Pseudopterygium: An Algorithm Approach Based on the Current Evidence
Abstract
1. Introduction
2. Materials and Methods
- Pseudopterygium: 67 articles.
- Pseudopterygia: 9 articles.
- ((pseudopterygium) OR (pseudopterygia)) AND (pterygium): 39 articles.
- ((pseudopterygium) OR (pseudopterygia)) AND (corneal degeneration): 10 articles.
- ((pseudopterygium) OR (pseudopterygia)) AND (trauma): 18 articles.
- ((pseudopterygium) OR (pseudopterygia)) AND (burn): 12 articles.
- ((pseudopterygium) OR (pseudopterygia)) AND (limbus): 20 articles.
- ((pseudopterygium) OR (pseudopterygia)) AND (chronic inflammation): 2 articles.
3. Definition of Pseudopterygium
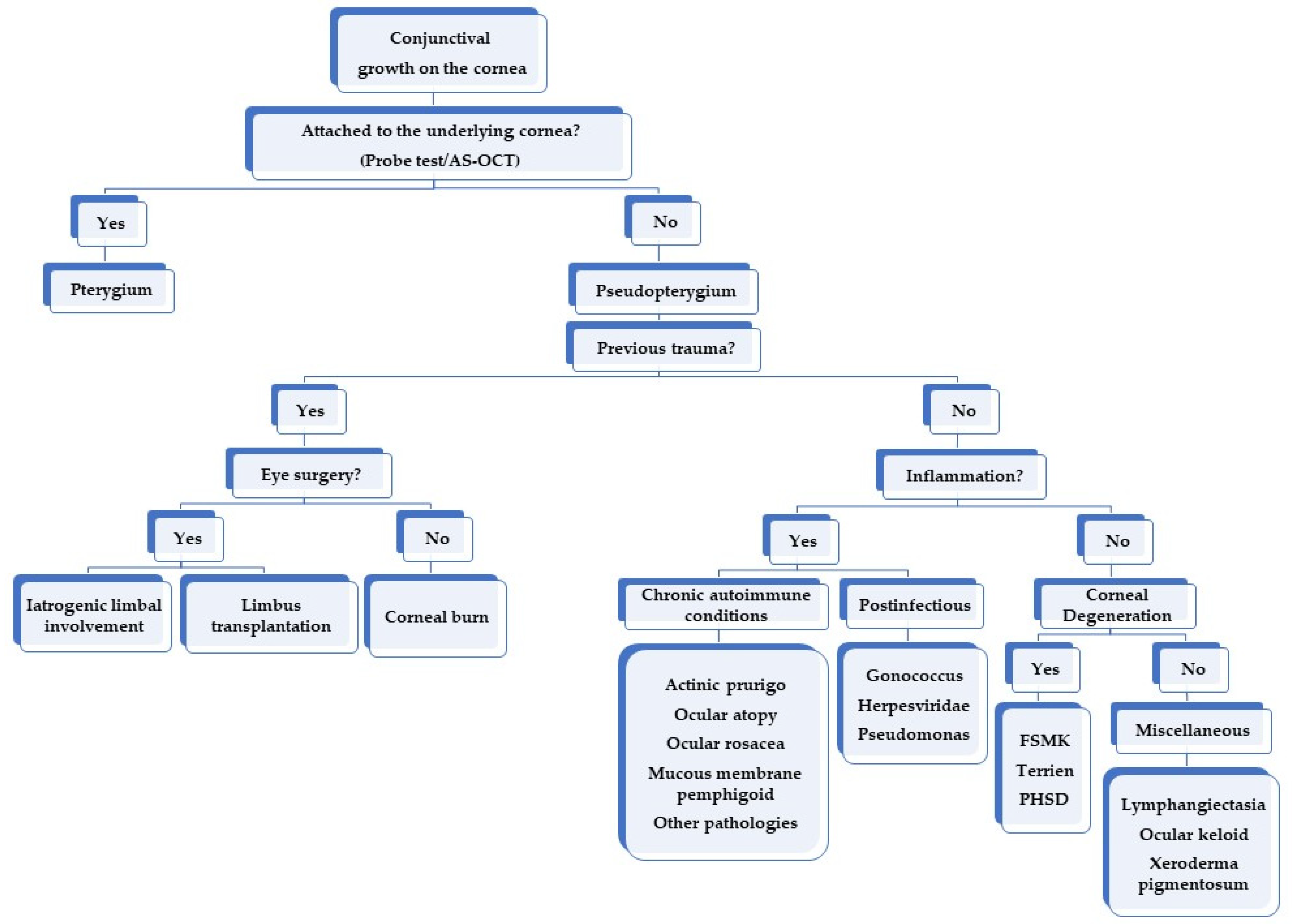
4. Differential Diagnosis
5. Causes of Pseudopterygium
5.1. Previous Eye Trauma
5.1.1. Iatrogenic Limbal Involvement
5.1.2. Ocular Burns
5.2. Inflammation-Related Causes
5.2.1. Chronic Autoimmune Conditions
5.2.2. Postinfectious
5.3. Corneal Degenerations
5.4. Other Causes
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Deng, S.X.; Kruse, F.; Gomes, J.A.P.; Chan, C.C.; Daya, S.; Dana, R.; Figueiredo, F.C.; Kinoshita, S.; Rama, P.; Sangwan, V.; et al. Global Consensus on the Management of Limbal Stem Cell Deficiency. Cornea 2020, 39, 1291–1302. [Google Scholar] [CrossRef]
- Le, Q.; Xu, J.; Deng, S.X. The diagnosis of limbal stem cell deficiency. Ocul. Surf. 2018, 16, 58–69. [Google Scholar] [CrossRef]
- Sejpal, K.; Bakhtiari, P.; Deng, S.X. Presentation, diagnosis and management of limbal stem cell deficiency. Middle East Afr. J. Ophthalmol. 2013, 20, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Sehu, K.W.; Lee, W.R. Ophthalmic Pathology: An Illustrated Guide for Clinicians; John Wiley & Sons: Hoboken, NJ, USA, 2012. [Google Scholar]
- Deng, S.X.; Borderie, V.; Chan, C.C.; Dana, R.; Figueiredo, F.C.; Gomes, J.A.P.; Pellegrini, G.; Shimmura, S.; Kruse, F.E. Global consensus on definition, classification, diagnosis, and staging of limbal stem cell deficiency. Cornea 2019, 38, 364–375. [Google Scholar] [CrossRef]
- Goldman, K.N.; Kaufman, H.E. Atypical Pterygium: A Clinical Feature of Terrien’s Marginal Degeneration. Arch. Ophthalmol. 1978, 96, 1027–1029. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.; Xia, X.; Wang, F.; Ye, S.; Zhou, S. Determination of surgical strategies for burn-induced conjunctivalized corneas using optical coherence tomography. Cornea 2015, 34, 1233–1239. [Google Scholar] [CrossRef]
- Soliman, W.; Mohamed, T.A. Spectral domain anterior segment optical coherence tomography assessment of pterygium and pinguecula. Acta Ophthalmol. 2012, 90, 461–465. [Google Scholar] [CrossRef]
- Prabhakar, S.K. Safety profile and complications of autologous limbal conjunctival transplantation for primary pterygium. Saudi J. Ophthalmol. 2014, 28, 262–267. [Google Scholar] [CrossRef]
- Jacob, S.; Dhawan, P.; Tsatsos, M.; Agarwal, A.; Narasimhan, S.; Kumar, A. Fibrin Glue-Assisted Closure of Macroperforation in Predescemetic Deep Anterior Lamellar Keratoplasty with a Donor Obtained from Small Incision Lenticule Extraction. Cornea 2019, 38, 775–779. [Google Scholar] [CrossRef] [PubMed]
- Akova, Y.A.; Jabbur, N.S.; Neumann, R.; Foster, C.S. Atypical Ocular Atopy. Ophthalmology 1993, 100, 1367–1371. [Google Scholar] [CrossRef]
- Mondino, B.J. Cicatricial Pemphigoid and Erythema Multiforme. Ophthalmology 1990, 97, 939–952. [Google Scholar] [CrossRef]
- Tong, L.; Hodgkins, P.R.; Denyer, J.; Brosnahan, D.; Harper, J.; Russell-Eggitt, I.; Taylor, D.S.I.; Atherton, D. The eye in epidermolysis bullosa. Br. J. Ophthalmol. 1999, 83, 323–326. [Google Scholar] [CrossRef] [PubMed]
- Bierly, J.R.; Dunn, J.P.; Dawson, C.R.; Ostler, H.B.; Wong, I.G. Fuchs’ superficial marginal keratitis. Am. J. Ophthalmol. 1992, 113, 541–545. [Google Scholar] [CrossRef]
- Brilakis, H.S.; Nordlund, M.L.; Holland, E.J. Recurrence of Fuchs marginal keratitis within a lamellar graft. Cornea 2004, 23, 639–640. [Google Scholar] [CrossRef]
- Keenan, J.D.; Mandel, M.R.; Margolis, T.P. Peripheral ulcerative keratitis associated with vasculitis manifesting asymmetrically as fuchs superficial marginal keratitis and terrien marginal degeneration. Cornea 2011, 30, 825–827. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Sarnicola, E.; Kurji, K.H.; Genereux, B.M.; Holland, E.J. Three hundred sixty-degree Fuchs superficial marginal keratitis managed with annular lamellar keratoplasty. Cornea 2018, 37, 260–262. [Google Scholar] [CrossRef] [PubMed]
- Qian, C.X.Y.; Bahar, I.; Levinger, E.; Rootman, D. Combined use of superficial keratectomy and subconjunctival bevacizumab injection for corneal neovascularization. Cornea 2008, 27, 1090–1092. [Google Scholar] [CrossRef]
- Chan, A.T.; Ulate, R.; Goldich, Y.; Rootman, D.S.; Chan, C.C. Terrien marginal degeneration: Clinical characteristics and outcomes. Am. J. Ophthalmol. 2015, 160, 867–872.e1. [Google Scholar] [CrossRef]
- Kursiah, M.R. Iatrogenic corneal perforation in terrien marginal degeneration. Med. J. Malays. 2013, 68, 173–174. [Google Scholar]
- Gore, D.M.; Iovieno, A.; Connell, B.J.; Alexander, R.; Meligonis, G.; Dart, J.K. Peripheral hypertrophic subepithelial corneal degeneration: Nomenclature, phenotypes, and long-term outcomes. Ophthalmology 2013, 120, 892–898. [Google Scholar] [CrossRef]
- Rommel, F.; Grisanti, S.; Ranjbar, M. Peripheral hypertrophic subepithelial corneal degeneration. JAMA Ophthalmol. 2017, 135, 936290. [Google Scholar] [CrossRef] [PubMed]
- Järventausta, P.J.; Tervo, T.M.T.; Kivelä, T.; Holopainen, J.M. Peripheral hypertrophic subepithelial corneal degeneration—clinical and histopathological features. Acta Ophthalmol. 2014, 92, 774–782. [Google Scholar] [CrossRef] [PubMed]
- Maust, H.A.; Raber, I.M. Peripheral hypertrophic subepithelial corneal degeneration. Eye Contact Lens 2003, 29, 266–269. [Google Scholar] [CrossRef] [PubMed]
- Raber, I.M.; Eagle, R.C., Jr. Peripheral Hypertrophic Subepithelial Corneal Degeneration. Cornea 2022, 41, 183–191. [Google Scholar] [CrossRef]
- Neame, H. Pseudo-pterygium. Proc. R Soc. Med. 1927, 20, 1793. [Google Scholar] [CrossRef] [PubMed]
- Witsberger, E.M.; Patel, S.V. Alkali Burn over a LASIK Flap. Cornea 2021, 40, 907–909. [Google Scholar] [CrossRef] [PubMed]
- Shi, W.; Wang, T.; Gao, H.; Xie, L. Management of severe ocular burns with symblepharon. Graefe’s Arch. Clin. Exp. Ophthalmol. 2009, 247, 101–106. [Google Scholar] [CrossRef]
- Borrelli, M.; Schröder, C.; Dart, J.K.G.; Collin, J.R.O.; Sieg, P.; Cree, I.A.; Matheson, M.A.; Tiffany, J.M.; Proctor, G.; Van Best, J.; et al. Long-term follow-up after submandibular gland transplantation in severe dry eyes secondary to cicatrizing conjunctivitis. Am. J. Ophthalmol. 2010, 150, 894–904. [Google Scholar] [CrossRef] [PubMed]
- Shimazaki, J.; Shinozaki, N.; Tsubota, K. Transplantation of amniotic membrane and limbal autograft for patients with recurrent pterygium associated with symblepharon. Br. J. Ophthalmol. 1998, 82, 235–240. [Google Scholar] [CrossRef]
- Autrata, R.; Rehurek, J.; Vodičková, K. Phototherapeutic keratectomy in children: 5-Year results. J. Cataract. Refract. Surg. 2004, 30, 1909–1916. [Google Scholar] [CrossRef] [PubMed]
- Gridley, M.J.; Perlman, E.M. A form of variable astigmatism induced by pseudopterygium. Ophthalmic Surg. 1986, 17, 794–795. [Google Scholar] [CrossRef]
- Deshmukh, R.; Ting, D.S.J.; Elsahn, A.; Mohammed, I.; Said, D.G.; Dua, H.S. Real-world experience of using ciclosporin-A 0.1% (Ikervis) in the management of ocular surface inflammatory diseases. Br. J. Ophthalmol. 2022, 106, 1087–1092. [Google Scholar] [CrossRef]
- Wang, M.; Wang, H.; Xiao, G.; Lin, J.; Wang, W.; Li, Y.; Hong, J. Severe Marginal Ulcerative Keratitis Induced by an Insect Foreign Body in the Cornea. Cornea 2020, 39, 1059–1061. [Google Scholar] [CrossRef] [PubMed]
- Dart, J.K. The 2016 Bowman Lecture Conjunctival curses: Scarring conjunctivitis 30 years on. Eye 2017, 31, 301–332. [Google Scholar] [CrossRef] [PubMed]
- Gonçalves dos Santos Martins, T.; Fontes de Azevedo Costa, A.L. A rare ocular complication of neisseria gonorrhoeae. Ir. J. Med. Sci. 2018, 187, 815–816. [Google Scholar] [CrossRef]
- Seitzman, G.D.; Strauss, E.C.; Margolis, T.P. “Steel wool keratopathy”: A clinical sign of chronic inflammation. Cornea 2006, 25, 742–744. [Google Scholar] [CrossRef] [PubMed]
- Mohammadpour, M.; Sabet, F.A. Long-term outcomes of amniotic membrane transplantation in contact lens-induced pseudomonas keratitis with impending corneal perforation. J. Ophthalmic Vis. Res. 2016, 11, 37–41. [Google Scholar] [CrossRef] [PubMed]
- Donnenfeld, E.D.; Perry, H.D.; Wallerstein, A.; Caronia, R.M.; Kanellopoulos, A.J.; Sforza, P.D.; D’Aversa, G. Subconjunctival mitomycin C for the treatment of ocular cicatricial pemphigoid. Ophthalmology 1999, 106, 72–79. [Google Scholar] [CrossRef]
- Ahmed, M.; Zein, G.; Khawaja, F.; Foster, C.S. Ocular cicatricial pemphigoid: Pathogenesis, diagnosis and treatment. Prog. Retin. Eye Res. 2004, 23, 579–592. [Google Scholar] [CrossRef]
- Pesudovs, K.; Phillips, A.J. The use of a rigid gas permeable lens in ocular cicatrical pemphigoid. Clin. Exp. Optom. 1992, 75, 188–191. [Google Scholar] [CrossRef]
- Mondino, B.J.; Brown, S.I. Ocular Cicatricial Pemphigoid. Ophthalmology 1981, 88, 95–100. [Google Scholar] [CrossRef]
- Heiligenhaus, A.; Shore, J.W.; Rubin, P.A.D.; Foster, C.S. Long-term Results of Mucous Membrane Grafting in Ocular Cicatricial Pemphigoid: Implications for Patient Selection and Surgical Considerations. Ophthalmology 1993, 100, 1283–1288. [Google Scholar] [CrossRef]
- Norn, M.S.; Kristensen, E.B. Benign mucous membrane pemphigoid. II. Cytology. Acta Ophthalmol. 1974, 52, 282–290. [Google Scholar] [CrossRef] [PubMed]
- Fine, J.D.; Mellerio, J.E. Extracutaneous manifestations and complications of inherited epidermolysis bullosa. Part I. Epithelial associated tissues. J. Am. Acad. Dermatol. 2009, 61, 367–384. [Google Scholar] [CrossRef]
- Dhingra, D.; Malhotra, C.; Jain, A.K. Ocular Rosacea—A Review. US Ophthalmic Rev. 2017, 10, 113. [Google Scholar] [CrossRef][Green Version]
- Foster, C.S.; Calonge, M. Atopic Keratoconjunctivitis. Ophthalmology 1990, 97, 992–1000. [Google Scholar] [CrossRef]
- Hojyo-Tomoka, M.T.; Vega-Memije, M.E.; Cortes-Franco, R.; Domínguez-Soto, L. Diagnosis and treatment of actinic prurigo. Dermatol. Ther. 2003, 16, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Ross, G.; Foley, P.; Baker, C. Actinic prurigo. Photodermatol. Photoimmunol. Photomed. 2008, 24, 272–275. [Google Scholar] [CrossRef] [PubMed]
- Ng, J.C.H.; Foley, P.A.; Crouch, R.B.; Baker, C.S. A case of severe actinic prurigo successfully treated with thalidomide. Australas. J. Dermatol. 2001, 42, 192–195. [Google Scholar] [CrossRef] [PubMed]
- McCoombes, J.A.; Hirst, L.W.; Green, W.R. Use of topical cyclosporin for conjunctival manifestations of actinic prurigo. Am. J. Ophthalmol. 2000, 130, 830–831. [Google Scholar] [CrossRef]
- Arrese, J.E.; Dominguez-Soto, L.; Hojyo-Tomoka, M.T.; Vega-Memije, E.; Cortés-Franco, R.; Guevara, E.; Piérard, G.E. Effectors of inflammation in actinic prurigo. J. Am. Acad. Dermatol. 2001, 44, 957–961. [Google Scholar] [CrossRef]
- Kitamura, G.; Tingey, C.; Golkar, L. Chronic actinic prurigo presenting in an adopted child. Pediatr. Dermatol. 2010, 27, 97–98. [Google Scholar] [CrossRef]
- Rodríguez-Ausín, P.; Antolín-Garcia, D.; Ruano del Salado, M.; Hita-Antón, C. Tacrolimus tópico al 0,03% en el tratamiento de la psoriasis ocular. Arch. Soc. Esp. Oftalmol. 2016, 91, 505–507. [Google Scholar] [CrossRef]
- Lekhanont, K.; Patarakittam, T.; Mantachote, K.; Waiyawatjamai, P.; Vongthongsri, A. Progressive keratolysis with pseudopterygium associated with erythema elevatum diutinum. Ophthalmology 2011, 118, 927–933. [Google Scholar] [CrossRef]
- Huang, L.C. Pseudopemphigoid as caused by topical drugs and pemphigus disease. World J. Ophthalmol. 2015, 5, 1. [Google Scholar] [CrossRef]
- Ramappa, M.; Bhalekar, S.; Chaurasia, S.; Mulay, K.; Trivedi, R.H.; Wilson, M.E. Presumed allograft stromal rejection after deep anterior lamellar keratoplasty in a boy presenting with interface fluid syndrome. J. AAPOS 2013, 17, 554–557. [Google Scholar] [CrossRef] [PubMed]
- Gupta, N.; Sachdev, R.; Tandon, R. Ocular surface squamous neoplasia in xeroderma pigmentosum: Clinical spectrum and outcome. Graefe’s Arch. Clin. Exp. Ophthalmol. 2011, 249, 1217–1221. [Google Scholar] [CrossRef] [PubMed]
- Booth, A.J.; Hodgkins, P.R. Pseudopterygium arising in a patient with multiple keloids. J. Pediatr. Ophthalmol. Strabismus 2006, 43, 49–51. [Google Scholar] [CrossRef] [PubMed]
- Dhooge, M.R.P.; Idema, A.J.S. Fibrodysplasia ossificans progressiva and corneal keloid. Cornea 2002, 21, 725–729. [Google Scholar] [CrossRef]
- Pastora, N.; Peralta-Calvo, J.; Yebenes-Gregorio, L.; Abelairas, J.; Hierro-Zarzuelo, A. Conjunctival lymphangiectasia presenting as pediatric pseudopterygium. Eye Contact Lens 2013, 39, 2012–2014. [Google Scholar] [CrossRef]
- Centre, Q.M.; Dua, H.S.; Saini, J.S.; Azuara-Blanco, A.; Gupta, P. Limbal stem cell deficiency: Concept, aetiology, clinical presentation, diagnosis and management. Indian J. Ophthalmol. 2000, 48, 83–92. [Google Scholar]
- Gagnon, M.R.; Dickinson, P.J. Ocular surface injury from a microwave superheated egg resulting in a pseudopterygium. Eye Contact Lens 2005, 31, 109–110. [Google Scholar] [CrossRef]
- Kim, Y.J.; Payal, A.R.; Daly, M.K. Effects of tear gases on the eye. Surv. Ophthalmol. 2016, 61, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Lang, S.J.; Böhringer, D.; Reinhard, T. Surgical management of corneal limbal dermoids: Retrospective study of different techniques and use of Mitomycin C. Eye 2014, 28, 857–862. [Google Scholar] [CrossRef] [PubMed]
- Choi, S.K.; Lee, D.; Kim, J.H.; Oh, S.H. A novel technique: Eccentric lamellar keratolimbal allografting using a femtosecond laser. Cornea 2010, 29, 1062–1065. [Google Scholar] [CrossRef]
- Umfress, A.C.; Mawn, L.A.; Joos, K.M.; Donahue, S.P.; Schmitt, A.D.; Shieh, C. Surgical management of large bilateral epibulbar dermoids with autologous oral mucous membrane transplantation. Am. J. Ophthalmol. Case Rep. 2020, 20, 100982. [Google Scholar] [CrossRef]
- Shields, C.L.; Regillo, A.C.; Mellen, P.L.; Kaliki, S.; Lally, S.E.; Shields, J.A. Giant conjunctival nevus: Clinical features and natural course in 32 cases. JAMA Ophthalmol. 2013, 131, 857–863. [Google Scholar] [CrossRef] [PubMed]
- Kaliki, S.; Mohammad, F.A.; Tahiliani, P.; Sangwan, V.S. Concomitant Simple Limbal Epithelial Transplantation After Surgical Excision of Ocular Surface Squamous Neoplasia. Am. J. Ophthalmol. 2017, 174, 68–75. [Google Scholar] [CrossRef]
- Wong, A.K.; Rao, S.K.; Leung, A.T.; Poon, A.S.; Lam, D.S. Inferior limbal-conjunctival autograft transplantation for recurrent pterygium. Indian J. Ophthalmol. 2000, 48, 21. [Google Scholar] [PubMed]
- Han, S.B.; Hyon, J.Y.; Hwang, J.M.; Wee, W.R. Efficacy and safety of limbal-conjunctival autografting with limbal fixation sutures after pterygium excision. Ophthalmologica 2012, 227, 210–214. [Google Scholar] [CrossRef] [PubMed]
- Gris, O.; Güell, J.L.; Del Campo, Z. Limbal-conjunctival autograft transplantation for the treatment of recurrent pterygium. Ophthalmology 2000, 107, 270–273. [Google Scholar] [CrossRef]
- Tseng, S.C.G.; Di Pascuale, M.A.; Liu, D.T.S.; Ying, Y.G.; Baradaran-Rafii, A. Intraoperative mitomycin C and amniotic membrane transplantation for fornix reconstruction in severe cicatricial ocular surface diseases. Ophthalmology 2005, 112, 896–903. [Google Scholar] [CrossRef] [PubMed]
- Pachigolla, G.; Prasher, P.; Di Pascuale, M.A.; McCulley, J.P.; McHenry, J.G.; Mootha, V.V. Evaluation of the role of prokera in the management of ocular surface and orbital disorders. Eye Contact Lens 2009, 35, 172–175. [Google Scholar] [CrossRef] [PubMed]
- Redd, T.K.; Seitzman, G.D. Ocular rosacea. Curr. Opin. Ophthalmol. 2020, 31, 503–507. [Google Scholar] [CrossRef]
- Wang, K.; Seitzman, G.; Gonzales, J.A. Ocular cicatricial pemphigoid. Curr. Opin. Ophthalmol. 2018, 29, 543–551. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.H.; Werth, V.P.; Parisi, E.; Sollecito, T.P. Mucous membrane pemphigoid. Dent. Clin. North Am. 2013, 57, 611–630. [Google Scholar] [CrossRef] [PubMed]
- Fuchs, E. Lehrbuch der Augenheilkunde, 7th ed.; Franz Deuticke: Vienna, Austria, 1898. [Google Scholar]
- Terrien, F. Dystrophie marginale symétrique des deux cornées avec astigmatisme régulier consécutive et guérison par la cautérization ignée. Arch. Ophthalmol. 1900, 20, 12–21. [Google Scholar]
- Ding, Y.; Murri, M.S.; Birdsong, O.C.; Ronquillo, Y.; Moshirfar, M. Terrien marginal degeneration. Surv. Ophthalmol. 2019, 64, 162–174. [Google Scholar] [CrossRef] [PubMed]
- Das, S.; Link, B.; Seitz, B. Salzmann’s nodular degeneration of the cornea: A review and case series. Cornea 2005, 24, 772–777. [Google Scholar] [CrossRef] [PubMed]
- Vanathi, M.; Panda, A.; Kai, S.; Sen, S. Corneal keloid. Ocul. Surf. 2008, 6, 186–197. [Google Scholar] [CrossRef]
- Mansour, A.M.; Barber, J.C.; Reinecke, R.D.; Wang, F.M. Ocular choristomas. Surv. Ophthalmol. 1989, 33, 339–358. [Google Scholar] [CrossRef]
- Yang, P.T.; Tucker, N.A.; Rootman, D.B.; Rootman, D.S.; McGowan, H.; Chan, C.C. Pagetoid spread of sebaceous cell carcinoma to the cornea. Can. J. Ophthalmol. 2012, 47, e46–e47. [Google Scholar] [CrossRef] [PubMed]
- Loeffler, K.U.; Perlman, J.I. Diffuse intraepithelial sebaceous carcinoma of the conjunctiva. Br. J. Ophthalmol. 1997, 81, 168. [Google Scholar] [CrossRef] [PubMed]
- Wright, P. Cicatrizing conjunctivitis. Trans. Ophthalmol. Soc. UK 1986, 105, 1–17. [Google Scholar] [PubMed]
- Gorouhi, F.; Davari, P.; Fazel, N. Cutaneous and mucosal lichen planus: A comprehensive review of clinical subtypes, risk factors, diagnosis, and prognosis. Sci. World J. 2014, 2014, 742826. [Google Scholar] [CrossRef] [PubMed]
- Burge, S.M.; Frith, P.A.; Juniper, R.P.; Wojnarowska, F. Mucosal involvement in systemic and chronic cutaneous lupus erythematosus. Br. J. Dermatol. 1989, 121, 727–741. [Google Scholar] [CrossRef] [PubMed]
- Shikari, H.; Antin, J.H.; Dana, R. Ocular graft-versus-host disease: A review. Surv. Ophthalmol. 2013, 58, 233–251. [Google Scholar] [CrossRef] [PubMed]
- Taylor, H.R.; Burton, M.J.; Haddad, D.; West, S.; Wright, H. Trachoma. Lancet 2014, 384, 2142–2152. [Google Scholar] [CrossRef]
- Hammer, L.H.; Perry, H.D.; Donnenfeld, E.D.; Rahn, E.K. Symblepharon formation in epidemic keratoconjunctivitis. Cornea 1990, 9, 338–340. [Google Scholar] [CrossRef] [PubMed]
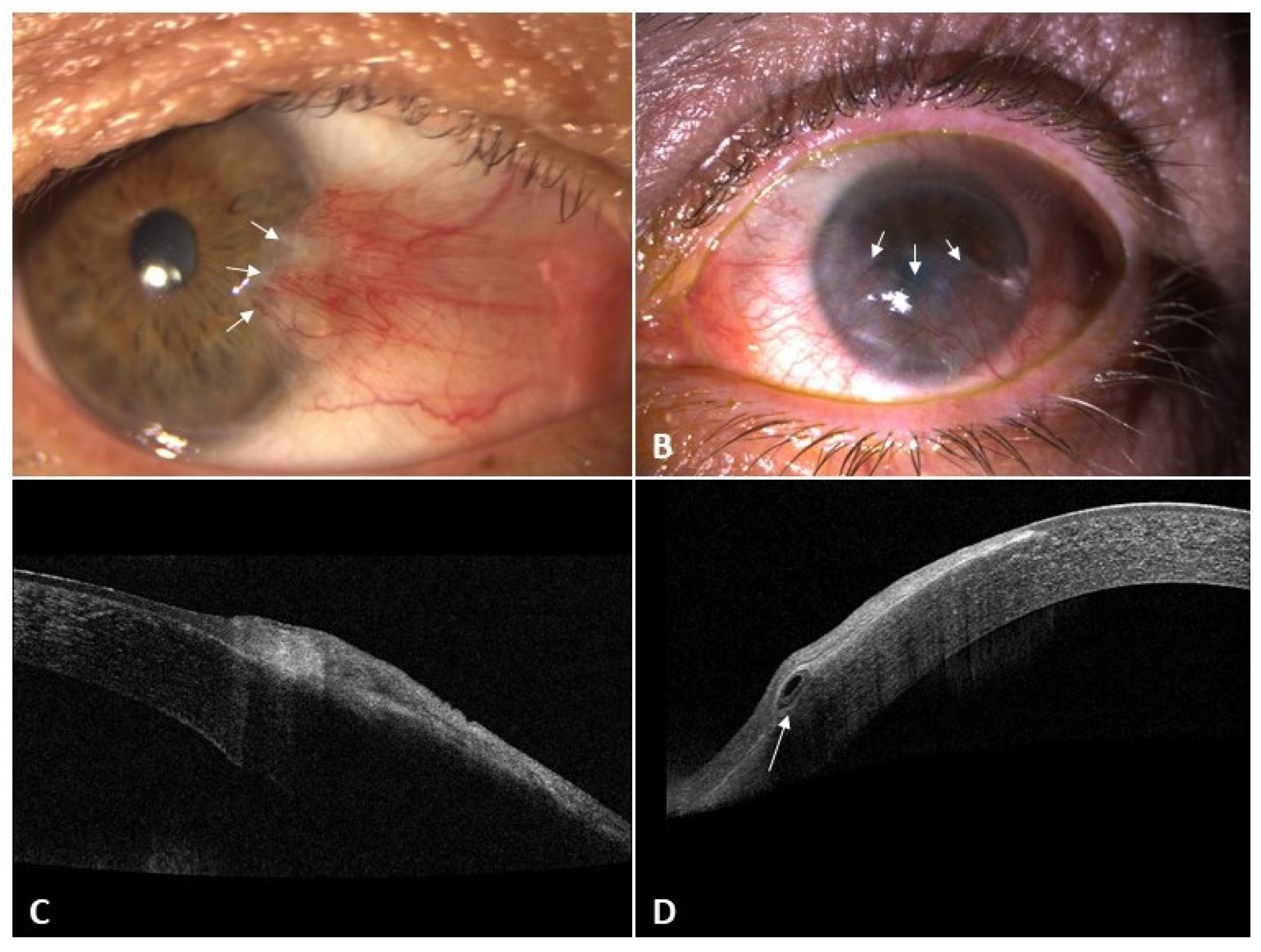
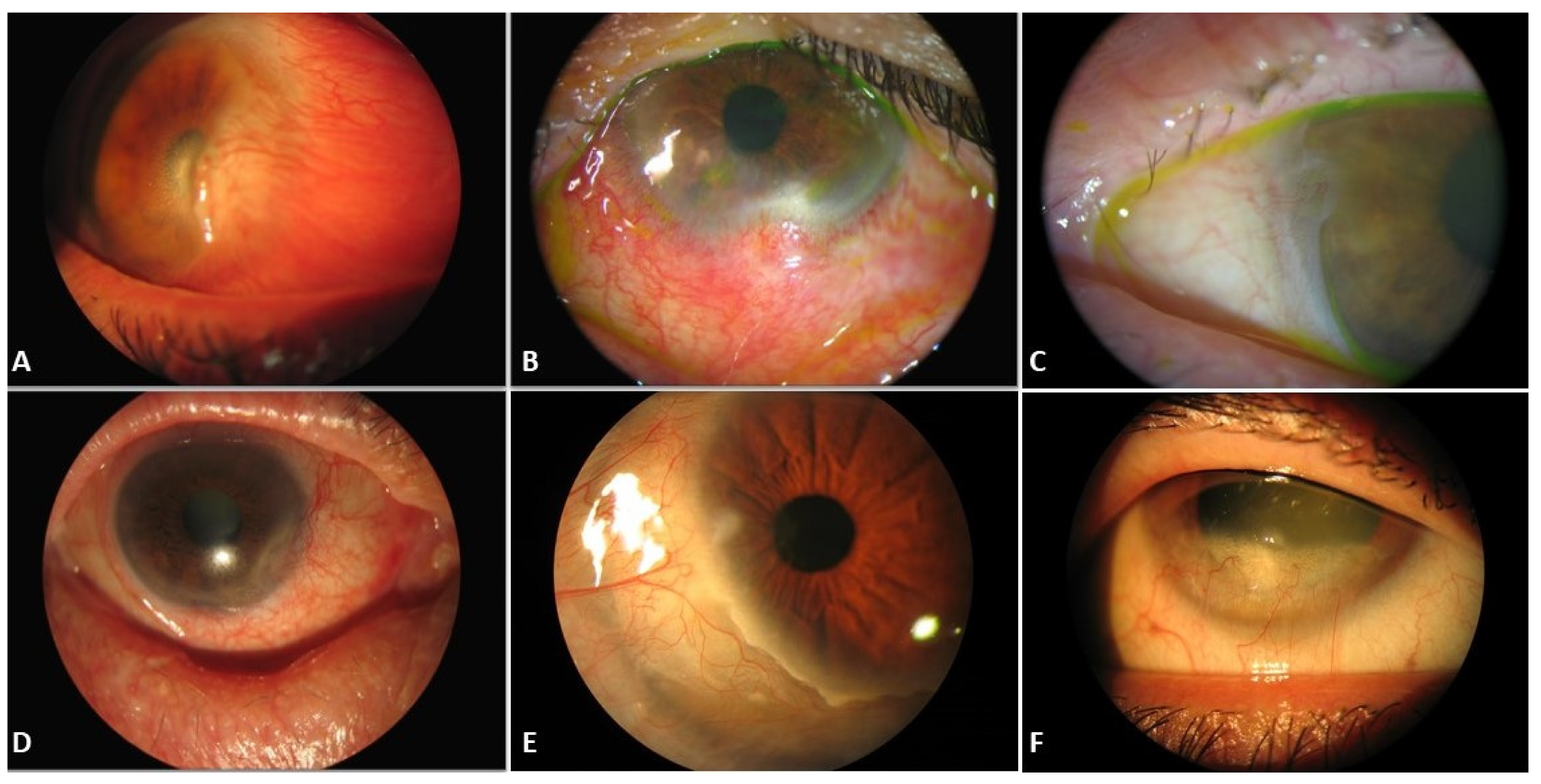
| Title | Pseudopterygium | Pterygium |
|---|---|---|
| Etiology | Secondary to corneal damage | Degenerative |
| Location | Anywhere along the 360 degrees | Nasal or temporal limbus |
| Condition | Stationary | Normally progressive |
| Form | Anterior portion broad and flat | Head pointed and well-defined |
| AS-OCT | Real plane of cleavage | Pseudoline of cleavage |
| Probe test | Positive | Negative |
| Histology | Fibrovascular tissue without foci of elastotic degeneration | Elastotic degeneration intermingled with epithelium and stroma |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Urbinati, F.; Borroni, D.; Rodríguez-Calvo-de-Mora, M.; Sánchez-González, J.-M.; García-Lorente, M.; Zamorano-Martín, F.; Rachwani-Anil, R.; Ortiz-Pérez, S.; Romano, V.; Rocha-de-Lossada, C. Pseudopterygium: An Algorithm Approach Based on the Current Evidence. Diagnostics 2022, 12, 1843. https://doi.org/10.3390/diagnostics12081843
Urbinati F, Borroni D, Rodríguez-Calvo-de-Mora M, Sánchez-González J-M, García-Lorente M, Zamorano-Martín F, Rachwani-Anil R, Ortiz-Pérez S, Romano V, Rocha-de-Lossada C. Pseudopterygium: An Algorithm Approach Based on the Current Evidence. Diagnostics. 2022; 12(8):1843. https://doi.org/10.3390/diagnostics12081843
Chicago/Turabian StyleUrbinati, Facundo, Davide Borroni, Marina Rodríguez-Calvo-de-Mora, José-María Sánchez-González, María García-Lorente, Francisco Zamorano-Martín, Rahul Rachwani-Anil, Santiago Ortiz-Pérez, Vito Romano, and Carlos Rocha-de-Lossada. 2022. "Pseudopterygium: An Algorithm Approach Based on the Current Evidence" Diagnostics 12, no. 8: 1843. https://doi.org/10.3390/diagnostics12081843
APA StyleUrbinati, F., Borroni, D., Rodríguez-Calvo-de-Mora, M., Sánchez-González, J.-M., García-Lorente, M., Zamorano-Martín, F., Rachwani-Anil, R., Ortiz-Pérez, S., Romano, V., & Rocha-de-Lossada, C. (2022). Pseudopterygium: An Algorithm Approach Based on the Current Evidence. Diagnostics, 12(8), 1843. https://doi.org/10.3390/diagnostics12081843











