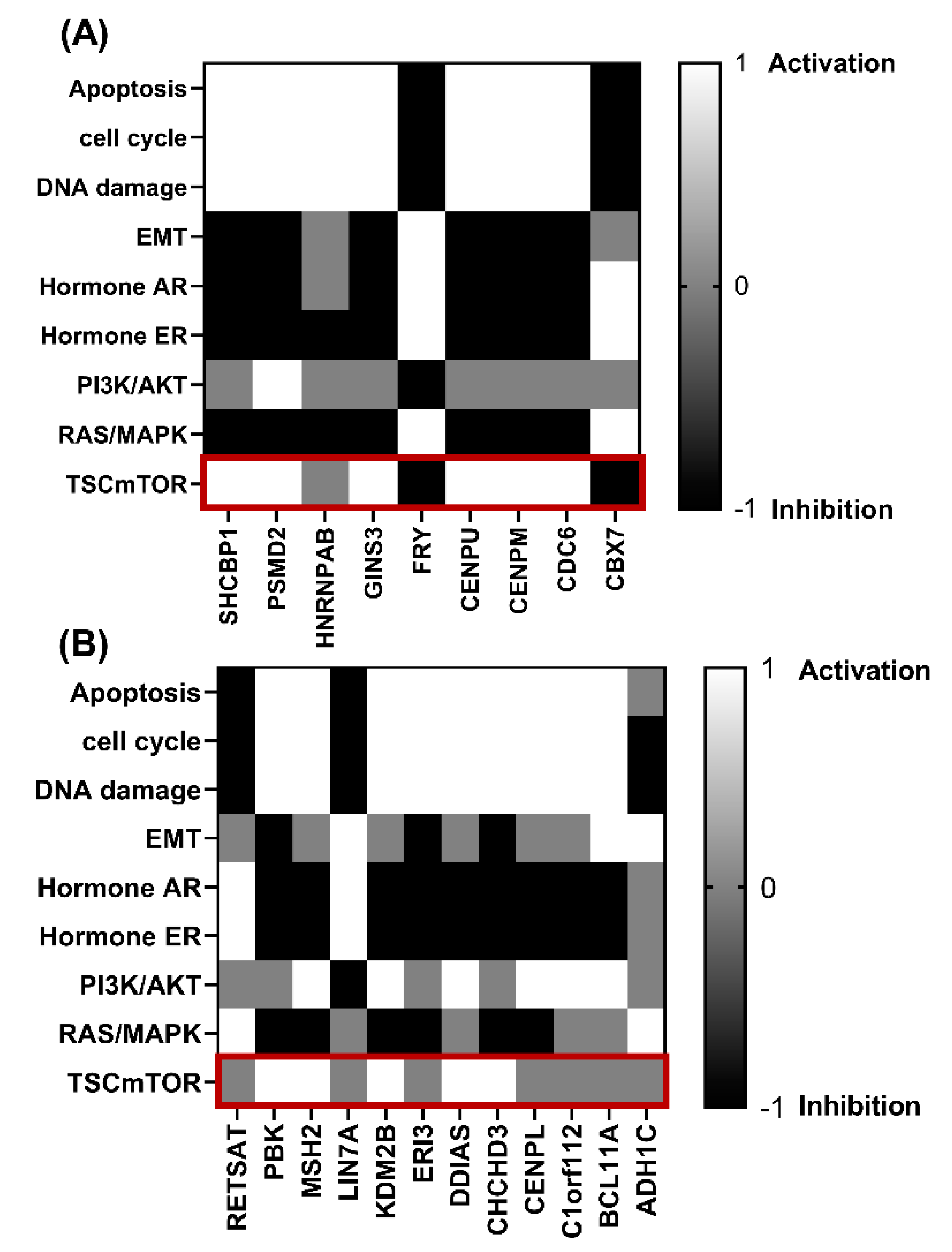
2.5. Association of Genes That Correlate Positively and Negatively with TUBB in ERα-Positive and ERα-Negative Breast Cancer and Show Similar KM Plots with Signaling Pathways
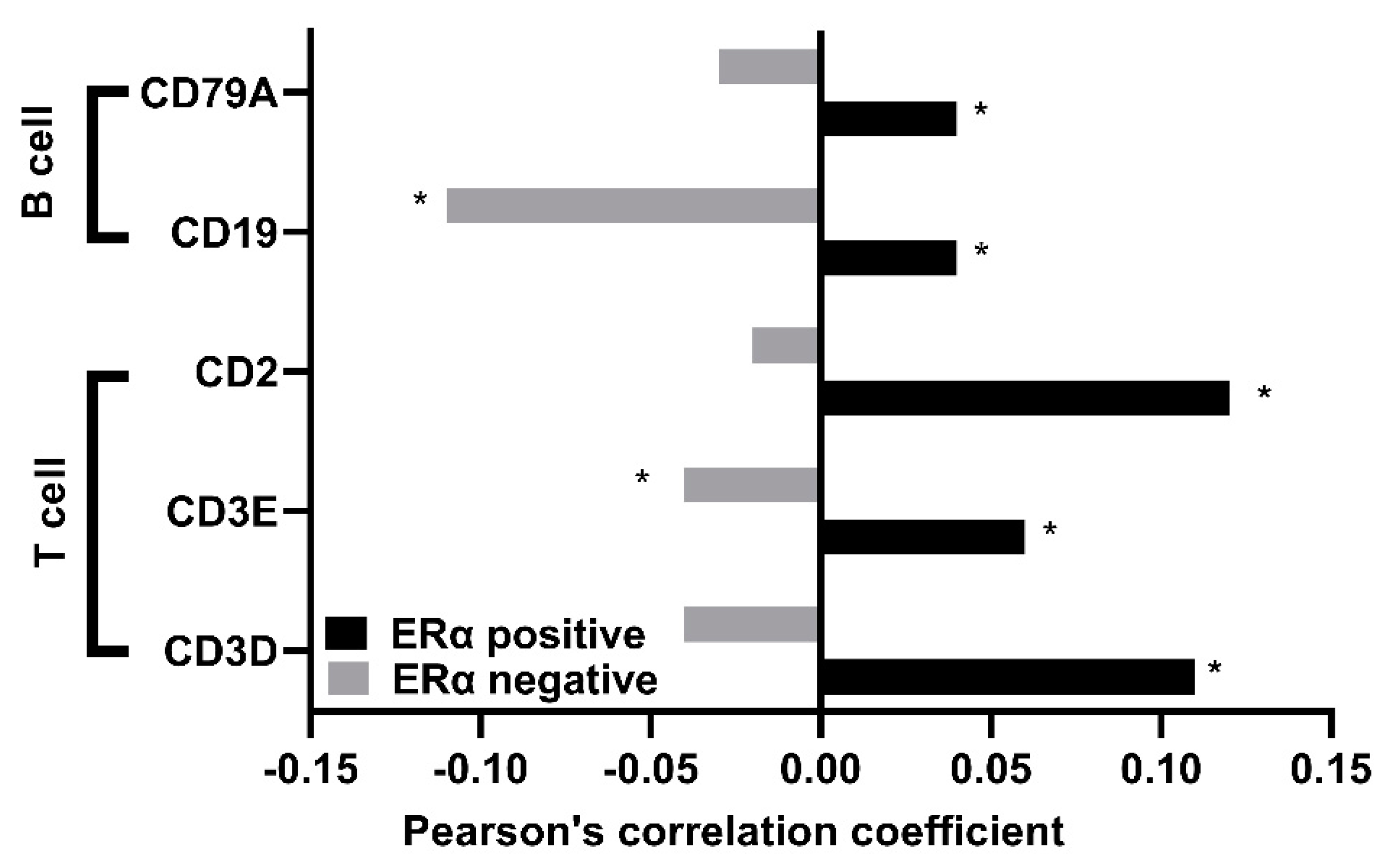
2.6. Immune Cells Infiltration
2.7. Identification of Repurposed Drugs
2.8. Statistical Analysis
3. Results
3.1. Higher TUBB mRNA in Breast Cancer Patients Compared to Normal Breast Tissue
3.2. Higher TUBB mRNA Expression Correlates with Poor Prognosis in ERα-Positive Patients and Better Prognosis in ERα-Negative Patients
3.3. TUBB Correlates Differentially with Lymph Nodes and Neoplasmic Histologic Grades in ERα-Positive and ERα-Negative Breast Cancer Patients
3.4. Identification of the Genes That Positively and Negatively Correlate with TUBB in ERα-Positive and ERα-Negative Breast Cancer Patients
3.5. Genes That Correlate with TUBB mRNA Expression in ERα-Positive and ERα-Negative Breast Cancer Patients Are Involved in Different Pathological Pathways
3.6. TUBB Correlates Positively with Several Gene Markers of Immune Cells in ERα-Positive Breast Cancer Patients and Negatively in ERα-Negative Breast Cancer Patients
3.7. Potential Clinical Targeting of TUBB
4. Discussion
5. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Findeisen, P.; Mühlhausen, S.; Dempewolf, S.; Hertzog, J.; Zietlow, A.; Carlomagno, T.; Kollmar, M. Six Subgroups and Extensive Recent Duplications Characterize the Evolution of the Eukaryotic Tubulin Protein Family. Genome Biol. Evol. 2014, 6, 2274–2288. [Google Scholar] [CrossRef] [PubMed]
- Desai, A.; Mitchison, T.J. Microtubule polymerization dynamics. Annu. Rev. Cell Dev. Biol. 1997, 13, 83–117. [Google Scholar] [CrossRef] [PubMed]
- Knossow, M.; Campanacci, V.; Khodja, L.A.; Gigant, B. The Mechanism of Tubulin Assembly into Microtubules: Insights from Structural Studies. Iscience 2020, 23, 101511. [Google Scholar] [CrossRef]
- Isrie, M.; Breuss, M.; Tian, G.; Hansen, A.H.; Cristofoli, F.; Morandell, J.; Kupchinsky, Z.A.; Sifrim, A.; Rodriguez-Rodriguez, C.M.; Dapena, E.P.; et al. Mutations in Either TUBB or MAPRE2 Cause Circumferential Skin Creases Kunze Type. Am. J. Hum. Genet. 2015, 97, 790–800. [Google Scholar] [CrossRef] [PubMed]
- Saussede-aim, J.; Dumontet, C.; Lyon, U. Regulation of tubulin expression: Multiple overlapping mechanisms. Int. J. Med. Med. Sci. 2009, 1, 290–296. [Google Scholar]
- Gasic, I.; Boswell, S.A.; Mitchison, T.J. Tubulin mRNA stability is sensitive to change in microtubule dynamics caused by multiple physiological and toxic cues. PLOS Biol. 2019, 17, e3000225. [Google Scholar] [CrossRef]
- Pechenino, A.S.; Frick, K.M. The effects of acute 17β-estradiol treatment on gene expression in the young female mouse hippocampus. Neurobiol. Learn. Mem. 2009, 91, 315–322. [Google Scholar] [CrossRef][Green Version]
- Yu, X.; Zhang, Y.; Wu, B.; Kurie, J.M.; Pertsemlidis, A. The miR-195 Axis Regulates Chemoresistance through TUBB and Lung Cancer Progression through BIRC5. Mol. Ther. Oncolytics 2019, 14, 288–298. [Google Scholar] [CrossRef]
- Luo, W.; Cao, Z.; Qiu, J.; Liu, Y.; Zheng, L.; Zhang, T. Novel Discoveries Targeting Pathogenic Gut Microbes and New Therapies in Pancreatic Cancer: Does Pathogenic E. coli Infection Cause Pancreatic Cancer Progression Modulated by TUBB/Rho/ROCK Signaling Pathway? A Bioinformatic Analysis. BioMed Res. Int. 2020, 2020, 2340124. [Google Scholar] [CrossRef]
- Nami, B.; Wang, Z. Genetics and Expression Profile of the Tubulin Gene Superfamily in Breast Cancer Subtypes and Its Relation to Taxane Resistance. Cancers 2018, 10, 274. [Google Scholar] [CrossRef]
- Iseri, O.D.; Kars, M.D.; Ufuk, G. Drug resistant MCF-7 cells have altered expression levels of ß-tubulin isotypes and mutations in TUBB gene. Uluslar. Hematol Derg. 2010, 20, 75–83. [Google Scholar]
- Gwo-Shu Lee, M.; Lewis, S.A.; Wilde, C.D.; Cowan, N.J. Evolutionary history of a multigene family: An expressed human β-tubulin gene and three processed pseudogenes. Cell 1983, 33, 477–487. [Google Scholar] [CrossRef]
- Borys, F.; Joachimiak, E.; Krawczyk, H.; Fabczak, H. Intrinsic and Extrinsic Factors Affecting Microtubule Dynamics in Normal and Cancer Cells. Molecules 2020, 25, 3705. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Ma, S.; Ma, Q.; Zhu, H. Silencing LINC00665 inhibits cutaneous melanoma in vitro progression and induces apoptosis via the miR-339-3p/TUBB. J. Clin. Lab. Anal. 2022, 1, e24630. [Google Scholar] [CrossRef] [PubMed]
- Barrón-Gallardo, C.; Garcia-Chagollán, M.; Morán-Mendoza, A.; Delgadillo-Cristerna, R.; Martínez-Silva, M.; Aguilar-Lemarroy, A.; Jave-Suárez, L. Transcriptomic Analysis of Breast Cancer Patients Sensitive and Resistant to Chemotherapy: Looking for Overall Survival and Drug Resistance Biomarkers. Technol. Cancer Res. Treat. 2022, 21, 1–14. [Google Scholar] [CrossRef]
- Trisciuoglio, D.; Degrassi, F. The Tubulin Code and Tubulin-Modifying Enzymes in Autophagy and Cancer. Cancers 2021, 14, 6. [Google Scholar] [CrossRef]
- Yin, F.; Bhattacharya, R. Human mutations that confer paclitaxel resistance. Mol. Cancer Ther. 2010, 9, 327–335. [Google Scholar] [CrossRef]
- Jiang, W.; Cai, G.; Hu, P.; Wang, Y. Personalized medicine of non-gene-specific chemotherapies for non-small cell lung cancer. Acta Pharm. Sin. B 2021, 11, 3406–3416. [Google Scholar] [CrossRef]
- Bakker, E.; Fujii, M.; Krstic-demonacos, M.; Demonacos, C.; Alhammad, R. Bioinformatic analysis of Protein Disulfide Isomerase A1 ( PDIA1 )-associated pathways towards developing stratified breast cancer therapies. Int. J. Oncol. 2022, 60, 16. [Google Scholar] [CrossRef]
- Ma, Y.; Dai, H.; Kong, X.; Wang, L. Impact of Thawing on Reference Gene Expression Stability in Renal Cell Carcinoma Samples. Diagn. Mol. Pathol. 2012, 21, 157–163. [Google Scholar] [CrossRef]
- Yang, J.; Yang, J.; Gao, Y.; Zhao, L.; Liu, L.; Qin, Y.; Wang, X.; Song, T.; Huang, C. Identification of Potential Serum Proteomic Biomarkers for Clear Cell Renal Cell Carcinoma. PLoS ONE 2014, 9, e111364. [Google Scholar] [CrossRef]
- Tang, Z.; Li, C.; Kang, B.; Gao, G.; Li, C.; Zhang, Z. GEPIA: A web server for cancer and normal gene expression profiling and interactive analyses. Nucleic Acids Res. 2017, 45, W98–W102. [Google Scholar] [CrossRef] [PubMed]
- Jézéquel, P.; Gouraud, W.; Ben Azzouz, F.; Guérin-Charbonnel, C.; Juin, P.P.; Lasla, H. Bc-GenExMiner 4.5: New mining module computes breast cancer differential gene expression analyses. Database 2021, 7, baab007. [Google Scholar] [CrossRef] [PubMed]
- Győrffy, B. Survival analysis across the entire transcriptome identifies biomarkers with the highest prognostic power in breast cancer. Comput. Struct. Biotechnol. J. 2021, 19, 4101–4109. [Google Scholar] [CrossRef] [PubMed]
- Li, Q.; Birkbak, N.J.; Gyorffy, B.; Szallasi, Z.; Eklund, A.C. Jetset: Selecting the optimal microarray probe set to represent a gene. BMC Bioinform. 2011, 12, 474. [Google Scholar] [CrossRef]
- Cerami, E.; Gao, J.; Dogrusoz, U.; Gross, B.E.; Sumer, S.O.; Aksoy, B.A.; Schultz, N. The cBio Cancer Genomics Portal: An Open Platform for Exploring Multidimensional Cancer Genomics Data. Cancer Discov. 2017, 32, 736–740. [Google Scholar] [CrossRef]
- Cereda, M.; Gambardella, G.; Benedetti, L.; Iannelli, F.; Patel, D.; Basso, G.; Guerra, R.F.; Mourikis, T.P.; Puccio, I.; Sinha, S.; et al. Patients with genetically heterogeneous synchronous colorectal cancer carry rare damaging germline mutations in immune-related genes. Nat. Commun. 2016, 7, 12072. [Google Scholar] [CrossRef]
- Liu, C.-J.; Hu, F.-F.; Xia, M.-X.; Han, L.; Zhang, Q.; Guo, A.-Y. GSCALite: A web server for gene set cancer analysis. Bioinformatics 2018, 34, 3771–3772. [Google Scholar] [CrossRef]
- Tang, Z.; Kang, B.; Li, C.; Chen, T.; Zhang, Z. GEPIA2: An enhanced web server for large-scale expression profiling and interactive analysis. Nucleic Acids Res. 2019, 47, W556–W560. [Google Scholar] [CrossRef]
- Amelio, I.; Gostev, M.; A Knight, R.; E Willis, A.; Melino, G.; Antonov, A.V. DRUGSURV: A resource for repositioning of approved and experimental drugs in oncology based on patient survival information. Cell Death Dis. 2014, 5, e1051. [Google Scholar] [CrossRef]
- Hsieh, A.C.; Liu, Y.; Edlind, M.P.; Ingolia, N.T.; Janes, M.R.; Sher, A.; Shi, E.Y.; Stumpf, C.R.; Christensen, C.; Bonham, M.J.; et al. The translational landscape of mTOR signalling steers cancer initiation and metastasis. Nature 2012, 485, 55–61. [Google Scholar] [CrossRef] [PubMed]
- Hare, S.H.; Harvey, A.J. mTOR function and therapeutic targeting in breast cancer. Am. J. Cancer Res. 2017, 7, 383–404. [Google Scholar] [PubMed]
- Zou, Z.; Tao, T.; Li, H.; Zhu, X. mTOR signaling pathway and mTOR inhibitors in cancer: Progress and challenges. Cell Biosci. 2020, 10, 31. [Google Scholar] [CrossRef]
- Iwata, T.; Ramírez-Komo, J.A.; Park, H.; Iritani, B.M. Control of B lymphocyte development and functions by the mTOR signaling pathways. Cytokine Growth Factor Rev. 2017, 35, 47–62. [Google Scholar] [CrossRef]
- Bian, T.; Zheng, M.; Jiang, D.; Liu, J.; Sun, H.; Li, X.; Liu, L.; Zhang, J.; Liu, Y. Prognostic biomarker TUBA1C is correlated to immune cell infiltration in the tumor microenvironment of lung adenocarcinoma. Cancer Cell Int. 2021, 21, 144. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhu, H.; Chen, B.; He, X.; Shen, Y.; Zhang, X.; Chen, W.; Liu, X.; Xu, Y.; Xu, X. Tubulin Alpha 1b Is Associated with the Immune Cell Infiltration and the Response of HCC Patients to Immunotherapy. Diagnostics 2022, 12, 858. [Google Scholar] [CrossRef]
- Jiang, L.; Zhu, X.; Yang, H.; Chen, T.; Lv, K. Bioinformatics Analysis Discovers Microtubular Tubulin Beta 6 Class V (TUBB6) as a Potential Therapeutic Target in Glioblastoma. Front. Genet. 2020, 11, 566579. [Google Scholar] [CrossRef]

| Gene Name | Correlation Coefficient 1 | p-Value | Number of Patients |
|---|---|---|---|
| SHCBP1 | 0.4589 | <0.0001 | 3685 |
| GINS3 | 0.4468 | <0.0001 | 3685 |
| CDC6 | 0.4441 | <0.0001 | 3685 |
| CENPM | 0.4228 | <0.0001 | 3685 |
| PSMD2 | 0.4178 | <0.0001 | 3685 |
| CENPU | 0.4102 | <0.0001 | 3685 |
| HNRNPAB | 0.4036 | <0.0001 | 3685 |
| CBX7 | −0.4316 | <0.0001 | 3685 |
| FRY | −0.412 | <0.0001 | 3685 |
| Gene Name | Correlation Coefficient 1 | p-Value | Number of Patients |
|---|---|---|---|
| MSH2 | 0.5704 | <0.0001 | 510 |
| CENPL | 0.5002 | <0.0001 | 510 |
| PBK | 0.4955 | <0.0001 | 510 |
| ERI3 | 0.4931 | <0.0001 | 510 |
| DDIAS | 0.4771 | <0.0001 | 510 |
| KDM2B | 0.4735 | <0.0001 | 510 |
| C1orf112 | 0.441 | <0.0001 | 510 |
| CHCHD3 | 0.4275 | <0.0001 | 510 |
| BCL11A | 0.4164 | <0.0001 | 510 |
| LOC100505942 | −0.432 | <0.0001 | 241 |
| RETSAT | −0.4088 | <0.0001 | 510 |
| LIN7A | −0.4068 | <0.0001 | 510 |
| ADH1C | −0.4006 | <0.0001 | 510 |
| TUBB-Targeting Drugs 1 | Chemical Formula | CID | Clinical Stage |
|---|---|---|---|
| Vincristine | C46H56N4O10 | 5978 | metastatic breast cancer (phase II) |
| Vinorelbine | C45H54N4O8 | 5311497 | approved for breast cancer |
| Vinblastine | C46H58N4O9 | 13342 | metastatic breast cancer (phase II) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alhammad, R. Bioinformatics Identification of TUBB as Potential Prognostic Biomarker for Worse Prognosis in ERα-Positive and Better Prognosis in ERα-Negative Breast Cancer. Diagnostics 2022, 12, 2067. https://doi.org/10.3390/diagnostics12092067
Alhammad R. Bioinformatics Identification of TUBB as Potential Prognostic Biomarker for Worse Prognosis in ERα-Positive and Better Prognosis in ERα-Negative Breast Cancer. Diagnostics. 2022; 12(9):2067. https://doi.org/10.3390/diagnostics12092067
Chicago/Turabian StyleAlhammad, Rashed. 2022. "Bioinformatics Identification of TUBB as Potential Prognostic Biomarker for Worse Prognosis in ERα-Positive and Better Prognosis in ERα-Negative Breast Cancer" Diagnostics 12, no. 9: 2067. https://doi.org/10.3390/diagnostics12092067
APA StyleAlhammad, R. (2022). Bioinformatics Identification of TUBB as Potential Prognostic Biomarker for Worse Prognosis in ERα-Positive and Better Prognosis in ERα-Negative Breast Cancer. Diagnostics, 12(9), 2067. https://doi.org/10.3390/diagnostics12092067





