Diagnostic Value of Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte, and Monocyte-to-Lymphocyte Ratios for the Assessment of Rheumatoid Arthritis in Patients with Undifferentiated Inflammatory Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Population
2.2. Clinical Variables
2.3. Statistical Analyses
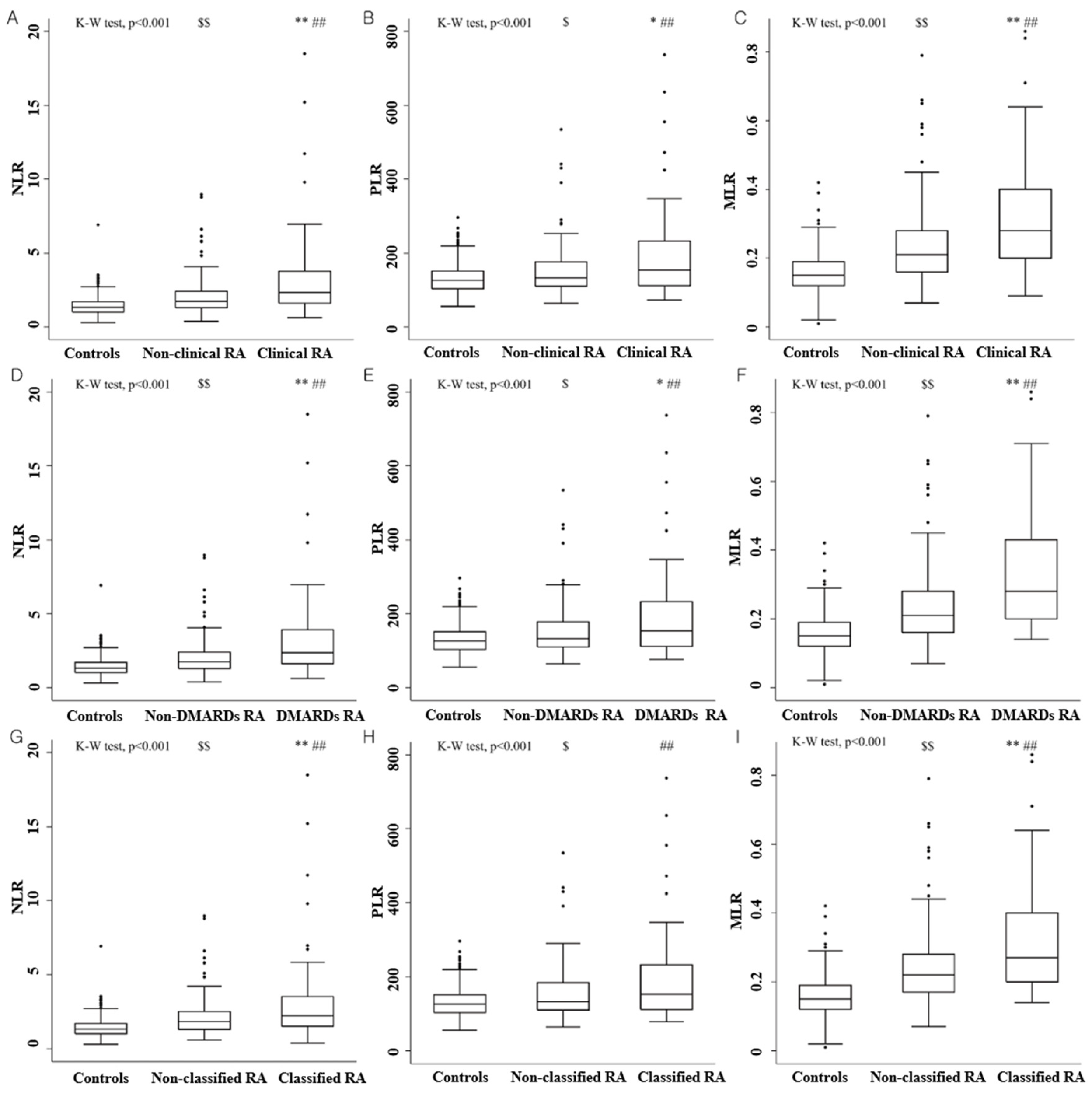
3. Results
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Kim, H.; Sung, Y.-K. Epidemiology of Rheumatoid Arthritis in Korea. J. Rheum. Dis. 2021, 28, 60–67. [Google Scholar] [CrossRef]
- Lee, Y.-K.; Bae, S.-C. Mortality in Korean Patients with Rheumatoid Arthritis. J. Rheum. Dis. 2021, 28, 113–118. [Google Scholar] [CrossRef]
- Kim, Y.; Kim, G.T.; Suh, Y.S.; Kim, H.O.; Lee, H.N.; Lee, S.G. The Impact of the Amendment of the Korean National Health Insurance Reimbursement Criteria for Anti-Tumor Necrosis Factor-Alpha Agents on Treatment Pattern, Clinical Response and Persistence in Patients With Rheumatoid Arthritis. J. Rheum. Dis. 2020, 27, 159–167. [Google Scholar] [CrossRef]
- Burgers, L.E.; Raza, K.; van der Helm-van Mil, A.H. Window of opportunity in rheumatoid arthritis—Definitions and supporting evidence: From old to new perspectives. RMD Open 2019, 5, e000870. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fraenkel, L.; Bathon, J.M.; England, B.R.; St Clair, E.W.; Arayssi, T.; Carandang, K.; Deane, K.D.; Genovese, M.; Huston, K.K.; Kerr, G.; et al. 2021 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Care Res. 2021, 73, 924–939. [Google Scholar] [CrossRef]
- Belluzzi, E.; Olivotto, E.; Toso, G.; Cigolotti, A.; Pozzuoli, A.; Biz, C.; Trisolino, G.; Ruggieri, P.; Grigolo, B.; Ramonda, R.; et al. Conditioned media from human osteoarthritic synovium induces inflammation in a synoviocyte cell line. Connect. Tissue Res. 2019, 60, 136–145. [Google Scholar] [CrossRef]
- Lee, H.N.; Kim, Y.K.; Kim, G.T.; Ahn, E.; So, M.W.; Sohn, D.H.; Lee, S.G. Neutrophil-to-lymphocyte and platelet-to-lymphocyte ratio as predictors of 12-week treatment response and drug persistence of anti-tumor necrosis factor-alpha agents in patients with rheumatoid arthritis: A retrospective chart review analysis. Rheumatol. Int. 2019, 39, 859–868. [Google Scholar] [CrossRef]
- Kim, A.; Kim, Y.; Kim, G.T.; Ahn, E.; So, M.W.; Sohn, D.H.; Lee, S.G. Platelet-to-lymphocyte ratio and neutrophil-to-lymphocyte ratio as potential makers for digital ulcers and interstitial lung disease in patients with systemic sclerosis: Cross-sectional analysis of data from a prospective cohort study. Rheumatol. Int. 2020, 40, 1071–1079. [Google Scholar] [CrossRef]
- Gao, K.; Zhu, W.; Liu, W.; Ma, D.; Li, H.; Yu, W.; Li, Q.; Cao, Y. The predictive role of monocyte-to-lymphocyte ratio in osteoporosis patient. Medicina 2019, 98, e16793. [Google Scholar] [CrossRef]
- Ma, L.; Pang, X.; Ji, G.; Ma, X.; Li, J.; Chang, Y.; Ma, C. Application of the neutrophil to lymphocyte ratio in the diagnosis and activity determination of ulcerative colitis: A meta-analysis and systematic review. Medicina 2021, 100, e27551. [Google Scholar] [CrossRef]
- Choe, J.Y.; Kim, S.K. Association between Hematological Indicesand Disease Activity in Patients with Rheumatoid Arthritis Treated with Janus Kinase Inhibitors for 24 Weeks. Medicina 2022, 58, 426. [Google Scholar] [CrossRef] [PubMed]
- Peng, Y.F.; Cao, L.; Zeng, Y.H.; Zhang, Z.X.; Chen, D.; Zhang, Q.; Zhu, Y.S. Platelet to lymphocyte ratio and neutrophil to lymphocyte ratio in patients with rheumatoid arthritis. Open Med. 2015, 10, 249–253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, H.; Qin, B.; Hu, Z.; Ma, N.; Yang, M.; Wei, T.; Tang, Q.; Huang, Y.; Huang, F.; Liang, Y.; et al. Neutrophil- and platelet-to-lymphocyte ratios are correlated with disease activity in rheumatoid arthritis. Clin. Lab. 2015, 61, 269–273. [Google Scholar] [CrossRef] [PubMed]
- Xu, Z.; Zhang, J.; Zhong, Y.; Mai, Y.; Huang, D.; Wei, W.; Huang, J.; Zhao, P.; Lin, F.; Jin, J. Predictive value of the monocyte-to-lymphocyte ratio in the diagnosis of prostate cancer. Medicina 2021, 100, e27244. [Google Scholar] [CrossRef]
- Deng, Y.; Li, W.; Liu, X.; Ma, G.; Wu, Q.; Chen, F.; Wang, Z.; Zhou, Q. The combination of platelet count and lymphocyte to monocyte ratio is a prognostic factor in patients with resected breast cancer. Medicina 2020, 99, e18755. [Google Scholar] [CrossRef]
- Velidedeoglu, M.; Kundaktepe, B.P.; Aksan, H.; Uzun, H. Preoperative Fibrinogen and Hematological Indexes in the Differential Diagnosis of Idiopathic Granulomatous Mastitis and Breast Cancer. Medicina 2021, 57, 698. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T.; Miyake, M.; Hori, S.; Ichikawa, K.; Omori, C.; Iemura, Y.; Owari, T.; Itami, Y.; Nakai, Y.; Anai, S.; et al. Clinical Impact of Sarcopenia and Inflammatory/Nutritional Markers in Patients with Unresectable Metastatic Urothelial Carcinoma Treated with Pembrolizumab. Diagnostics 2020, 10, 310. [Google Scholar] [CrossRef] [PubMed]
- Radulescu, D.; Baleanu, V.D.; Padureanu, V.; Radulescu, P.M.; Bordu, S.; Patrascu, S.; Socea, B.; Bacalbasa, N.; Surlin, M.V.; Georgescu, I.; et al. Neutrophil/Lymphocyte Ratio as Predictor of Anastomotic Leak after Gastric Cancer Surgery. Diagnostics 2020, 10, 799. [Google Scholar] [CrossRef]
- Li, L.H.; Chen, C.T.; Chang, Y.C.; Chen, Y.J.; Lee, I.H.; How, C.K. Prognostic role of neutrophil-to-lymphocyte ratio, platelet-to-lymphocyte ratio, and systemic immune inflammation index in acute ischemic stroke: A STROBE-compliant retrospective study. Medicina 2021, 100, e26354. [Google Scholar] [CrossRef]
- Yang, Z.; Zhang, Z.; Lin, F.; Ren, Y.; Liu, D.; Zhong, R.; Liang, Y. Comparisons of neutrophil-, monocyte-, eosinophil-, and basophil-lymphocyte ratios among various systemic autoimmune rheumatic diseases. APMIS 2017, 125, 863–871. [Google Scholar] [CrossRef]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010, 62, 2569–2581. [Google Scholar] [CrossRef] [PubMed]
- Fischer, J.E.; Bachmann, L.M.; Jaeschke, R. A readers’ guide to the interpretation of diagnostic test properties: Clinical example of sepsis. Intensive Care Med. 2003, 29, 1043–1051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.Y.; Yin, Y.M.; Kuai, S.G.; Shan, Z.B.; Pei, H.; Wang, J. Combination of neutrophil to lymphocyte ratio and platelet to lymphocyte ratio as diagnostic biomarker for rheumatoid arthritis. Int. J. Clin. Exp. Med. 2016, 9, 22076–22081. [Google Scholar]
- Chen, Q.; Chen, D.Y.; Xu, X.Z.; Liu, Y.Y.; Yin, T.T.; Li, D. Platelet/Lymphocyte, Lymphocyte/Monocyte, and Neutrophil/Lymphocyte Ratios as Biomarkers in Patients with Rheumatoid Arthritis and Rheumatoid Arthritis-Associated Interstitial Lung Disease. Med. Sci. Monit. 2019, 25, 6474–6481. [Google Scholar] [CrossRef]
- Erre, G.L.; Buscetta, G.; Mangoni, A.A.; Castagna, F.; Paliogiannis, P.; Oggiano, M.; Carru, C.; Passiu, G.; Zinellu, A. Diagnostic accuracy of different blood cells-derived indexes in rheumatoid arthritis: A cross-sectional study. Medicina 2020, 99, e22557. [Google Scholar] [CrossRef]
- Jin, Z.; Cai, G.; Zhang, P.; Li, X.; Yao, S.; Zhuang, L.; Ren, M.; Wang, Q.; Yu, X. The value of the neutrophil-to-lymphocyte ratio and platelet-to-lymphocyte ratio as complementary diagnostic tools in the diagnosis of rheumatoid arthritis: A multicenter retrospective study. J. Clin. Lab. Anal. 2021, 35, e23569. [Google Scholar] [CrossRef]
- Targonska-Stepniak, B.; Zwolak, R.; Piotrowski, M.; Grzechnik, K.; Majdan, M. The Relationship between Hematological Markers of Systemic Inflammation (Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte, Lymphocyte-to-Monocyte Ratios) and Ultrasound Disease Activity Parameters in Patients with Rheumatoid Arthritis. J. Clin. Med. 2020, 9, 2760. [Google Scholar] [CrossRef]
- Li, M.; Xie, L. Correlation between NLR, PLR, and LMR and Disease Activity, Efficacy Assessment in Rheumatoid Arthritis. Evid. Based Complement Altern. Med. 2021, 2021, 4433141. [Google Scholar] [CrossRef]
- Lijuan, W.; Yuting, Z.; Chaoyang, L.; Ju, Y. Neutrophil-lymphocyte, platelet-lymphocyte and lymphocyte-monocyte ratios may not be useful markers to assess disease activity in rheumatoid arthritis: A STROBE-compliant article. Medicina 2021, 100, e27631. [Google Scholar] [CrossRef]
- Fu, X.; Liu, H.; Huang, G.; Dai, S.S. The emerging role of neutrophils in autoimmune-associated disorders: Effector, predictor, and therapeutic targets. MedComm 2021, 2, 402–413. [Google Scholar] [CrossRef]
- O’Neil, L.J.; Kaplan, M.J. Neutrophils in Rheumatoid Arthritis: Breaking Immune Tolerance and Fueling Disease. Trends Mol. Med. 2019, 25, 215–227. [Google Scholar] [CrossRef] [PubMed]
- Olumuyiwa-Akeredolu, O.O.; Page, M.J.; Soma, P.; Pretorius, E. Platelets: Emerging facilitators of cellular crosstalk in rheumatoid arthritis. Nat. Rev. Rheumatol. 2019, 15, 237–248. [Google Scholar] [CrossRef] [PubMed]
- Kondo, N.; Kuroda, T.; Kobayashi, D. Cytokine Networks in the Pathogenesis of Rheumatoid Arthritis. Int. J. Mol. Sci. 2021, 22, 922. [Google Scholar] [CrossRef]
- Tu, J.; Huang, W.; Zhang, W.; Mei, J.; Zhu, C. A Tale of Two Immune Cells in Rheumatoid Arthritis: The Crosstalk Between Macrophages and T Cells in the Synovium. Front. Immunol. 2021, 12, 655477. [Google Scholar] [CrossRef] [PubMed]
- Kemble, S.; Croft, A.P. Critical Role of Synovial Tissue-Resident Macrophage and Fibroblast Subsets in the Persistence of Joint Inflammation. Front. Immunol. 2021, 12, 715894. [Google Scholar] [CrossRef] [PubMed]
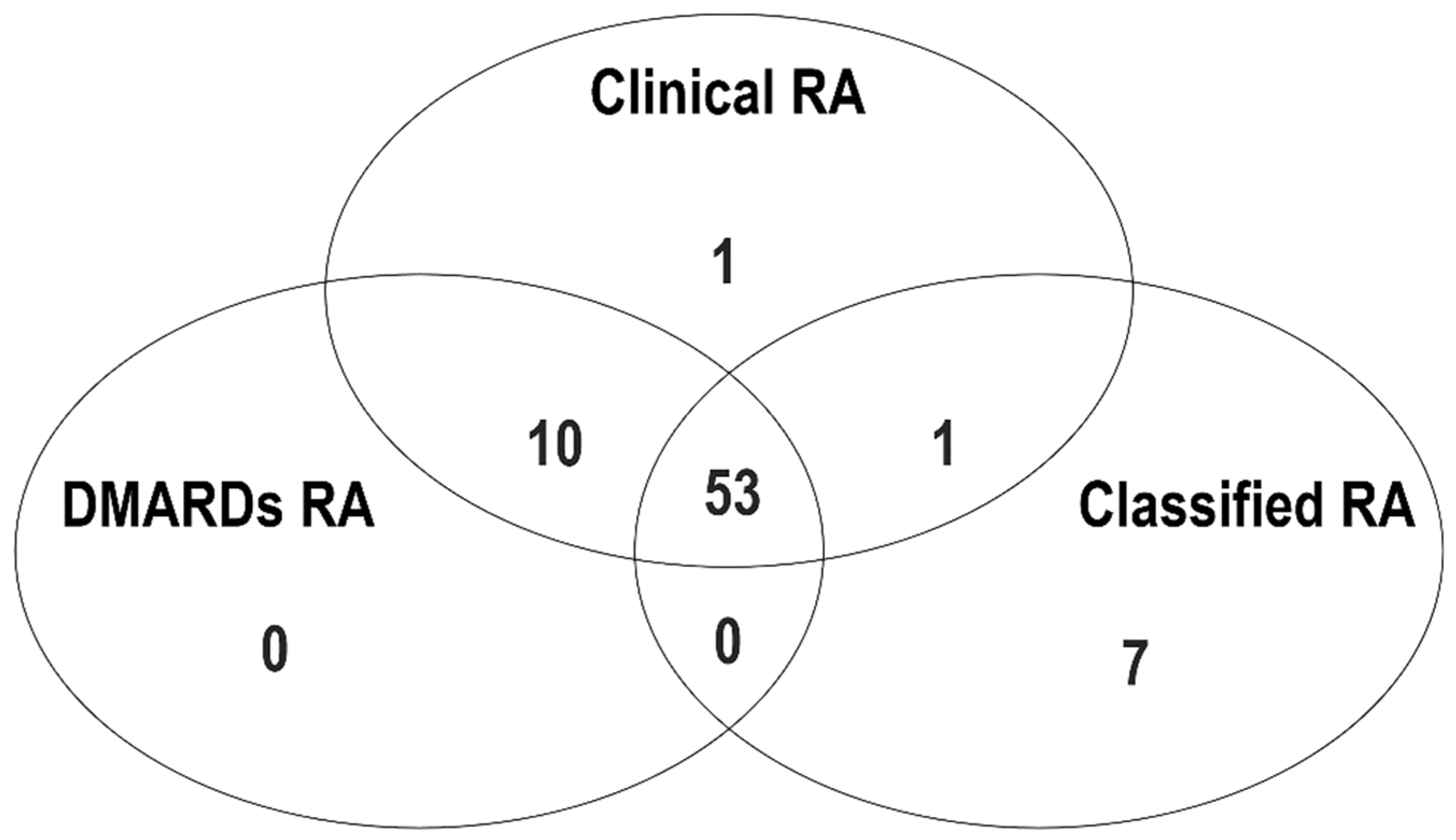
| UIA (n = 201) | Controls (n = 280) | p Value | |
|---|---|---|---|
| Age, years, mean ± SD | 58.8 ± 12.9 | 58 ± 8.2 | 0.621 |
| WBC, 103/uL, median, (IQR) | 6.46 (5.00–8.14) | 5.01 (4.24–5.83) | <0.001 |
| Platelet, 106/uL, median, (IQR) | 265 (230–324) | 239 (216–277) | <0.001 |
| NLR, median, (IQR) | 1.96 (1.34–2.69) | 1.32 (1.01–1.70) | <0.001 |
| PLR, median, (IQR) | 135.54 (110.1–188.2) | 126.18 (102.99–151.2) | <0.001 |
| MLR, median, (IQR) | 0.23 (0.18–0.32) | 0.15 (0.12–0.19) | <0.001 |
| CRP, mg/dL, median, (IQR) | 0.08 (0.03–0.62) | 0.03 (0.02–0.07) | <0.001 |
| ESR, mm/h, median, (IQR) | 16 (7–34) | ||
| Sx duration, month, median, (IQR) | 12 (5–24) | ||
| SJC, n, median, (IQR) | 1 (1–2) | ||
| TJC, n, median, (IQR) | 2 (1–4) | ||
| Small-joint involvement, n, median, (IQR) | 2 (1–2) | ||
| Large-joint involvement, n, median, (IQR) | 0 (0–1) | ||
| RF, IU/mL, median, (IQR) | 9.5 (7.0–23.2) | ||
| RF positivity, n (%) | 69 (34.3) | ||
| Anti-CCP, U/mL, median, (IQR) | 28.4 (0.2–10.8) | ||
| Anti-CCP positivity, n (%) | 55 (27.4) | ||
| 2010 ACR/EULAR criteria score, median, (IQR) | 4.0 (3–6) | ||
| Clinical RA, n (%) | 65 (32.3) | ||
| DMARDs RA, n (%) | 63 (31.3) | ||
| Classified RA, n (%) | 61 (30.3) |
| Parameter | SJC | TJC | Sx Duration, Month | ESR, mm/h | CRP, mg/dL | RF, IU/mL | Anti-CCP Ab, U/mL | Criteria Score | |
|---|---|---|---|---|---|---|---|---|---|
| NLR | ρ p-value | 0.251 <0.001 | 0.123 0.081 | −0.14 0.047 | 0.395 <0.001 | 0.513 <0.001 | 0.197 0.005 | 0.229 0.001 | 0.208 0.003 |
| PLR | ρ p-value | 0.181 0.01 | 0.055 0.441 | −0.114 0.108 | 0.231 <0.001 | 0.332 <0.001 | 0.124 0.079 | 0.164 0.02 | 0.138 0.05 |
| MLR | ρ p-value | 0.295 <0.001 | 0.229 0.001 | −0.036 0.61 | 0.329 <0.001 | 0.456 <0.001 | 0.241 0.001 | 0.239 0.001 | 0.313 <0.001 |
| Diagnosis | Parameter | Cut-Off | Sensitivity | Specificity | PPV | NPV | AUC | p Value |
|---|---|---|---|---|---|---|---|---|
| Clinical RA | NLR | 2.07 | 66.2% | 64% | 46.7% | 79.8% | 0.661 | <0.001 |
| PLR | 143.26 | 56.9% | 61.8% | 41.6% | 75% | 0.593 | 0.034 | |
| MLR | 0.24 | 64.6% | 62.5% | 45.7% | 78.7% | 0.687 | <0.001 | |
| DMARDs RA | NLR | 2.07 | 66.7% | 63.8% | 45.7% | 80.7% | 0.671 | <0.001 |
| PLR | 143.26 | 57.1% | 61.6% | 40.4% | 75.9% | 0.600 | 0.023 | |
| MLR | 0.24 | 65.1% | 62.3% | 44.1% | 79.6% | 0.701 | <0.001 | |
| Classified RA | NLR | 2.07 | 62.3% | 61.4% | 41.3% | 78.9% | 0.622 | 0.006 |
| PLR | 143.26 | 55.7% | 60.7% | 38.2% | 75.9% | 0.580 | 0.070 | |
| MLR | 0.24 | 62.3% | 60.7% | 40.9% | 78.7% | 0.663 | <0.001 |
| Clinical RA (n = 65) | DMARDs RA (n = 63) | Classified RA (n = 61) | |||||
|---|---|---|---|---|---|---|---|
| NLR ≥ 2.07 | NLR < 2.07 | NLR ≥ 2.07 | NLR < 2.07 | NLR ≥ 2.07 | NLR < 2.07 | ||
| MLR ≥ 0.24 | PLR ≥ 143.26 | 30 (46.1) | 2 (3.1) | 29 (44.6) | 2 (3.1) | 28 (45.9) | 1 (1.5) |
| PLR < 143.26 | 6 (9.2) | 4 (6.2) | 6 (9.2) | 4 (6.2) | 5 (7.7) | 4 (6.2) | |
| MLR < 0.24 | PLR ≥ 143.26 | 3 (4.6) | 1 (1.5) | 3 (4.6) | 1 (1.5) | 2 (3.1) | 3 (4.6) |
| PLR < 143.26 | 4 (6.2) | 15 (23.8) | 4 (6.2) | 14 (21.5) | 3 (4.6) | 15 (23.1) | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, B.-W.; Kim, A.-R.; Kim, Y.-K.; Kim, G.-T.; Ahn, E.-Y.; So, M.-W.; Lee, S.-G. Diagnostic Value of Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte, and Monocyte-to-Lymphocyte Ratios for the Assessment of Rheumatoid Arthritis in Patients with Undifferentiated Inflammatory Arthritis. Diagnostics 2022, 12, 1702. https://doi.org/10.3390/diagnostics12071702
Song B-W, Kim A-R, Kim Y-K, Kim G-T, Ahn E-Y, So M-W, Lee S-G. Diagnostic Value of Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte, and Monocyte-to-Lymphocyte Ratios for the Assessment of Rheumatoid Arthritis in Patients with Undifferentiated Inflammatory Arthritis. Diagnostics. 2022; 12(7):1702. https://doi.org/10.3390/diagnostics12071702
Chicago/Turabian StyleSong, Byung-Wook, A-Ran Kim, Yun-Kyung Kim, Geun-Tae Kim, Eun-Young Ahn, Min-Wook So, and Seung-Geun Lee. 2022. "Diagnostic Value of Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte, and Monocyte-to-Lymphocyte Ratios for the Assessment of Rheumatoid Arthritis in Patients with Undifferentiated Inflammatory Arthritis" Diagnostics 12, no. 7: 1702. https://doi.org/10.3390/diagnostics12071702
APA StyleSong, B.-W., Kim, A.-R., Kim, Y.-K., Kim, G.-T., Ahn, E.-Y., So, M.-W., & Lee, S.-G. (2022). Diagnostic Value of Neutrophil-to-Lymphocyte, Platelet-to-Lymphocyte, and Monocyte-to-Lymphocyte Ratios for the Assessment of Rheumatoid Arthritis in Patients with Undifferentiated Inflammatory Arthritis. Diagnostics, 12(7), 1702. https://doi.org/10.3390/diagnostics12071702






