Strong Correlations between the Binding Antibodies against Wild-Type and Neutralizing Antibodies against Omicron BA.1 and BA.2 Variants of SARS-CoV-2 in Individuals Following Booster (Third-Dose) Vaccination
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants and Ethical Considerations
2.2. Measurement Anti-RBD IgG and sVNT
2.3. Foci-Reduction Neutralization Test (FRNT50)
2.4. Statistical Analysis
3. Results
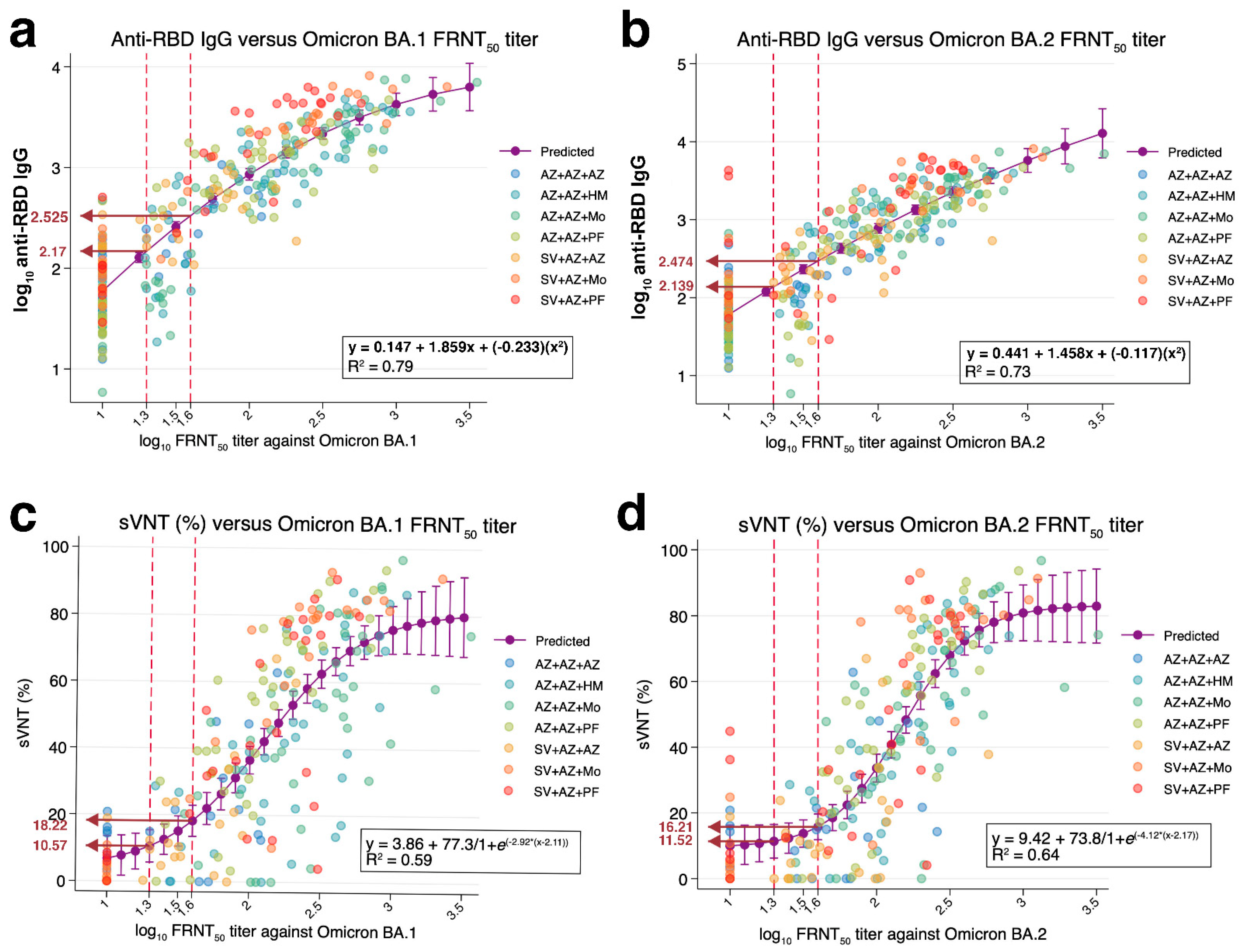
3.1. Correlations between Anti-RBD IgG against Wild-Type and FRNT50 Titers against Omicron
3.2. Correlations between sVNT against Omicron and FRNT50 Titers against Omicron
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Feng, S.; Phillips, D.J.; White, T.; Sayal, H.; Aley, P.K.; Bibi, S.; Dold, C.; Fuskova, M.; Gilbert, S.C.; Hirsch, I.; et al. Correlates of protection against symptomatic and asymptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 2032–2040. [Google Scholar] [CrossRef]
- Gallais, F.; Gantner, P.; Bruel, T.; Velay, A.; Planas, D.; Wendling, M.J.; Bayer, S.; Solis, M.; Laugel, E.; Reix, N.; et al. Evolution of antibody responses up to 13 months after SARS-CoV-2 infection and risk of reinfection. EBioMedicine 2021, 71, 103561. [Google Scholar] [CrossRef] [PubMed]
- Khoury, D.S.; Cromer, D.; Reynaldi, A.; Schlub, T.E.; Wheatley, A.K.; Juno, J.A.; Subbarao, K.; Kent, S.J.; Triccas, J.A.; Davenport, M.P. Neutralizing antibody levels are highly predictive of immune protection from symptomatic SARS-CoV-2 infection. Nat. Med. 2021, 27, 1205–1211. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, R.; Suzuki, E.; Murai, R.; Tanaka, M.; Fujiya, Y.; Takahashi, S. Performance analysis among multiple fully automated anti-SARS-CoV-2 antibody measurement reagents: A potential indicator for the correlation of protection in the antibody titer. J. Infect. Chemother. 2022, 28, 1295–1303. [Google Scholar] [CrossRef]
- Tan, C.W.; Chia, W.N.; Qin, X.; Liu, P.; Chen, M.I.; Tiu, C.; Hu, Z.; Chen, V.C.; Young, B.E.; Sia, W.R.; et al. A SARS-CoV-2 surrogate virus neutralization test based on antibody-mediated blockage of ACE2–spike protein–protein interaction. Nat. Biotechnol. 2020, 38, 1073–1078. [Google Scholar] [CrossRef]
- Lee, B.; Ko, J.H.; Park, J.; Moon, H.W.; Baek, J.Y.; Jung, S.; Lim, H.Y.; Kim, K.C.; Huh, K.; Cho, S.Y.; et al. Estimating the neutralizing effect and titer correlation of semi-quantitative anti-SARS-CoV-2 Antibody Immunoassays. Front. Cell. Infect. Microbiol. 2022, 12, 822599. [Google Scholar] [CrossRef]
- Morinaga, Y.; Tani, H.; Terasaki, Y.; Nomura, S.; Kawasuji, H.; Shimada, T.; Igarashi, E.; Saga, Y.; Yoshida, Y.; Yasukochi, R.; et al. Correlation of the commercial anti-SARS-CoV-2 receptor binding domain antibody test with the chemiluminescent reduction neutralizing test and possible detection of antibodies to emerging variants. Microbiol. Spectr. 2021, 9, e00560-21. [Google Scholar] [CrossRef]
- Ramos, A.; Cardoso, M.J.; Ribeiro, L.; Guimarães, J.T. Assessing SARS-CoV-2 neutralizing antibodies after BNT162b2 vaccination and their correlation with SARS-CoV-2 IgG anti-S1, anti-RBD and anti-S2 serological titers. Diagnostics 2022, 12, 205. [Google Scholar] [CrossRef]
- Assawakosri, S.; Kanokudom, S.; Chansaenroj, J.; Suntronwong, N.; Auphimai, C.; Nilyanimit, P.; Vichaiwattana, P.; Thongmee, T.; Duangchinda, T.; Chantima, W.; et al. Persistence of immunity against omicron BA.1 and BA.2 following homologous and heterologous COVID-19 booster vaccines in healthy adults after a two-doses AZD1222 vaccination. Int. J. Infect. Dis. 2022. [Google Scholar] [CrossRef]
- Suntronwong, N.; Kanokudom, S.; Auphimai, C.; Assawakosri, S.; Thongmee, T.; Vichaiwattana, P.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; Chansaenroj, J.; et al. Effects of boosted mRNA and adenoviral-vectored vaccines on immune responses to omicron BA.1 and BA.2 following the heterologous CoronaVac/AZD1222 vaccination. medRxiv 2022. [Google Scholar] [CrossRef]
- Dolscheid-Pommerich, R.; Bartok, E.; Renn, M.; Kümmerer, B.M.; Schulte, B.; Schmithausen, R.M.; Stoffel-Wagner, B.; Streeck, H.; Saschenbrecker, S.; Steinhagen, K.; et al. Correlation between a quantitative anti-SARS-CoV-2 IgG ELISA and neutralization activity. J. Med. Virol. 2022, 94, 388–392. [Google Scholar] [CrossRef] [PubMed]
- Guiomar, R.; Santos, A.J.; Melo, A.; Costa, I.; Matos, R.; Rodrigues, A.P.; Kislaya, I.; Silva, A.S.; Roque, C.; Silva, C.; et al. High correlation between binding IgG (anti-RBD/S) and neutralizing antibodies against SARS-CoV-2 six months after vaccination. medRxiv 2021. [Google Scholar] [CrossRef]
- Rockstroh, A.; Wolf, J.; Fertey, J.; Kalbitz, S.; Schroth, S.; Lübbert, C.; Ulbert, S.; Borte, S. Correlation of humoral immune responses to different SARS-CoV-2 antigens with virus neutralizing antibodies and symptomatic severity in a German COVID-19 cohort. Emerg. Microbes Infect. 2021, 10, 774–781. [Google Scholar] [CrossRef] [PubMed]
- Wajnberg, A.; Amanat, F.; Firpo, A.; Altman, D.R.; Bailey, M.J.; Mansour, M.; McMahon, M.; Meade, P.; Mendu, D.R.; Muellers, K.; et al. Robust neutralizing antibodies to SARS-CoV-2 infection persist for months. Science 2020, 370, 1227–1230. [Google Scholar] [CrossRef] [PubMed]
- Poon, R.W.; Lu, L.; Fong, C.H.; Ip, T.C.; Chen, L.L.; Zhang, R.R.; Yip, C.C.; Cheng, V.C.; Chan, K.H.; Yuen, K.Y.; et al. Correlation between commercial anti-RBD IgG titer and neutralization titer against SARS-CoV-2 beta variant. Diagnostics 2021, 11, 2216. [Google Scholar] [CrossRef] [PubMed]
- Ou, J.; Lan, W.; Wu, X.; Zhao, T.; Duan, B.; Yang, P.; Ren, Y.; Quan, L.; Zhao, W.; Seto, D.; et al. Tracking SARS-CoV-2 Omicron diverse spike gene mutations identifies multiple inter-variant recombination events. Signal Transduct. Target. Ther. 2022, 7, 138. [Google Scholar] [CrossRef]
- Medigeshi, G.R.; Batra, G.; Murugesan, D.R.; Thiruvengadam, R.; Chattopadhyay, S.; Das, B.; Gosain, M.; Ayushi; Singh, J.; Anbalagan, A.; et al. Sub-optimal neutralisation of omicron (B. 1.1. 529) variant by antibodies induced by vaccine alone or SARS-CoV-2 Infection plus vaccine (hybrid immunity) post 6-months. EBioMedicine 2022, 78, 103938. [Google Scholar] [CrossRef]
- Takheaw, N.; Liwsrisakun, C.; Chaiwong, W.; Laopajon, W.; Pata, S.; Inchai, J.; Duangjit, P.; Pothirat, C.; Bumroongkit, C.; Deesomchok, A.; et al. Correlation analysis of anti-SARS-CoV-2 RBD IgG and neutralizing antibody against SARS-CoV-2 Omicron variants after vaccination. Diagnostics 2022, 12, 1315. [Google Scholar] [CrossRef]
- GeurtsvanKessel, C.H.; Geers, D.; Schmitz, K.S.; Mykytyn, A.Z.; Lamers, M.M.; Bogers, S.; Scherbeijn, S.; Gommers, L.; Sablerolles, R.S.G.; Nieuwkoop, N.N.; et al. Divergent SARS-CoV-2 Omicron-reactive T and B cell responses in COVID-19 vaccine recipients. Sci. Immunol. 2022, 7, eabo2202. [Google Scholar] [CrossRef]
- Iketani, S.; Liu, L.; Guo, Y.; Liu, L.; Chan, J.F.; Huang, Y.; Wang, M.; Luo, Y.; Yu, J.; Chu, H.; et al. Antibody evasion properties of SARS-CoV-2 Omicron sublineages. Nature 2022, 604, 553–556. [Google Scholar] [CrossRef]
- Lusvarghi, S.; Pollett, S.D.; Neerukonda, N.D.; Wang, W.; Wang, R.; Vassell, R.; Epsi, N.J.; Fries, A.C.; Agan, B.K.; Lindholm, D.A.; et al. SARS-CoV-2 Omicron neutralization by therapeutic antibodies, convalescent sera, and post-mRNA vaccine booster. bioRxiv 2022. [Google Scholar] [CrossRef]

| Booster Groups | Anti-RBD IgG | sVNT | FRNT50 Titers BA.1 | FRNT50 Titers BA.2 | |
|---|---|---|---|---|---|
| n | GMT (95%CI) | Median (IQR) | GMT (95%CI) | GMT (95%CI) | |
| AZ + AZ + AZ | |||||
| Pre-boost | 20 | 67.4 (47.1–96.4) | NA | 13 (10.4–16.2) | 12.5 (10–15.6) |
| 28 d post-boost | 20 | 298.5 (204.2–436.2) | 15 (4.8–21.7) | 32.2 (20.1–51.6) | 45.6 (28.8–72.3) |
| AZ + AZ + HM | |||||
| Pre-boost | 20 | 46.8 (37.1–59) | NA | 14.6 (11.6–18.5) | 13 (10.4–16.1) |
| 28 d post-boost | 20 | 2160 (1649–2829) | 65.7 (32–77) | 396.5 (275.4–570.7) | 224.5 (156.4–322.2) |
| 90 d post-boost | 20 | 901.2 (657–1236) | 38 (22.5 -52.1) | 119.1 (78.5–180.8) | 110.5 (75–163) |
| AZ + AZ + Mo | |||||
| Pre-boost | 20 | 43.6 (31.1–61.3) | NA | 16.6 (13.2–21) | 11 (9.6–12.6) |
| 28 d post-boost | 20 | 3034 (2418–3806) | 67.7 (50.5–80.4) | 547.8 (415.2–723) | 324.2 (213.6–492.2) |
| 90 d post-boost | 20 | 916 (675–1243) | 54.4 (35.5–88.9) | 141 (89.6–221.6) | 122 (71.4–208) |
| AZ + AZ + PF | |||||
| Pre-boost | 20 | 43.3 (31.4–56.7) | NA | 10 (10) | 15 (11.8–19.1) |
| 28 d post-boost | 20 | 1876 (1581–2227) | 70.3 (56.9–78) | 166.3 (114–243.3) | 247.7 (179.2–342.4) |
| 90 d post-boost | 20 | 556 (460–673) | 32.5 (17.9–53.8) | 78.1(47.5–128.2) | 73.8 (56.1–97.2) |
| SV + AZ + AZ | |||||
| Pre-boost | 10 | 146.5 (77.6–276.3) | 11.48 (0.3–18.9) | 12.8 (8.8–18.5) | 24.4 (16.8–35.4) |
| 28 d post-boost | 20 | 315.8 (233.5–427) | 11.4 (2.6–23.8) | 40.3 (27.3–59.6) | 59.3 (39.7–88.5) |
| SV + AZ + Mo | |||||
| Pre-boost | 10 | 98 (66.2–145) | 3.58 (1.0–6.6) | 11 (8.9–13.5) | 12 (9.1–15.7) |
| 28 d post-boost | 20 | 2930 (2156–3983) | 79.7 (61.1–82.4) | 271.6 (173–427) | 235 (144–385.4) |
| SV + AZ + PF | |||||
| Pre-boost | 10 | 135.4 (67.7–270.8) | 8.1 (4.4–17.3) | 17.3 (8.7–34.1) | 28.3 (14.8–53.8) |
| 28 d post-boost | 20 | 3049 (2322–4005) | 58.4 (33.1–78.5) | 171 (120–243.3) | 130.7 (78.9–216.8) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suntronwong, N.; Assawakosri, S.; Kanokudom, S.; Yorsaeng, R.; Auphimai, C.; Thongmee, T.; Vichaiwattana, P.; Duangchinda, T.; Chantima, W.; Pakchotanon, P.; et al. Strong Correlations between the Binding Antibodies against Wild-Type and Neutralizing Antibodies against Omicron BA.1 and BA.2 Variants of SARS-CoV-2 in Individuals Following Booster (Third-Dose) Vaccination. Diagnostics 2022, 12, 1781. https://doi.org/10.3390/diagnostics12081781
Suntronwong N, Assawakosri S, Kanokudom S, Yorsaeng R, Auphimai C, Thongmee T, Vichaiwattana P, Duangchinda T, Chantima W, Pakchotanon P, et al. Strong Correlations between the Binding Antibodies against Wild-Type and Neutralizing Antibodies against Omicron BA.1 and BA.2 Variants of SARS-CoV-2 in Individuals Following Booster (Third-Dose) Vaccination. Diagnostics. 2022; 12(8):1781. https://doi.org/10.3390/diagnostics12081781
Chicago/Turabian StyleSuntronwong, Nungruthai, Suvichada Assawakosri, Sitthichai Kanokudom, Ritthideach Yorsaeng, Chompoonut Auphimai, Thanunrat Thongmee, Preeyaporn Vichaiwattana, Thaneeya Duangchinda, Warangkana Chantima, Pattarakul Pakchotanon, and et al. 2022. "Strong Correlations between the Binding Antibodies against Wild-Type and Neutralizing Antibodies against Omicron BA.1 and BA.2 Variants of SARS-CoV-2 in Individuals Following Booster (Third-Dose) Vaccination" Diagnostics 12, no. 8: 1781. https://doi.org/10.3390/diagnostics12081781
APA StyleSuntronwong, N., Assawakosri, S., Kanokudom, S., Yorsaeng, R., Auphimai, C., Thongmee, T., Vichaiwattana, P., Duangchinda, T., Chantima, W., Pakchotanon, P., Chansaenroj, J., Nilyanimit, P., Srimuan, D., Thatsanatorn, T., Sudhinaraset, N., Wanlapakorn, N., Mongkolsapaya, J., & Poovorawan, Y. (2022). Strong Correlations between the Binding Antibodies against Wild-Type and Neutralizing Antibodies against Omicron BA.1 and BA.2 Variants of SARS-CoV-2 in Individuals Following Booster (Third-Dose) Vaccination. Diagnostics, 12(8), 1781. https://doi.org/10.3390/diagnostics12081781






