Gene Expression Profile of Stromal Factors in Cancer-Associated Fibroblasts from Prostate Cancer
Abstract
1. Introduction
2. Material and Methods
2.1. Patients
- Seven patients with BPH underwent prostate TUR (transurethral resection; mean age: 72 years; sd: 9.38), where TUR samples were collected.
- Twenty-three patients with suspected PCa who underwent radical prostatectomy (mean age: 66.96 years; sd: 6.57). These patients were selected with an estimated survival of more than 10 years since the tumor was clinically localized (cT2 or less; PSA < 20 nanograms per milliliter (ng/mL). Patients with estimated survival under 10 years, with significant local involvement by rectal examination or transrectal ultrasound, with PSA > 20 ng/mL and/or suspected lymph node or metastasis in imaging studies (CT and bone scan), with previous neoplasms, with neoadjuvant therapy, with development of a second primary tumor, or with an insufficient amount of tissue obtained for culture cells were excluded. In the eventually selected patients, open radical or laparoscopic prostatectomy was performed using standardized techniques. Pelvic lymphadenectomy was performed when the PSA was above 10 ng/mL and/or a Gleason score greater than 6 was found in the biopsy sample. Once the radical prostatectomy was performed, the stage and the Gleason pattern of the specimen, the presence of perineural or seminal vesicles invasion, and the occurrence of positive margins or pathological lymph node involvement (when lymphadenectomy was performed) were analyzed.
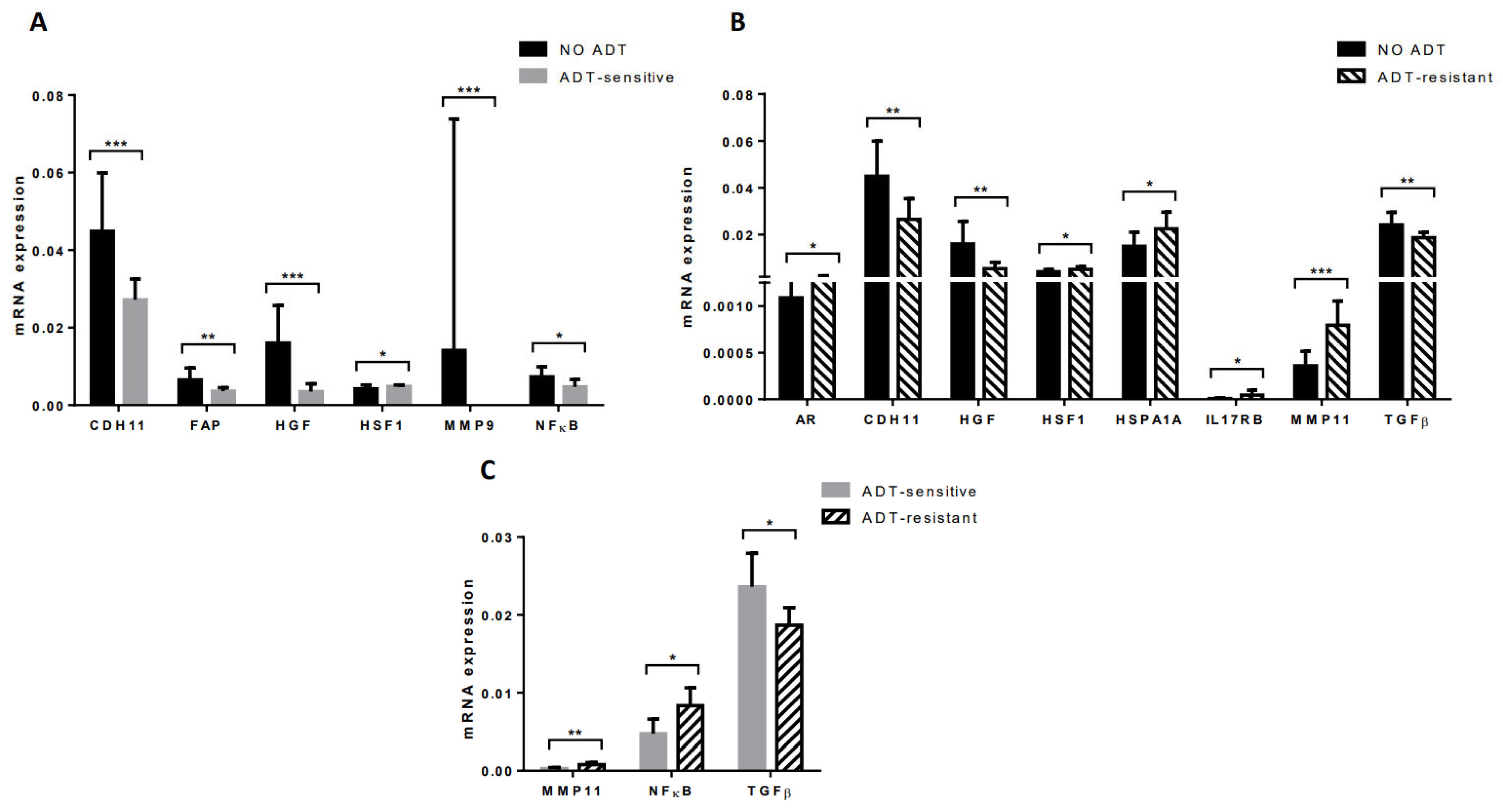
- Fourteen advanced PCa patients already diagnosed and treated with androgen deprivation therapy (ADT; mean age: 76.71 years; sd: 8.07). A new transrectal ultrasound biopsy for prostate carcinoma was performed as described below. ADT-sensitive prostate tumors were defined as having indetectable PSA and tumor disappearance upon image-based diagnosis. ADT-resistant prostate tumors were selected based on the European Association of Urology (EAU) criteria: serum testosterone castration levels (testosterone < 50 ng/dL) and three consecutive rises of PSA 1 week apart, resulting in two 50% increases over the nadir, with a PSA > 2 ng/mL or appearance of two or more lesions on bone scan or soft tissue lesions. The median follow-up for patients with ADT-sensible tumors was 38 months (range of 2–93) and 21 months (range of 20–30) for patients with ADT-resistant tumors.
2.2. Isolation and Culture of Fibroblasts from Surgical or Biopsy Specimens
2.3. qRT-PCR
2.4. Immunostaining Analyses
2.5. Statistical Analyses
3. Results
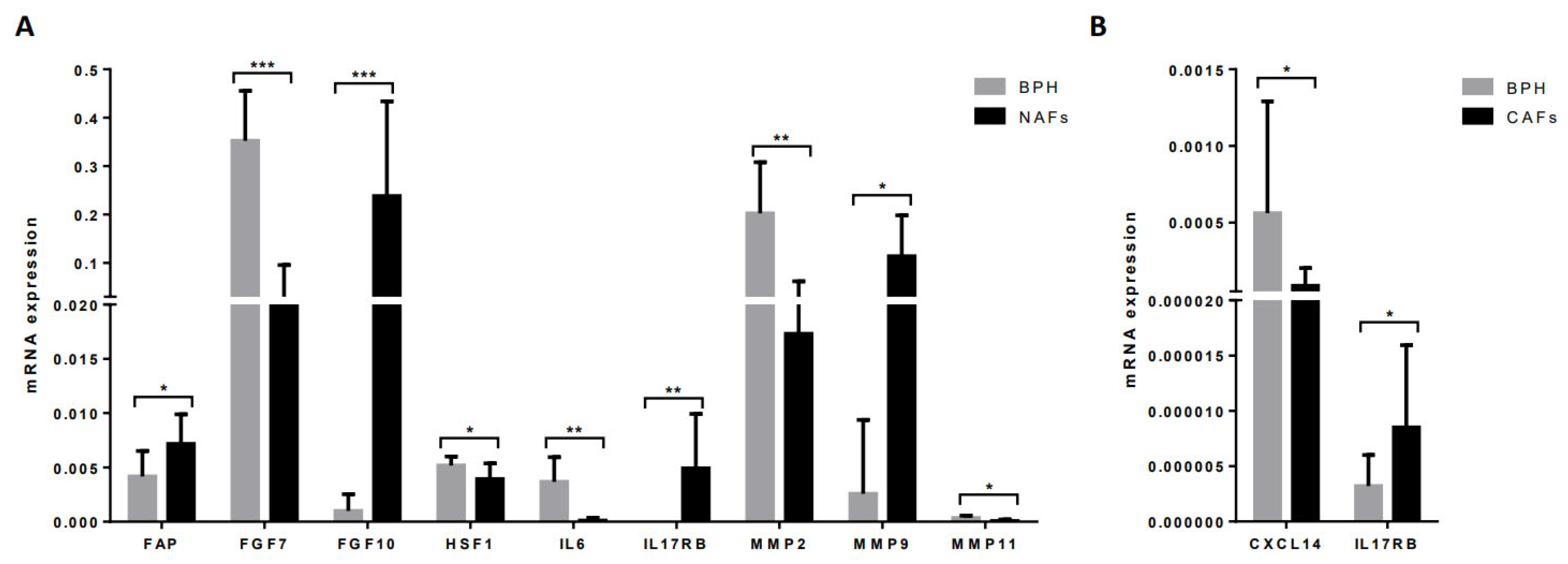
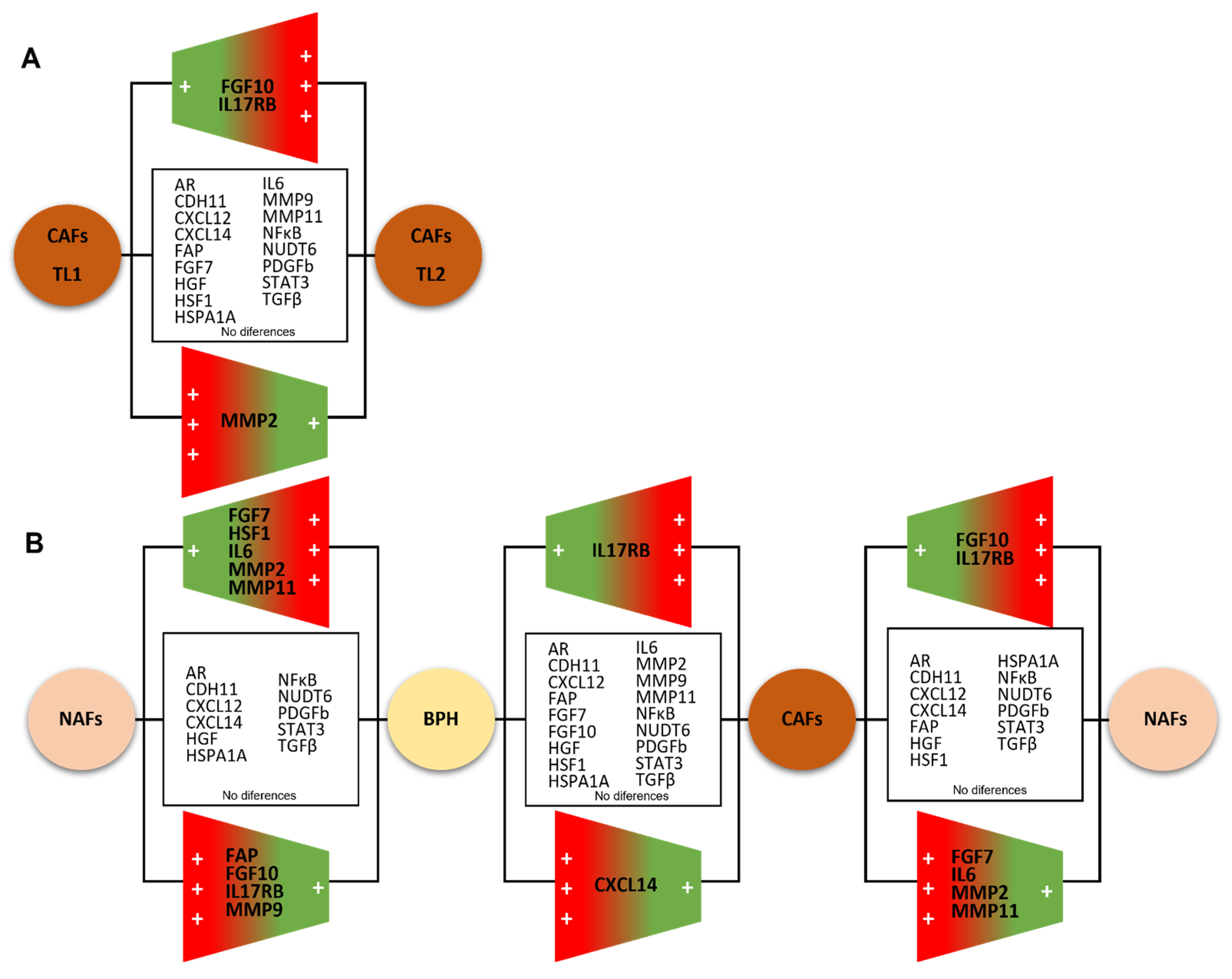
3.1. Molecular Profile of CAFs and Paired NAFs
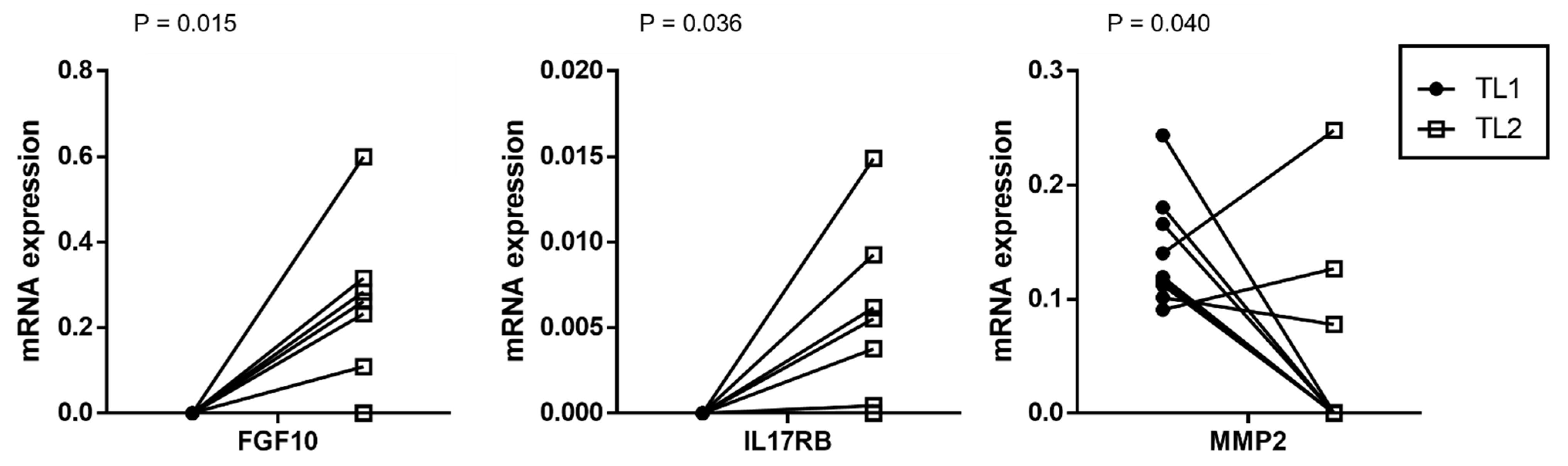
3.2. Comparing the Gene Expression Profile of CAFs from Multifocal Tumors
3.3. Comparing the Gene Expression Profile of NAFs or CAFs with Fibroblasts from BPH
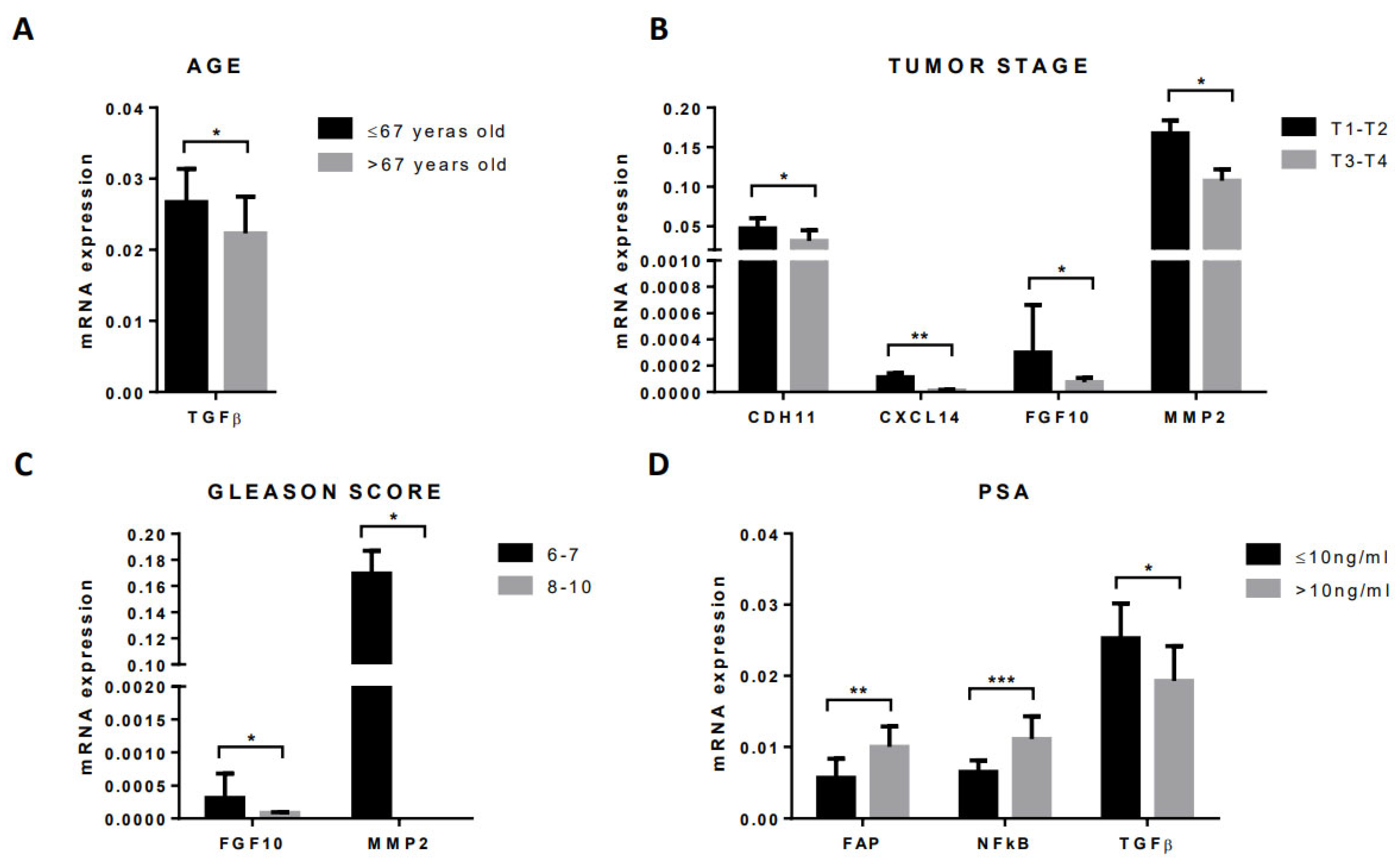
3.4. Gene Expression Profile of CAFs According to Clinical–Pathological Characteristics of Patients and Tumors
3.5. Gene Expression Profile of CAFs from Tumors of Patients Undergoing Androgen Deprivation Therapy
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Amling, C.L.; Blute, M.L.; Bergstralh, E.J.; Seay, T.M.; Slezak, J.; Zincke, H. Long-term hazard of progression after radical prostatectomy for clinically localized prostate cancer: Continued risk of biochemical failure after 5 years. J. Urol. 2000, 164, 101–105. [Google Scholar] [CrossRef]
- Freedland, S.J.; Humphreys, E.B.; Mangold, L.A.; Eisenberger, M.; Dorey, F.J.; Walsh, P.C.; Partin, A.W. Risk of prostate cancer-specific mortality following biochemical recurrence after radical prostatectomy. JAMA 2005, 294, 433–439. [Google Scholar] [CrossRef]
- Costa-Almeida, R.; Soares, R.; Granja, P.L. Fibroblasts as maestros orchestrating tissue regeneration. J. Tissue Eng. Regen. Med. 2018, 12, 240–251. [Google Scholar] [CrossRef] [PubMed]
- Bonollo, F.; Thalmann, G.N.; Kruithof-de Julio, M.; Karkampouna, S. The Role of Cancer-Associated Fibroblasts in Prostate Cancer Tumorigenesis. Cancers 2020, 12, 1887. [Google Scholar] [CrossRef] [PubMed]
- Bhowmick, N.A.; Neilson, E.G.; Moses, H.L. Stromal fibroblasts in cancer initiation and progression. Nature 2004, 432, 332–337. [Google Scholar] [CrossRef]
- Erez, N.; Truitt, M.; Olson, P.; Arron, S.T.; Hanahan, D. Cancer-Associated Fibroblasts Are Activated in Incipient Neoplasia to Orchestrate Tumor-Promoting Inflammation in an NF-kappaB-Dependent Manner. Cancer Cell 2010, 17, 135–147. [Google Scholar] [CrossRef] [PubMed]
- Planche, A.; Bacac, M.; Provero, P.; Fusco, C.; Delorenzi, M.; Stehle, J.C.; Stamenkovic, I. Identification of prognostic molecular features in the reactive stroma of human breast and prostate cancer. PLoS ONE 2011, 6, e18640. [Google Scholar] [CrossRef] [PubMed]
- Wikstrom, P.; Marusic, J.; Stattin, P.; Bergh, A. Low stroma androgen receptor level in normal and tumor prostate tissue is related to poor outcome in prostate cancer patients. Prostate 2009, 69, 799–809. [Google Scholar] [CrossRef] [PubMed]
- Eiro, N.; Fernandez-Gomez, J.; Sacristan, R.; Fernandez-Garcia, B.; Lobo, B.; Gonzalez-Suarez, J.; Quintas, A.; Escaf, S.; Vizoso, F.J. Stromal factors involved in human prostate cancer development, progression and castration resistance. J. Cancer Res. Clin. Oncol. 2017, 143, 351–359. [Google Scholar] [CrossRef]
- Bussard, K.M.; Mutkus, L.; Stumpf, K.; Gomez-Manzano, C.; Marini, F.C. Tumor-associated stromal cells as key contributors to the tumor microenvironment. Breast Cancer Res. 2016, 18, 84. [Google Scholar] [CrossRef]
- Lovf, M.; Zhao, S.; Axcrona, U.; Johannessen, B.; Bakken, A.C.; Carm, K.T.; Hoff, A.M.; Myklebost, O.; Meza-Zepeda, L.A.; Lie, A.K.; et al. Multifocal Primary Prostate Cancer Exhibits High Degree of Genomic Heterogeneity. Eur. Urol. 2019, 75, 498–505. [Google Scholar] [CrossRef]
- Wei, L.; Wang, J.; Lampert, E.; Schlanger, S.; DePriest, A.D.; Hu, Q.; Gomez, E.C.; Murakam, M.; Glenn, S.T.; Conroy, J.; et al. Intratumoral and Intertumoral Genomic Heterogeneity of Multifocal Localized Prostate Cancer Impacts Molecular Classifications and Genomic Prognosticators. Eur. Urol. 2017, 71, 183–192. [Google Scholar] [CrossRef]
- Gonzalez, L.; Eiro, N.; Fernandez-Garcia, B.; Gonzalez, L.O.; Dominguez, F.; Vizoso, F.J. Gene expression profile of normal and cancer-associated fibroblasts according to intratumoral inflammatory cells phenotype from breast cancer tissue. Mol. Carcinog. 2016, 55, 1489–1502. [Google Scholar] [CrossRef]
- Eiro, N.; Gonzalez, L.; Martinez-Ordonez, A.; Fernandez-Garcia, B.; Gonzalez, L.O.; Cid, S.; Dominguez, F.; Perez-Fernandez, R.; Vizoso, F.J. Cancer-associated fibroblasts affect breast cancer cell gene expression, invasion and angiogenesis. Cell Oncol. 2018, 41, 369–378. [Google Scholar] [CrossRef]
- Macklin, M.T. The genetic basis of human mammary cancer. In Proceedings of Second National Cancer Conference; American Chemical Society: New York, NY, USA, 1954; pp. 1074–1087. [Google Scholar]
- Lee, S.O.; Lou, W.; Johnson, C.S.; Trump, D.L.; Gao, A.C. Interleukin-6 protects LNCaP cells from apoptosis induced by androgen deprivation through the Stat3 pathway. Prostate 2004, 60, 178–186. [Google Scholar] [CrossRef]
- Nakashima, J.; Tachibana, M.; Horiguchi, Y.; Oya, M.; Ohigashi, T.; Asakura, H.; Murai, M. Serum interleukin 6 as a prognostic factor in patients with prostate cancer. Clin. Cancer Res. 2000, 6, 2702–2706. [Google Scholar]
- Gong, Y.; Chippada-Venkata, U.D.; Oh, W.K. Roles of matrix metalloproteinases and their natural inhibitors in prostate cancer progression. Cancers 2014, 6, 1298–1327. [Google Scholar] [CrossRef]
- Vizoso, F.J.; Gonzalez, L.O.; Corte, M.D.; Rodriguez, J.C.; Vazquez, J.; Lamelas, M.L.; Junquera, S.; Merino, A.M.; Garcia-Muniz, J.L. Study of matrix metalloproteinases and their inhibitors in breast cancer. Br. J. Cancer 2007, 96, 903–911. [Google Scholar] [CrossRef]
- Gonzalez, L.O.; Gonzalez-Reyes, S.; Marin, L.; Gonzalez, L.; Gonzalez, J.M.; Lamelas, M.L.; Merino, A.M.; Rodriguez, E.; Pidal, I.; del Casar, J.M.; et al. Comparative analysis and clinical value of the expression of metalloproteases and their inhibitors by intratumour stromal mononuclear inflammatory cells and those at the invasive front of breast carcinomas. Histopathology 2010, 57, 862–876. [Google Scholar] [CrossRef]
- Eiro, N.; Fernandez-Garcia, B.; Vazquez, J.; Del Casar, J.M.; Gonzalez, L.O.; Vizoso, F.J. A phenotype from tumor stroma based on the expression of metalloproteases and their inhibitors, associated with prognosis in breast cancer. Oncoimmunology 2015, 4, e992222. [Google Scholar] [CrossRef]
- Eiro, N.; Gonzalez, L.; Gonzalez, L.O.; Fernandez-Garcia, B.; Lamelas, M.L.; Marin, L.; Gonzalez-Reyes, S.; del Casar, J.M.; Vizoso, F.J. Relationship between the inflammatory molecular profile of breast carcinomas and distant metastasis development. PLoS ONE 2012, 7, e49047. [Google Scholar] [CrossRef]
- Eiro, N.; Pidal, I.; Fernandez-Garcia, B.; Junquera, S.; Lamelas, M.L.; del Casar, J.M.; Gonzalez, L.O.; Lopez-Muniz, A.; Vizoso, F.J. Impact of CD68/(CD3+CD20) ratio at the invasive front of primary tumors on distant metastasis development in breast cancer. PLoS ONE 2012, 7, e52796. [Google Scholar] [CrossRef]
- Escaff, S.; Fernandez, J.M.; Gonzalez, L.O.; Suarez, A.; Gonzalez-Reyes, S.; Gonzalez, J.M.; Vizoso, F.J. Study of matrix metalloproteinases and their inhibitors in prostate cancer. Br. J. Cancer 2010, 102, 922–929. [Google Scholar] [CrossRef]
- Fernandez-Gomez, J.; Escaf, S.; Gonzalez, L.O.; Suarez, A.; Gonzalez-Reyes, S.; Gonzalez, J.; Miranda, O.; Vizoso, F. Relationship between metalloprotease expression in tumour and stromal cells and aggressive behaviour in prostate carcinoma: Simultaneous high-throughput study of multiple metalloproteases and their inhibitors using tissue array analysis of radical prostatectomy samples. Scand. J. Urol. Nephrol. 2011, 45, 171–176. [Google Scholar] [CrossRef] [PubMed]
- Peruzzi, D.; Mori, F.; Conforti, A.; Lazzaro, D.; De Rinaldis, E.; Ciliberto, G.; La Monica, N.; Aurisicchio, L. MMP11: A novel target antigen for cancer immunotherapy. Clin. Cancer Res. 2009, 15, 4104–4113. [Google Scholar] [CrossRef] [PubMed]
- Brasse, D.; Mathelin, C.; Leroux, K.; Chenard, M.P.; Blaise, S.; Stoll, I.; Tomasetto, C.; Rio, M.C. Matrix metalloproteinase 11/stromelysin-3 exerts both activator and repressor functions during the hematogenous metastatic process in mice. Int. J. Cancer 2010, 127, 1347–1355. [Google Scholar] [CrossRef] [PubMed]
- Boulay, A.; Masson, R.; Chenard, M.P.; El Fahime, M.; Cassard, L.; Bellocq, J.P.; Sautes-Fridman, C.; Basset, P.; Rio, M.C. High cancer cell death in syngeneic tumors developed in host mice deficient for the stromelysin-3 matrix metalloproteinase. Cancer Res. 2001, 61, 2189–2193. [Google Scholar]
- Kanharat, N.; Tuamsuk, P. Correlation between Microvascular Density and Matrix Metalloproteinase 11 Expression in Prostate Cancer Tissues: A Preliminary Study in Thailand. Asian Pac. J. Cancer Prev. 2015, 16, 6639–6643. [Google Scholar] [CrossRef][Green Version]
- Ishii, K.; Takahashi, S.; Sugimura, Y.; Watanabe, M. Role of Stromal Paracrine Signals in Proliferative Diseases of the Aging Human Prostate. J. Clin. Med. 2018, 7, 68. [Google Scholar] [CrossRef]
- Gasinska, A.; Jaszczynski, J.; Rychlik, U.; Luczynska, E.; Pogodzinski, M.; Palaczynski, M. Prognostic Significance of Serum PSA Level and Telomerase, VEGF and GLUT-1 Protein Expression for the Biochemical Recurrence in Prostate Cancer Patients after Radical Prostatectomy. Pathol. Oncol. Res. 2020, 26, 1049–1056. [Google Scholar] [CrossRef]
- Verzella, D.; Fischietti, M.; Capece, D.; Vecchiotti, D.; Del Vecchio, F.; Cicciarelli, G.; Mastroiaco, V.; Tessitore, A.; Alesse, E.; Zazzeroni, F. Targeting the NF-kappaB pathway in prostate cancer: A promising therapeutic approach? Curr. Drug Targets 2016, 17, 311–320. [Google Scholar] [CrossRef]
- Niu, Y.; Altuwaijri, S.; Yeh, S.; Lai, K.P.; Yu, S.; Chuang, K.H.; Huang, S.P.; Lardy, H.; Chang, C. Targeting the stromal androgen receptor in primary prostate tumors at earlier stages. Proc. Natl. Acad. Sci. USA 2008, 105, 12188–12193. [Google Scholar] [CrossRef]
- Vilamaior, P.S.; Taboga, S.R.; Carvalho, H.F. Modulation of smooth muscle cell function: Morphological evidence for a contractile to synthetic transition in the rat ventral prostate after castration. Cell Biol. Int. 2005, 29, 809–816. [Google Scholar] [CrossRef]
- Yu, S.; Xia, S.; Yang, D.; Wang, K.; Yeh, S.; Gao, Z.; Chang, C. Androgen receptor in human prostate cancer-associated fibroblasts promotes prostate cancer epithelial cell growth and invasion. Med. Oncol. 2013, 30, 674. [Google Scholar] [CrossRef]
- Thalmann, G.N.; Rhee, H.; Sikes, R.A.; Pathak, S.; Multani, A.; Zhau, H.E.; Marshall, F.F.; Chung, L.W. Human prostate fibroblasts induce growth and confer castration resistance and metastatic potential in LNCaP Cells. Eur. Urol. 2010, 58, 162–171. [Google Scholar] [CrossRef]
- Chen, P.-Y.; Wei, W.-F.; Wu, H.-Z.; Fan, L.-S.; Wang, W. Cancer-Associated Fibroblast Heterogeneity: A Factor That Cannot Be Ignored in Immune Microenvironment Remodeling. Front. Immunol. 2021, 12, 2760. [Google Scholar] [CrossRef]
- De, P.; Aske, J.; Sulaiman, R.; Dey, N. Bête Noire of Chemotherapy and Targeted Therapy: CAF-Mediated Resistance. Cancers 2022, 14, 1519. [Google Scholar] [CrossRef]
- Bianchi-Frias, D.; Basom, R.; Delrow, J.J.; Coleman, I.M.; Dakhova, O.; Qu, X.; Fang, M.; Franco, O.E.; Ericson, N.G.; Bielas, J.H.; et al. Cells Comprising the Prostate Cancer Microenvironment Lack Recurrent Clonal Somatic Genomic Aberrations. Mol. Cancer Res. 2016, 14, 374–384. [Google Scholar] [CrossRef]
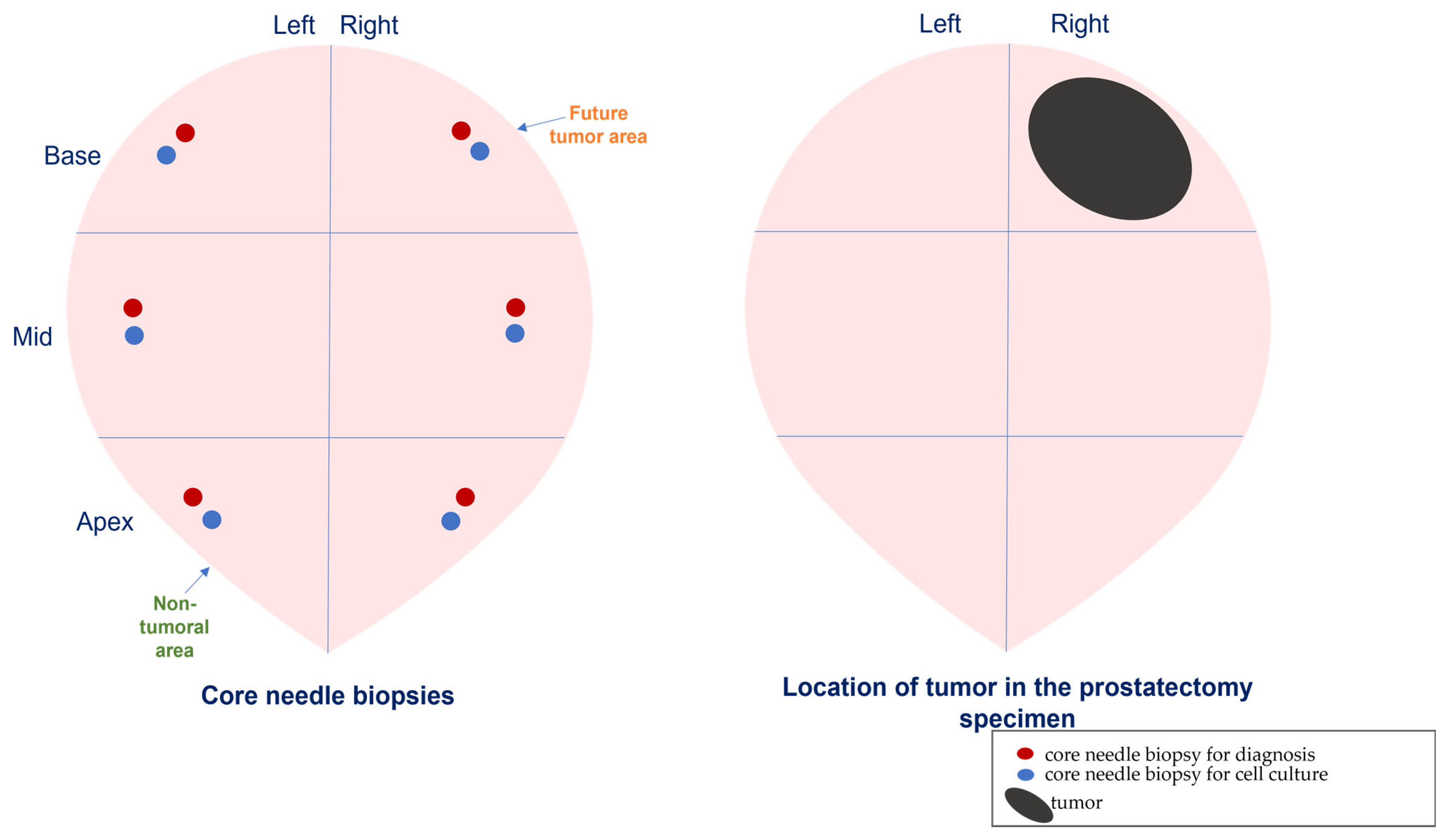

| Characteristics | Localized PCa (n = 23) | PCa ADT-Sensitive (n = 7) | PCa ADT-Resistant (n = 7) |
|---|---|---|---|
| N (%) | N (%) | N (%) | |
| Mean Age (years) | 66.9 ± 1.3 | 78.8 ± 1.9 | 74.5 ± 3.8 |
| Tumor Stage (T) * | |||
| T1-T2 | 17 (73.9) | - | - |
| T3-T4 | 4 (17.4) | - | - |
| Gleason Score * | |||
| 6-7 | 16 (69.6) | 1 (14.3) | 1 (14.3) |
| 8-10 | 5 (21.7) | 6 (85.7) | 6 (85.7) |
| PSA (ng/mL) | |||
| ≤10 | 19 (82.6) | 4 (57.1) | 1 (14.3) |
| >10 | 4 (17.4) | 3 (42.9) | 6 (85.7) |
| Factor | Gene Name | Reference |
|---|---|---|
| Hormone Receptors | ||
| AR | Androgen Receptor | 102544 |
| Proteins Implicated in Stroma/Epithelium Interactions | ||
| CDH11 | Cadherin-11 | 112371 |
| CXCL12 | Stromal cell derived factor 1 | 110618 |
| CXCL14 | Chemokine ligand 14 | 111260 |
| FAP | Fibroblast activation protein alpha | 108274 |
| Growth Factors | ||
| FGF7 | Fibroblast growth factor 7 | 113109 |
| FGF10 | Fibroblast growth factor 10 | 118274 |
| HGF | Hepatocyte growth factor | 108357 |
| FGF2 | Fibroblast growth factor 2 (NUTD6) | 118274 |
| PDGFB | Platelet-derived growth factor beta | 110713 |
| TGFβ | Transforming growth factor beta | 101210 |
| Chaperones | ||
| HSPA1A | Heat Shock Protein Family A (Hsp70) Member 1A | 145875 |
| HSF1 | Heat shock transcription factor 1 | 110674 |
| Proteins Implicated in Inflammation | ||
| IL6 | Interleukin 6 | 113614 |
| IL17RB | Receptor of interleukine 17 | 140392 |
| NFkB | Nuclear factor kappa B | 100646 |
| STAT3 | Signal transducer and activation of transcripcion 3 | 110694 |
| Proteins Implicated in Invasion | ||
| MMP2 | Matrix metalloprotease 2 | 103899 |
| MMP9 | Matrix metalloprotease 9 | 139820 |
| MMP11 | Matrix metalloprotease 11 | 103163 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eiro, N.; Fernández-Gómez, J.M.; Gonzalez-Ruiz de León, C.; Fraile, M.; Gonzalez-Suarez, J.; Lobo-Rodríguez, B.; García-Rodríguez, J.; Escaf, S.; Vizoso, F.J. Gene Expression Profile of Stromal Factors in Cancer-Associated Fibroblasts from Prostate Cancer. Diagnostics 2022, 12, 1605. https://doi.org/10.3390/diagnostics12071605
Eiro N, Fernández-Gómez JM, Gonzalez-Ruiz de León C, Fraile M, Gonzalez-Suarez J, Lobo-Rodríguez B, García-Rodríguez J, Escaf S, Vizoso FJ. Gene Expression Profile of Stromal Factors in Cancer-Associated Fibroblasts from Prostate Cancer. Diagnostics. 2022; 12(7):1605. https://doi.org/10.3390/diagnostics12071605
Chicago/Turabian StyleEiro, Noemi, Jesús María Fernández-Gómez, Cristina Gonzalez-Ruiz de León, Maria Fraile, Jorge Gonzalez-Suarez, Beatriz Lobo-Rodríguez, Jorge García-Rodríguez, Safwan Escaf, and Francisco J. Vizoso. 2022. "Gene Expression Profile of Stromal Factors in Cancer-Associated Fibroblasts from Prostate Cancer" Diagnostics 12, no. 7: 1605. https://doi.org/10.3390/diagnostics12071605
APA StyleEiro, N., Fernández-Gómez, J. M., Gonzalez-Ruiz de León, C., Fraile, M., Gonzalez-Suarez, J., Lobo-Rodríguez, B., García-Rodríguez, J., Escaf, S., & Vizoso, F. J. (2022). Gene Expression Profile of Stromal Factors in Cancer-Associated Fibroblasts from Prostate Cancer. Diagnostics, 12(7), 1605. https://doi.org/10.3390/diagnostics12071605






