Clinical and Serological Findings of COVID-19 Participants in the Region of Makkah, Saudi Arabia
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Ethical Approval
2.2. Sample Collection
2.3. Serological Assays
2.3.1. Microneutralization Test (MNT)
2.3.2. Enzyme-Linked Immunosorbent Assay (ELISA) for SARS-CoV-2 S Protein
2.4. Statistical Analysis
3. Results
3.1. Clinical and Demographic Data of Patients
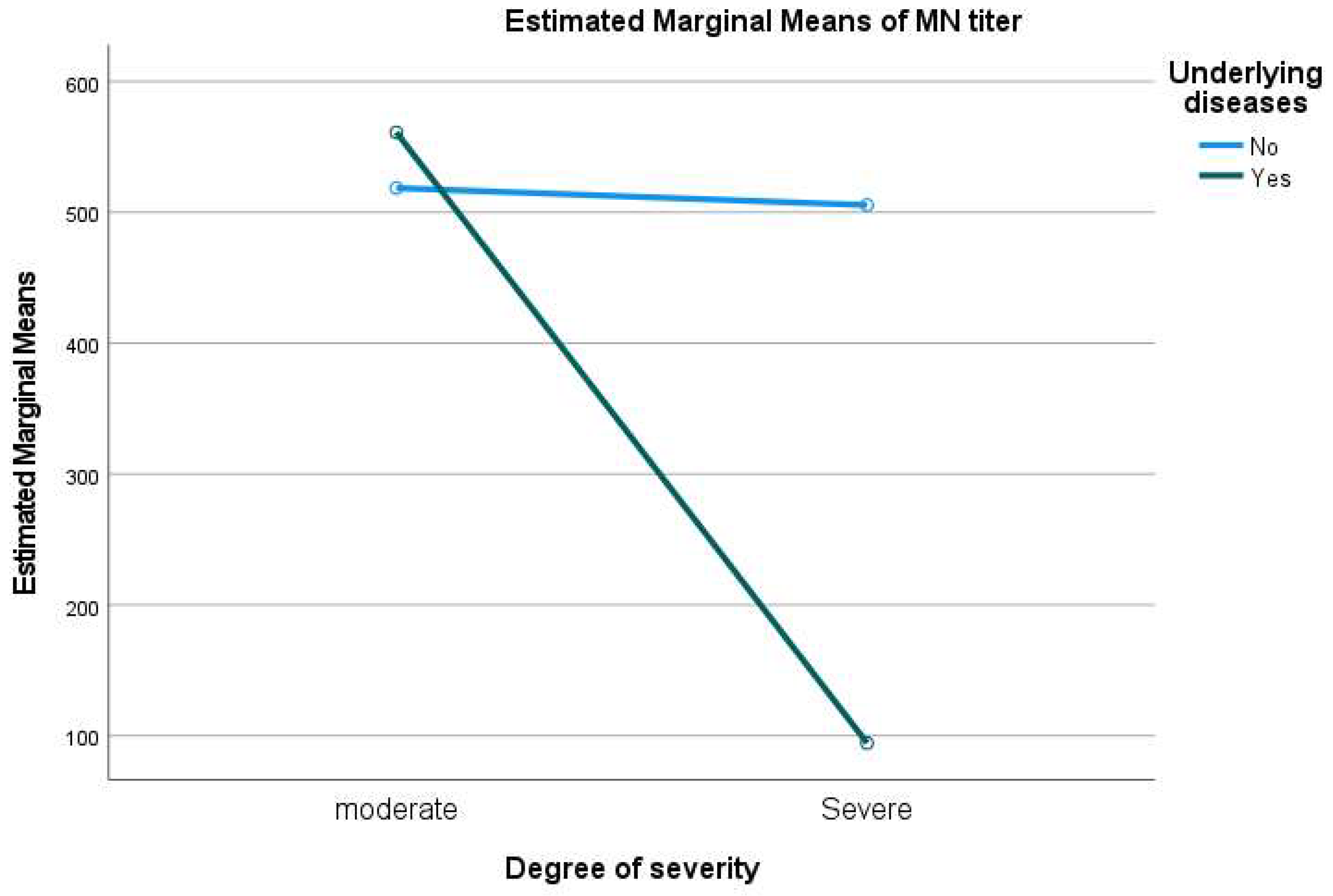
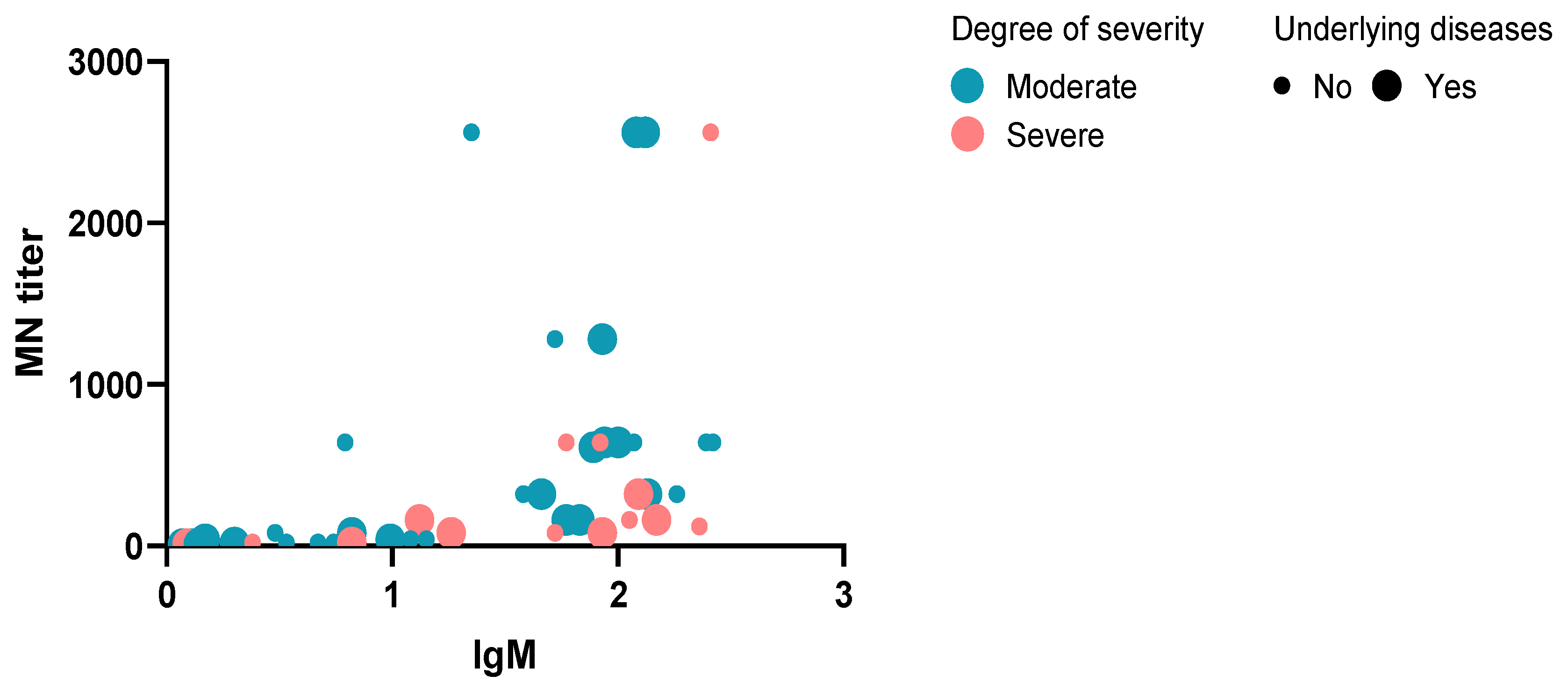
3.2. MNT Results
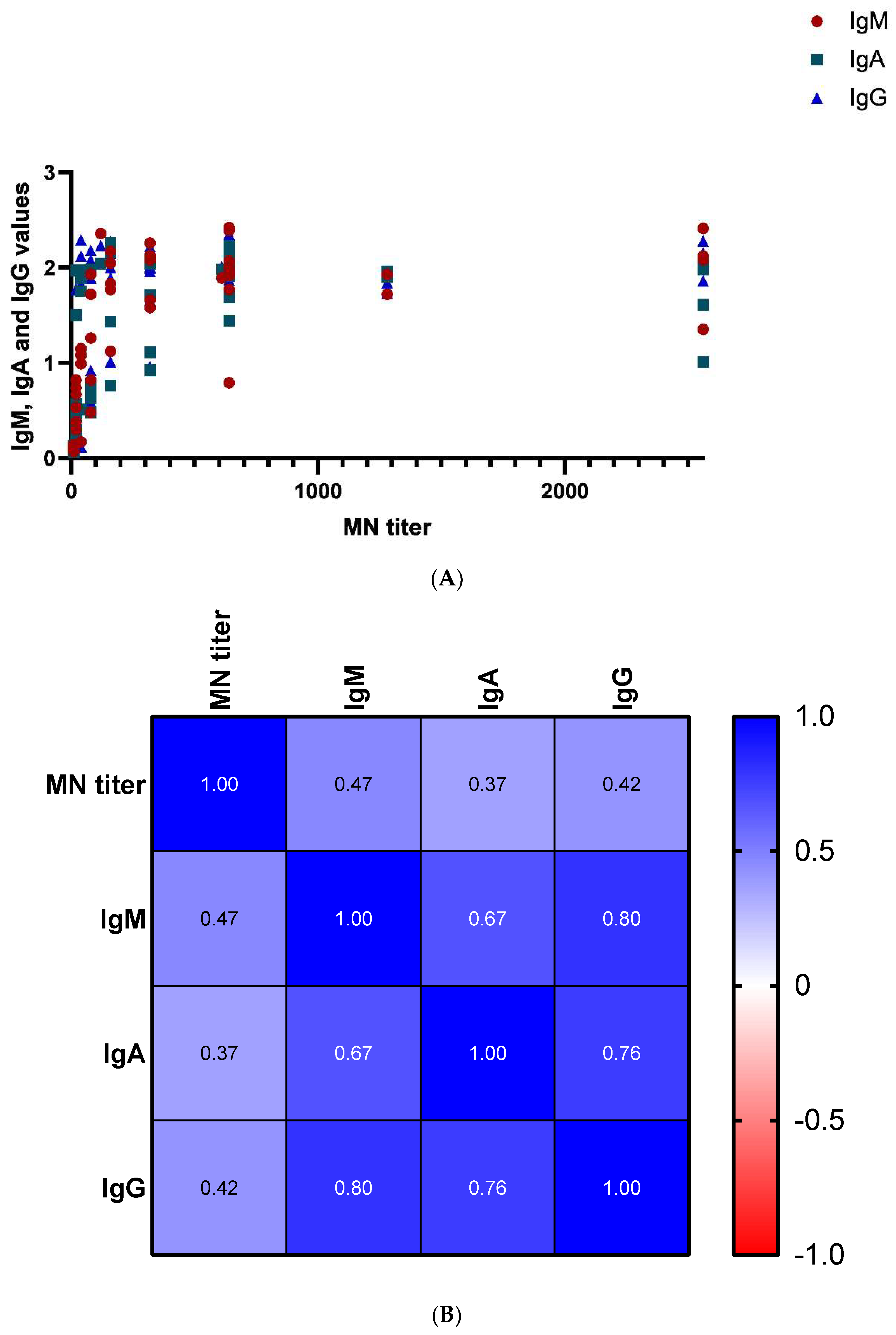
3.3. ELISA IgM, IgA, and IgG Assays Results
4. Discussion
5. Study Limitations
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Phelan, A.L.; Katz, R.; Gostin, L.O. The Novel Coronavirus Originating in Wuhan, China: Challenges for Global Health Governance. JAMA 2020, 323, 709–710. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, N.; Zhang, D.; Wang, W.; Li, X.; Yang, B.; Song, J.; Zhao, X.; Huang, B.; Shi, W.; Lu, R.; et al. A Novel Coronavirus from Patients with Pneumonia in China, 2019. N. Engl. J. Med. 2020, 382, 727–733. [Google Scholar] [CrossRef] [PubMed]
- Barry, M.; Al Amri, M.; Memish, Z.A. COVID-19 in the Shadows of MERS-CoV in the Kingdom of Saudi Arabia. J. Epidemiol. Glob. Health 2020, 10, 1–3. [Google Scholar] [CrossRef] [Green Version]
- Gautret, P.; Al-Tawfiq, J.A.; Hoang, V.T. COVID 19: Will the 2020 Hajj pilgrimage and Tokyo Olympic Games be cancelled? Travel Med. Infect. Dis. 2020, 34, 101622. [Google Scholar] [CrossRef]
- Aljahdali, M.O.; Alhassan, A.B.; Albeladi, M.N. Impact of Novel coronavirus disease (COVID-19) lockdown on ambient air quality of Saudi Arabia: A case study of nine cities. Saudi J. Biol. Sci. 2020, 28, 1356–1364. [Google Scholar] [CrossRef] [PubMed]
- Ebrahim, S.H.; Ahmed, Y.; Alqahtani, S.A.; Memish, Z.A. The Hajj pilgrimage during the COVID-19 pandemic in 2020: Event hosting without the mass gathering. J. Travel Med. 2020, 28, taaa194. [Google Scholar] [CrossRef] [PubMed]
- Available online: https://covid19.moh.gov.sa/ (accessed on 27 May 2022).
- WHO. Middle East Respiratory Syndrome Coronavirus (MERS-CoV)—The Kingdom of Saudi Arabia, in Emergencies Preparedness, Response; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- WHO. Laboratory testing for coronavirus disease (COVID-19) in suspected human cases. In Interim Guidance 19 March 2020; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Alzahrani, F.A.; Shait Mohammed, M.R.; Alkarim, S.; Azhar, E.I.; El-Magd, M.A.; Hawsawi, Y.; Abdulaal, W.H.; Yusuf, A.; Alhatmi, A.; Albiheyri, R.; et al. Untargeted Metabolic Profiling of Extracellular Vesicles of SARS-CoV-2-Infected Patients Shows Presence of Potent Anti-Inflammatory Metabolites. Int. J. Mol. Sci. 2021, 22, 10467. [Google Scholar] [CrossRef]
- Alzahrani, F.A.; Alkarim, S.A.; Hawsawi, Y.M.; Abdulaal, W.H.; Albiheyri, R.; Kurdi, B.; Alguridi, H.; El-Magd, M.A. 25 (S)-Hydroxycholesterol acts as a possible dual enzymatic inhibitor of SARS-CoV-2 M(pro) and RdRp-: An insight from molecular docking and dynamics simulation approaches. J. Biomol. Struct. Dyn. 2022; in press. [Google Scholar]
- Yassine, H.M.; Al-Jighefee, H.; Al-Sadeq, D.W.; Dargham, S.R.; Younes, S.N.; Shurrab, F.; Marei, R.M.; Hssain, A.A.; Taleb, S.; Alhussain, H.; et al. Performance evaluation of five ELISA kits for detecting anti-SARS-CoV-2 IgG antibodies. Int. J. Infect. Dis. 2020, 102, 181–187. [Google Scholar] [CrossRef]
- Van Honacker, E.; Coorevits, L.; Boelens, J.; Verhasselt, B.; Van Braeckel, E.; Bauters, F.; De Bus, L.; Schelstraete, P.; Willems, J.; Vandendriessche, S.; et al. Sensitivity and specificity of 14 SARS-CoV-2 serological assays and their diagnostic potential in RT-PCR negative COVID-19 infections. Acta Clin. Belg. 2022, 77, 315–320. [Google Scholar] [CrossRef]
- Wajnberg, A.; Mansour, M.; Leven, E.; Bouvier, N.M.; Patel, G.; Firpo-Betancourt, A.; Mendu, R.; Jhang, J.; Arinsburg, S.; Gitman, M.; et al. Humoral response and PCR positivity in patients with COVID-19 in the New York City region, USA: An observational study. Lancet Microbe 2020, 1, e283–e289. [Google Scholar] [CrossRef]
- Pollán, M.; Pérez-Gómez, B.; Pastor-Barriuso, R.; Oteo, J.; Hernán, M.A.; Perez-Olmeda, M.; Sanmartín, J.L.; Fernández-García, A.; Cruz, I.; de Larrea, N.F.; et al. Prevalence of SARS-CoV-2 in Spain (ENE-COVID): A nationwide, population-based seroepidemiological study. Lancet 2020, 396, 535–544. [Google Scholar] [CrossRef]
- Al-Tawfiq, J.A.; Memish, Z.A. Serologic testing of coronaviruses from MERS-CoV to SARS-CoV-2: Learning from the past and anticipating the future. Travel Med. Infect. Dis. 2020, 37, 101785. [Google Scholar] [CrossRef] [PubMed]
- Amanat, F.; Nguyen, T.; Chromikova, V.; Strohmeier, S.; Stadlbauer, D.; Javier, A.; Jiang, K.; Asthagiri-Arunkumar, G.; Jurczyszak, D.; Polanco, J.; et al. A serological assay to detect SARS-CoV-2 seroconversion in humans. Nat. Med. 2020, 26, 1033–1036. [Google Scholar] [CrossRef] [PubMed]
- Manenti, A.; Maggetti, M.; Casa, E.; Martinuzzi, D.; Torelli, A.; Trombetta, C.M.; Marchi, S.; Montomoli, E. Evaluation of SARS-CoV-2 neutralizing antibodies using a CPE-based colorimetric live virus micro-neutralization assay in human serum samples. J. Med. Virol. 2020, 92, 2096–2104. [Google Scholar] [CrossRef] [PubMed]
- Haveri, A.; Smura, T.; Kuivanen, S.; Osterlund, P.; Hepojoki, J.; Ikonen, N.; Pitkapaasi, M.; Blomqvist, S.; Rönkkö, E.; Kantele, A.; et al. Serological and molecular findings during SARS-CoV-2 infection: The first case study in Finland, January to February 2020. Euro Surveill. 2020, 25, 2000266. [Google Scholar] [CrossRef]
- Stadlbauer, D.; Amanat, F.; Chromikova, V.; Jiang, K.; Strohmeier, S.; Arunkumar, G.A.; Tan, J.; Bhavsar, D.; Capuano, C.; Kirkpatrick, E.; et al. SARS-CoV-2 Seroconversion in Humans: A Detailed Protocol for a Serological Assay, Antigen Production, and Test Setup. Curr. Protoc. Microbiol. 2020, 57, e100. [Google Scholar] [CrossRef] [Green Version]
- Rusanen, J.; Kareinen, L.; Levanov, L.; Mero, S.; Pakkanen, S.H.; Kantele, A.; Amanat, F.; Krammer, F.; Hedman, K.; Vapalahti, O.; et al. Rapid homogeneous assay for detecting antibodies against SARS-CoV-2. medRxiv 2020. [Google Scholar] [CrossRef]
- Javelle, E.; Raoult, D. COVID-19 pandemic more than a century after the Spanish flu. Lancet Infect. Dis. 2020, 21, e78. [Google Scholar] [CrossRef]
- Memish, Z.A.; Steffen, R.; White, P.; Dar, O.; Azhar, E.I.; Sharma, A.; Zumla, A. Mass gatherings medicine: Public health issues arising from mass gathering religious and sporting events. Lancet 2019, 393, 2073–2084. [Google Scholar] [CrossRef]
- Okba, N.M.A.; Müller, M.A.; Li, W.; Wang, C.; GeurtsvanKessel, C.H.; Corman, V.M.; Lamers, M.M.; Sikkema, R.S.; De Bruin, E.; Chandler, F.D.; et al. Severe Acute Respiratory Syndrome Coronavirus 2-Specific Antibody Responses in Coronavirus Disease Patients. Emerg. Infect Dis. 2020, 26, 1478–1488. [Google Scholar] [CrossRef]
- Jiang, C.; Wang, Y.; Hu, M.; Wen, L.; Wen, C.; Wang, Y.; Zhu, W.; Tai, S.; Jiang, Z.; Xiao, K.; et al. Antibody seroconversion in asymptomatic and symptomatic patients infected with severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2). Clin. Transl. Immunol. 2020, 9, e1182. [Google Scholar] [CrossRef]
- Zhang, W.; Du, R.-H.; Li, B.; Zheng, X.-S.; Yang, X.-L.; Hu, B.; Wang, Y.-Y.; Xiao, G.-F.; Yan, B.; Shi, Z.-L.; et al. Molecular and serological investigation of 2019-nCoV infected patients: Implication of multiple shedding routes. Emerg. Microbes Infect. 2020, 9, 386–389. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, M.; Gilboa, T.; Ogata, A.F.; Maley, A.M.; Cohen, L.; Busch, E.L.; Lazarovits, R.; Mao, C.-P.; Cai, Y.; Zhang, J.; et al. Ultrasensitive high-resolution profiling of early seroconversion in patients with COVID-19. Nat. Biomed. Eng. 2020, 4, 1180–1187. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Pan, Z.; Yue, S.; Yu, F.; Zhang, J.; Yang, Y.; Li, R.; Liu, B.; Yang, X.; Gao, L.; et al. Disease severity dictates SARS-CoV-2-specific neutralizing antibody responses in COVID-19. Signal Transduct. Target Ther. 2020, 5, 180. [Google Scholar] [CrossRef] [PubMed]
- Ko, J.-H.; Joo, E.-J.; Park, S.-J.; Baek, J.Y.; Kim, W.D.; Jee, J.; Kim, C.J.; Jeong, C.; Kim, Y.-J.; Shon, H.J.; et al. Neutralizing Antibody Production in Asymptomatic and Mild COVID-19 Patients, in Comparison with Pneumonic COVID-19 Patients. J. Clin. Med. 2020, 9, 2268. [Google Scholar] [CrossRef]
- Tighe, P.J.; Urbanowicz, R.A.; Fairclough, L.; McClure, C.P.; Thomson, B.J.; Gomez, N.; Chappell, J.G.; Tsoleridis, T.; Loose, M.; Carlile, M.; et al. Potent anti-SARS-CoV-2 Antibody Responses are Associated with Better Prognosis in Hospital Inpatient COVID-19 Disease. medRxiv 2020. [Google Scholar] [CrossRef]
- Khamis, R.Y.; Hughes, A.D.; Caga-Anan, M.; Chang, C.L.; Boyle, J.J.; Kojima, C.; Welsh, P.; Sattar, N.; Johns, M.; Sever, P.; et al. High Serum Immunoglobulin G and M Levels Predict Freedom From Adverse Cardiovascular Events in Hypertension: A Nested Case-Control Substudy of the Anglo-Scandinavian Cardiac Outcomes Trial. EBioMedicine 2016, 9, 372–380. [Google Scholar] [CrossRef] [Green Version]
- Staudt, A.; Staudt, Y.; Dorr, M.; Bohm, M.; Knebel, F.; Hummel, A.; Wunderle, L.; Tiburcy, M.; Wernecke, K.D.; Baumannet, G.; et al. Potential role of humoral immunity in cardiac dysfunction of patients suffering from dilated cardiomyopathy. J. Am. Coll. Cardiol. 2004, 44, 829–836. [Google Scholar] [CrossRef] [Green Version]
- Lin, D.; Bridgeman, M.B.; Brunetti, L. Evaluation of alterations in serum immunoglobulin concentrations in components of metabolic syndrome, obesity, diabetes, and dyslipidemia. BMC Cardiovasc. Disord. 2019, 19, 319. [Google Scholar] [CrossRef] [Green Version]
| Characteristics | Number and Percentage |
|---|---|
| Demographic data | |
| Age range | |
| ≤20 | 2 (4.0%) |
| 21–30 | 4 (8.0%) |
| 31–40 | 9 (18.0%) |
| 41–50 | 8 (16.0%) |
| 51–60 | 11 (22.0%) |
| ≥61 | 16 (32.0%) |
| Sex | |
| Male | 44 (88.0%) |
| Female | 6 (12.0%) |
| Origin | |
| Saudi | 13 (26.0%) |
| non-Saudi | 37 (74.0%) |
| Smoking | |
| Smoker | 22 (46.0%) |
| Nonsmoker | 27 (44.0%) |
| Not known | 1 (2.0%) |
| Outcome | |
| Died | 5 (10.0%) |
| Survived | 45 (90.0%) |
| Other underlying medical conditions | |
| Autoimmune diseases | 1 (2.0%) |
| Liver diseases | 2 (4.0%) |
| Malignancy | 1 (2.0%) |
| Kidney diseases | 3 (6.0%) |
| Cerbro-vascular diseases | 5 (10.0%) |
| Lung diseases | 3 (6.0%) |
| Cardiovascular diseases | 14 (28.0%) |
| Diabetes mellitus | 12 (24.0%) |
| Arterial Systematic Hypertension | 25 (50.0%) |
| Clinical manifestations | |
| Fever (>38.0 °C) | 10 (20%) |
| Headache | 6 (12%) |
| GIT symptoms (nausea, vomiting, diarrhea) | 7 (14%) |
| Sore throat | 6 (12%) |
| Nasal congestion | 6 (12%) |
| Runny nose | 2 (4%) |
| Cough (dry or productive) | 28 (56%) |
| Hemoptysis | 5 (10%) |
| Shortness of breath | 23 (46%) |
| Conjunctival congestion | 2 (4%) |
| Intubated individuals | 18 (36%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alzahrani, O.R.; Alanazi, A.D.; Kareinen, L.; Hawsawi, Y.M.; Alhadrami, H.A.; Khogeer, A.A.; Alatwi, H.E.; Alharbi, A.A.; Sironen, T.; Vapalahti, O.; et al. Clinical and Serological Findings of COVID-19 Participants in the Region of Makkah, Saudi Arabia. Diagnostics 2022, 12, 1725. https://doi.org/10.3390/diagnostics12071725
Alzahrani OR, Alanazi AD, Kareinen L, Hawsawi YM, Alhadrami HA, Khogeer AA, Alatwi HE, Alharbi AA, Sironen T, Vapalahti O, et al. Clinical and Serological Findings of COVID-19 Participants in the Region of Makkah, Saudi Arabia. Diagnostics. 2022; 12(7):1725. https://doi.org/10.3390/diagnostics12071725
Chicago/Turabian StyleAlzahrani, Othman R., Abdullah D. Alanazi, Lauri Kareinen, Yousef M. Hawsawi, Hani A. Alhadrami, Asim A. Khogeer, Hanan E. Alatwi, Amnah A. Alharbi, Tarja Sironen, Olli Vapalahti, and et al. 2022. "Clinical and Serological Findings of COVID-19 Participants in the Region of Makkah, Saudi Arabia" Diagnostics 12, no. 7: 1725. https://doi.org/10.3390/diagnostics12071725
APA StyleAlzahrani, O. R., Alanazi, A. D., Kareinen, L., Hawsawi, Y. M., Alhadrami, H. A., Khogeer, A. A., Alatwi, H. E., Alharbi, A. A., Sironen, T., Vapalahti, O., Hepojoki, J., & Zakham, F. (2022). Clinical and Serological Findings of COVID-19 Participants in the Region of Makkah, Saudi Arabia. Diagnostics, 12(7), 1725. https://doi.org/10.3390/diagnostics12071725









