Abstract
Primary soft-tissue lymphoma (PSTL) is a rare extranodal non-Hodgkin lymphoma, characterized by a mass growing within soft-tissue, which is connective tissue, adipose tissue, and skeletal muscle. Here, we describe a case of biphenotypic lymphoblastic lymphoma arising from soft tissue of the popliteal fossa in an 11-year-old boy. A pediatric review about PSTL revealed that anaplastic large cell lymphoma is the most common histological type and a biphenotypic lymphoblastic lymphoma has not yet been reported in childhood. Lymphoma should always be considered in patients presenting with a soft-tissue mass, and a comprehensive immunohistochemical evaluation, including B-cell, T-cell, and myeloid markers, is needed to make a correct diagnosis and establish the most suitable treatment.
1. Introduction
Primary extranodal non-Hodgkin lymphoma (PE-NHL) typically arises from tissue other than lymph nodes and even from sites that normally do not contain lymphoid tissue; the incidence rate ranges from 24% to 48% of all NHL [1,2]. A variety of PE-NHL is primary soft-tissue lymphoma (PSTL), representing about 0.1% of all malignant lymphomas [3]. It is characterized by a primary mass growing within soft-tissue, which includes connective tissue, adipose tissue, and skeletal muscle. This rare condition must be distinguished from the most frequent event in which soft-tissue is involved secondarily, as a consequence of direct spreading or hematogenous dissemination from a nodal or extranodal lymphoma [4]. The majority of PSTL occurs in adulthood, while children are exceptionally affected [5].
We describe a case of a pediatric PSTL localized in the popliteal fossa, with an outstanding histological characterization; a review about this topic was conducted thereafter.
2. Materials and Methods
Detailed immunophenotyping was performed on paraffin sections from tissue sample; the staining panel included CD2, CD3, CD4, CD5, CD7, CD8, CD10, CD11c, CD13, CD14, CD15, CD19, CD20, CD22, CD33, CD34, CD38, CD43, CD45, CD68, CD79a, CD99, CD117, CD163, myeloperoxidase (MPO), terminal deoxynucleotidyl transferase (TdT), cytoplasmic µ chains, vimentin, PAX5, FLI-1, lysozyme, Myf4, MND116, and EMA.
As regards the molecular cytogenetic screening for chromosomal abnormalities associated with myeloid and lymphoid leukemias, total RNA was isolated using Trizol (Invitrogen); one microgram of RNA was reverse-transcribed into cDNA using the SuperScript II system (Invitrogen) according to the manufacturer’s instructions. Reverse transcriptase-polymerase chain reaction (RT-PCR) was performed for the evaluation of all rearrangements.
A literature review about pediatric PSTL was conducted using PubMed, Scopus, and Web of Science, combining the terms “soft-tissue” AND “non-Hodgkin lymphoma”, “connective tissue” AND “non-Hodgkin lymphoma”, and “skeletal muscle” AND “non-Hodgkin lymphoma”; 12 cases were retrieved.
3. Case Presentation
An 11-year-old boy suffered from a 2-months history of a growing mass in his right popliteal fossa; he did not complain of any other symptoms. Due to progressive increase in size of the popliteal mass, a magnetic resonance imaging (MRI) of the right knee was performed; it revealed a 5 × 4 × 10 cm lesion, localized in the distal region of the posterior thigh and in the popliteal fossa, isointense to muscle on T1-weighted images and with intermediate signal intensity on T2-weighted images, showing intense and not homogeneous contrast enhancement; concomitant edema in subcutaneous adipose tissue and in gastrocnemius muscle fascia was detected (Figure 1A,B). The boy was admitted to another hospital; a needle biopsy was performed, which showed an undifferentiated malignant tumor with small round cells, positive for vimentin and CD99, negative for CD45, CD20, and CD3, suggesting initially the diagnosis of Ewing’s sarcoma/peripheral primitive neuroectodermal tumors (pPNET). Ten days after, the boy came to our attention. On physical examination, a painless, hard, and fixed mass was palpable in the posterior side of the right knee, with evident venous reticulum of the overlying skin, and a clear discrepancy of circumference between the two legs (29 cm in the right one versus 25 cm in the left one) was found; no enlarged lymph nodes were noticed. Blood examinations were all in normal range as well as bilateral bone marrow aspirates; a new incisional biopsy of the mass was performed, and the immunohistochemical evaluation revealed positivity for TdT, B-lineage markers (CD79a, PAX5, CD19, CD22, and CD10), myeloid marker (MPO), CD34, CD43, FLI-1, and, at least focally, CD33 and CD117 (Figure 2, Figure 3 and Figure 4); the tissue was negative for CD38, intracytoplasmic mu chains, CD13, CD15, monocyte/macrophage lineage markers (CD68, CD11c, CD14, lysozyme, and CD163), T-lineage markers (CD2, CD3, CD4, CD5, CD7, and CD8), Myf4, MND116, and EMA. Proliferation index (Ki67) was 50%. Clonal rearrangement of immunoglobulin heavy chain genes was identified by polymerase chain reaction (PCR) of framework regions (FR) and a dominant peak of 107 bp and 247 bp was seen in FR3 and FR2, respectively. Notably, no residual lymph nodal architecture was observed in all examined tissue sections. Biopsy and bone marrow samples were also screened for the presence of core-binding factor (CBF)-β abnormalities, t(8;21)RUNX1-RUNX1T1, inv(16)CBFB-MYH11, t(15;17)PML-RARA, t(1;19)E2A-PBX1, t(9;22)BCR-ABL p190/p210, t(12;21)TEL-AML1, MLL translocations, and several KMT2A and NUP98 rearrangements [6,7,8]; the internal tandem duplication of FLT3 mutation (FLT3-ITD) and NPM1 mutations were also screened. No genetic mutation was found. According to the WHO criteria [9], a biphenotypic lymphoblastic lymphoma (B/Myeloid) was diagnosed. The staging procedure was completed with thorax–abdomen computerized tomography (CT) scan, whole-body positron emission tomography (PET)-CT scan, and 99Tc-MDP bone scan; no other organ involvement was ascertained. Based upon these findings, in order to use chemotherapy agents effective in both lymphoid and myeloid neoplasms, the patient was started on AIEOP LLA REC 2003 protocol, S2 group, arm B (Table 1). After induction therapy, the clinical and radiological disappearance of the popliteal lesion was appreciated; furthermore, at the end of the chemotherapy blocks, knee MRI and PET-CT confirmed no evidence of disease. The patient has been in continuous complete remission for more than 10 years from stop therapy.
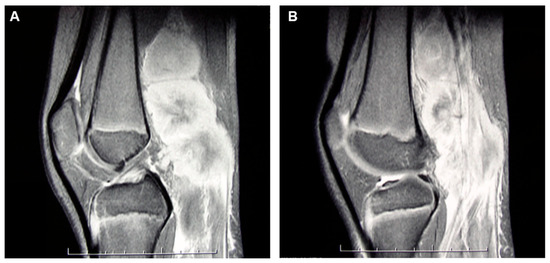
Figure 1.
(A,B). MRI of the right knee revealed a 5 × 4 × 10 cm multinodular lesion localized in the distal region of the posterior thigh and in the popliteal fossa, isointense to muscle on T1-weighted images and with intermediate signal intensity on T2-weighted images, showing intense and not homogeneous contrast enhancement.
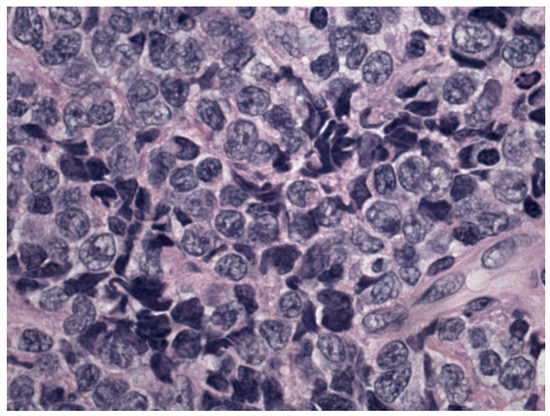
Figure 2.
Morphological details investigated using hematoxylin and eosin staining: monomorphic population of blasts.
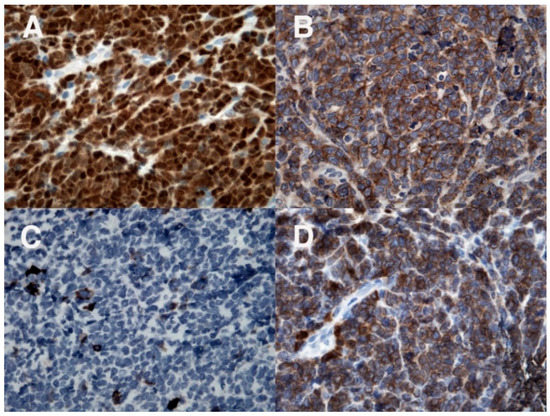
Figure 3.
Immunohistochemical evaluation: positivity for TdT (A) and B-cell markers CD19 (B) and CD79a (D); CD20 (C) is negative.
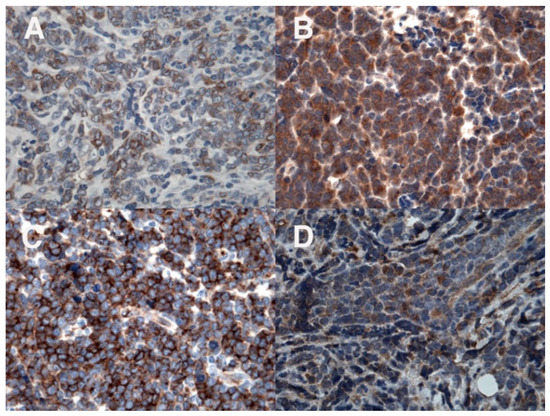
Figure 4.
Immunohistochemical evaluation: positivity for the myeloid marker MPO (A); in addition, positivity for CD33 (B), CD34 (C), and CD117 (D).

Table 1.
Therapy courses according to the S2 group/arm B of the AIEOP ALL REC/2003 trial. Our patient received the following chemotherapy: phase IA-IDA, R2 block, R1 block, R2 block, R1 block, R2 block, R1 block, maintenance.
4. Discussion
Soft-tissues are uncommon primary sites for NHL, and most of the available information concerns the adult population. In adulthood, PSTL more often affects the extremities, especially the lower limbs, without any sex difference, diffuse large B-cell lymphoma (DLBCL) being the most frequent histological subtype [10].
After literature search, no pediatric review about this topic was found; the discovered case reports are listed in Table 2 [11,12,13,14,15,16,17,18,19,20,21,22] and, including our patient, we identified 13 pediatric PSTL, with a slight predominance of female sex (54%). The median and mean age were 11 and 11.3 years, respectively. In 9 out of 13 children (69%), lymphoma was localized in the extremities, five in the lower limb, two in the upper limb, and two in both upper and lower limbs. Except in our case, all the patients had anaplastic large cell lymphoma (ALCL) as histological subtype (ALK-positive in seven patients). The treatment of choice was chemotherapy alone in 10/13 (77%); chemotherapy was associated with surgery in one case and with autologous stem cell transplantation in another child. In one patient, therapy was not specified [13]. Follow-up was detailed in nine children: seven (78%) achieved complete remission, one relapsed after 6 months from diagnosis and another one died of disease progression less than 2 months after diagnosis. Based on the collected data, pediatric PSTL differs from its adult counterpart in at least two main respects: in adulthood, the prognosis is poor and DLBCL occurs in more than half of all cases [5], while in children, PSTL presents a high rate of survival and ALCL is the predominant histology. In a large international cohort of 420 children and adolescents with ALCL, 48 patients without lymph node involvement at the diagnosis were recognized, of which 40% were soft tissue masses; also in this study, a better prognosis of this rare site of presentation was reported [23].

Table 2.
Pediatric patients affected by PSTL reported in the literature.
In a patient presenting with a soft-tissue mass, the most common diagnostic hypothesis is soft-tissue sarcoma, especially desmoid tumors, rhabdomyosarcoma, synovial sarcoma, and Ewing’s sarcoma/pPNET; another differential diagnosis includes neuroblastoma. All these neoplasms belong to the group of small round blue cells tumors, characterized by some degree of cytomorphologic and immunophenotypical overlap [24], but with radically different therapeutic approaches. For example, in lymphoblastic lymphoma (LBL), malignant cells can form rosette-like structures and express CD99 and FLI-1, mimicking Ewing sarcoma [25,26]. The use of a limited immunohistochemical panel is a potential diagnostic pitfall and should be avoided, because a misdiagnosis leads to incorrect treatment and devastating outcomes [27]. To prevent this, small round blue cells tumors should be tested for TdT, CD79a, and CD43; the study of B and T-cell receptor gene rearrangements can also be helpful to distinguish LBL from Ewing sarcoma [28,29].
LBL is the second most common type of NHL in children and adolescents; in around 70–80% of cases, it is of T-lymphoblastic origin and in 20–30%, it arises from B lymphoblasts [30]. Biphenotypic LBL or mixed lineage lymphoma is defined by the presence of tumor cells expressing markers of two lineages, including B-lymphoid, T-lymphoid, and myeloid. It is very rare, even though the frequency is probably underestimated; in a study on 146 pediatric LBLs, an unexpected large number of mixed lineage lymphomas was recognized (7%), B-cell/myeloid differentiation being the most frequent phenotype [31]. For the identification of biphenotypic LBL, an extensive histological evaluation with a systematic staining for all the hematopoietic lineages and molecular genetic analysis should always be performed, as demonstrated in our patient. As regards the treatment, the use of drugs active against lymphoid and myeloid neoplasms seems to be effective in achieving complete remission in the majority of leukemias of ambiguous lineage [32]; this clinical evidence justifies our therapeutic choice, which proved successful.
5. Conclusions
Pediatric PSTL is an extremely rare disease, characterized by a favorable prognosis in the majority of patients. In the presence of a soft-tissue mass, histological examination, including an extensive immunohistochemistry panel for lymphoma, and molecular genetic analysis are essential to make a correct diagnosis. In our case, unique because of both onset site (popliteal fossa) and histological characterization (biphenotypic LBL), the choice of a chemotherapy regimen active against both lymphoid and myeloid neoplastic cells showed to be highly effective.
Author Contributions
Conceptualization, P.F., M.P. (Marta Pillon) and G.A.R.; methodology, L.M., A.Z., M.P. (Martina Pigazzi) and E.S.G.D.; validation, P.D.; investigation, P.F. and G.A.R.; data curation, E.C.; writing—original draft, P.F. and G.A.R.; writing—review and editing, L.M., P.D., A.Z., M.P. (Martina Pigazzi), E.C., E.S.G.D. and M.P. (Marta Pillon). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
All procedures followed were in accordance with the ethical standards of the committee in charge of human experimentation (institutional and national) and the Declaration of Helsinki.
Informed Consent Statement
Informed consent was obtained from the subject involved in the study.
Data Availability Statement
The data that support the findings of this study are available on request from the corresponding author. The data are not publicly available due to privacy or ethical restrictions.
Acknowledgments
The Parents’ Association A.S.L.T.I. (Associazione Siciliana Leucemie e Tumori Infantili) is acknowledged for supporting the activity of the Pediatric Onco-Hematology Unit of A.R.N.A.S. Ospedali Civico, Di Cristina e Benfratelli.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Zucca, E.; Roggero, E.; Bertoni, F.; Cavalli, F. Primary extranodal non-Hodgkin’s lymphomas. Part 1: Gastrointestinal, cutaneous and genitourinary lymphomas. Ann. Oncol. 1997, 8, 727–737. [Google Scholar] [CrossRef]
- Zucca, E.; Roggero, E.; Bertoni, F.; Conconi, A.; Cavalli, F. Primary extranodal non-Hodgkin’s lymphomas. Part 2: Head and neck, central nervous system and other less common sites. Ann. Oncol. 1999, 10, 1023–1033. [Google Scholar] [CrossRef] [PubMed]
- Travis, W.D.; Banks, P.M.; Reiman, H.M. Primary extranodal soft tissue lymphoma of the extremities. Am. J. Surg. Pathol. 1987, 11, 359–366. [Google Scholar] [CrossRef] [PubMed]
- Salamao, D.R.; Nascimento, A.G.; Lloyd, R.V.; Chen, M.G.; Habermann, T.M.; Strickler, J.G. Lymphoma in soft tissue: A clinicopathologic study of 19 cases. Hum. Pathol. 1996, 27, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Derenzini, E.; Casadei, B.; Pellegrini, C.; Argnani, L.; Pileri, S.; Zinzani, P.L. Non-hodgkin lymphomas presenting as soft tissue masses: A single center experience and meta-analysis of the published series. Clin. Lymphoma Myeloma Leuk. 2013, 13, 258–265. [Google Scholar] [CrossRef]
- Bisio, V.; Zampini, M.; Tregnago, C.; Manara, E.; Salsi, V.; Di Meglio, A.; Masetti, R.; Togni, M.; Di Giacomo, D.; Minuzzo, S.; et al. NUP98-fusion transcripts characterize different biological entities within acute myeloid leukemia: A report from the AIEOP-AML group. Leukemia 2017, 31, 974–977. [Google Scholar] [CrossRef] [PubMed]
- Pession, A.; Masetti, R.; Rizzari, C.; Putti, M.C.; Casale, F.; Fagioli, F.; Luciani, M.; Lo Nigro, L.; Menna, G.; Micalizzi, C.; et al. Results of the AIEOP AML 2002/01 multicenter prospective trial for the treatment of children with acute myeloid leukemia. Blood 2013, 122, 170–178. [Google Scholar] [CrossRef] [PubMed]
- Pigazzi, M.; Manara, E.; Bisio, V.; Aveic, S.; Masetti, R.; Menna, G.; Zecca, M.; Pession, A.; Locatelli, F.; Basso, G. Screening of novel genetic aberrations in pediatric acute myeloid leukemia: A report from the AIEOP AML-2002 study group. Blood 2012, 120, 3860–3862. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Borowitz, M.J.; Bene, M.C.; Harris, N.L.; Porwit, A.; Matutes, E. Acute leukemias of ambiguous lineage. In WHO Classification of Tumours of Haematopoietic and Lymphoid Tissues; Swerdlow, S.H., Campo, E., Harris, N.L., Jaffe, E.S., Pileri, S.A., Stein, H., Thiele, J., Eds.; IARC Press: Lyon, France, 2017. [Google Scholar]
- Yang, J.; Zhang, F.; Fang, H.; Ye, Z.; Lin, S.; Han, A. Clinicopathologic features of primary lymphoma in soft tissue. Leuk. Lymphoma 2010, 51, 2039–2046. [Google Scholar] [CrossRef]
- Winter, S.S.; Duncan, M.H.; Foucar, E.; McConnell, T.S.; Cartwright, K.C. Childhood Ki-1 lymphoma: Presentation as a buttock mass. Am. J. Pediatr. Hematol. Oncol. 1991, 13, 334–337. [Google Scholar] [CrossRef]
- Evans, G.R.; King, J.C.; McGinnis, M.R.; Wilgis, E.F. Primary non-Hodgkin’s lymphoma presenting in the upper extremity: A case report. J. Hand Surg. Am. 1993, 18, 612–614. [Google Scholar] [CrossRef]
- Chew, F.S.; Schellingerhout, D.; Keel, S.B. Primary lymphoma of skeletal muscle. AJR Am. J. Roentgenol. 1999, 172, 1370. [Google Scholar] [CrossRef][Green Version]
- Ishii, E.; Honda, K.; Nakagawa, A.; Urago, K.; Oshima, K. Primary CD30/Ki-1 positive anaplastic large cell lymphoma of skeletal muscle with der(17)t(1;17)(q11;p11). Cancer Genet. Cytogenet. 2000, 122, 116–120. [Google Scholar] [CrossRef]
- Menon, B.S.; Maziah, W.; Samarendra, M.; Toha, A. Pathological case of the month. Ki-1-positive anaplastic large cell lymphoma involving muscle. Arch. Pediatr. Adolesc. Med. 2001, 155, 411–412. [Google Scholar] [CrossRef][Green Version]
- Driss, M.; Abbes, I.; Mrad, K.; Sassi, S.; Oubich, F.; Barsaoui, S.; Romdhane, K.B. Primary CD30/ALK-1 positive anaplastic large cell lymphoma of the skeletal muscle in a child. Pathologica 2009, 101, 97–100. [Google Scholar]
- Wu, L.; Wang, Y.; Fu, S.L.; Huang, L.; Tongji, F.C.; Qi, J.Y. Anaplastic large cell lymphoma with primary involvement of skeletal muscle: A rare case report and review of the literature. Pediatr. Hematol. Oncol. 2009, 26, 142–149. [Google Scholar] [CrossRef] [PubMed]
- Rekhi, B.; Sridhar, E.; Viswanathan, S.; Shet, T.M.; Jambhekar, N.A. ALK+ anaplastic large cell lymphoma with cohesive, perivascular arrangements on cytology, mimicking a soft tissue sarcoma: A report of 2 cases. Acta Cytol. 2010, 54, 75–78. [Google Scholar] [CrossRef]
- Gaiser, T.; Geissinger, E.; Schattenberg, T.; Scharf, H.P.; Dürken, M.; Dinter, D.; Rosenwald, A.; Marx, A. Case report: A unique pediatric case of a primary CD8 expressing ALK-1 positive anaplastic large cell lymphoma of skeletal muscle. Diagn. Pathol. 2012, 7, 38. [Google Scholar] [CrossRef]
- Kounami, S.; Shibuta, K.; Yoshiyama, M.; Mitani, Y.; Watanabe, T.; Takifuji, K.; Yoshikawa, N. Primary anaplastic large cell lymphoma of the psoas muscle: A case report and literature review. Acta Haematol. 2012, 127, 186–188. [Google Scholar] [CrossRef]
- Pasricha, S.; Gandhi, J.S.; Gupta, G.; Mehta, A. Small cell variant of anaplastic large cell lymphoma presenting as arm mass in a child: A rare entity with diagnostic challenge. J. Cancer Res. Ther. 2013, 9, 317–319. [Google Scholar] [CrossRef]
- Gupta, D.; Vishwajeet, V.; Ramzan, M.; Elhence, P.A. ALK-positive anaplastic large cell lymphoma of skeletal muscle masquerading as soft tissue sarcoma. BMJ Case Rep. 2022, 15, e245685. [Google Scholar] [CrossRef] [PubMed]
- Mussolin, L.; Le Deley, M.C.; Carraro, E.; Damm-Welk, C.; Attarbaschi, A.; Williams, D.; Burke, A.; Horibe, K.; Nakazawa, A.; Wrobel, G.; et al. Prognostic Factors in Childhood Anaplastic Large Cell Lymphoma: Long Term Results of the International ALCL99 Trial. Cancers 2020, 12, 2747. [Google Scholar] [CrossRef]
- McGahey, B.E.; Moriarty, A.T.; Nelson, W.A.; Hull, M.T. Fine-needle aspiration biopsy of small round blue cell tumors of childhood. Cancer 1992, 69, 1067–1073. [Google Scholar] [CrossRef] [PubMed]
- Ozdemirli, M.; Fanburg-Smith, J.C.; Hartmann, D.P.; Shad, A.T.; Lage, J.M.; Magrath, I.T.; Azumi, N.; Harris, N.L.; Cossman, J.; Jaffe, E.S. Precursor B-Lymphoblastic lymphoma presenting as a solitary bone tumor and mimicking Ewing’s sarcoma: A report of four cases and review of the literature. Am. J. Surg. Pathol. 1998, 22, 795–804. [Google Scholar] [CrossRef]
- Folpe, A.L.; Hill, C.E.; Parham, D.M.; O’Shea, P.A.; Weiss, S.W. Immunohistochemical detection of FLI-1 protein expression: A study of 132 round cell tumors with emphasis on CD99-positive mimics of Ewing’s sarcoma/primitive neuroectodermal tumor. Am. J. Surg. Pathol. 2000, 24, 1657–1662. [Google Scholar] [CrossRef]
- Magro, G.; Longo, F.R.; Angelico, G.; Spadola, S.; Amore, F.F.; Salvatorelli, L. Immunohistochemistry as potential diagnostic pitfall in the most common solid tumors of children and adolescents. Acta Histochem. 2015, 117, 397–414. [Google Scholar] [CrossRef]
- Ozdemirli, M.; Fanburg-Smith, J.C.; Hartmann, D.P.; Azumi, N.; Miettinen, M. Differentiating lymphoblastic lymphoma and Ewing’s sarcoma: Lymphocyte markers and gene rearrangement. Mod. Pathol. 2001, 14, 1175–1182. [Google Scholar] [CrossRef][Green Version]
- Lucas, D.R.; Bentley, G.; Dan, M.E.; Tabaczka, P.; Poulik, J.M.; Mott, M.P. Ewing sarcoma vs lymphoblastic lymphoma. A comparative immunohistochemical study. Am. J. Clin. Pathol. 2001, 115, 11–17. [Google Scholar] [CrossRef]
- Burkhardt, B.; Hermiston, M.L. Lymphoblastic lymphoma in children and adolescents: Review of current challenges and future opportunities. Br. J. Haematol. 2019, 185, 1158–1170. [Google Scholar] [CrossRef]
- Oschlies, I.; Burkhardt, B.; Chassagne-Clement, C.; d’Amore, E.S.; Hansson, U.; Hebeda, K.; Mc Carthy, K.; Kodet, R.; Maldyk, J.; Müllauer, L.; et al. Diagnosis and immunophenotype of 188 pediatric lymphoblastic lymphomas treated within a randomized prospective trial: Experiences and preliminary recommendations from the European childhood lymphoma pathology panel. Am. J. Surg. Pathol. 2011, 35, 836–844. [Google Scholar] [CrossRef]
- Al-Seraihy, A.S.; Owaidah, T.M.; Ayas, M.; El-Solh, H.; Al-Mahr, M.; Al-Ahmari, A.; Belgaumi, A.F. Clinical characteristics and outcome of children with biphenotypic acute leukemia. Haematologica 2009, 94, 1682–1690. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).