Evaluation of Respiratory Particle Emission during Otorhinolaryngological Procedures in the Context of the SARS-CoV-2 Pandemic
Abstract
:1. Introduction
2. Materials and Methods
2.1. Measurement Techniques
2.2. Subjects
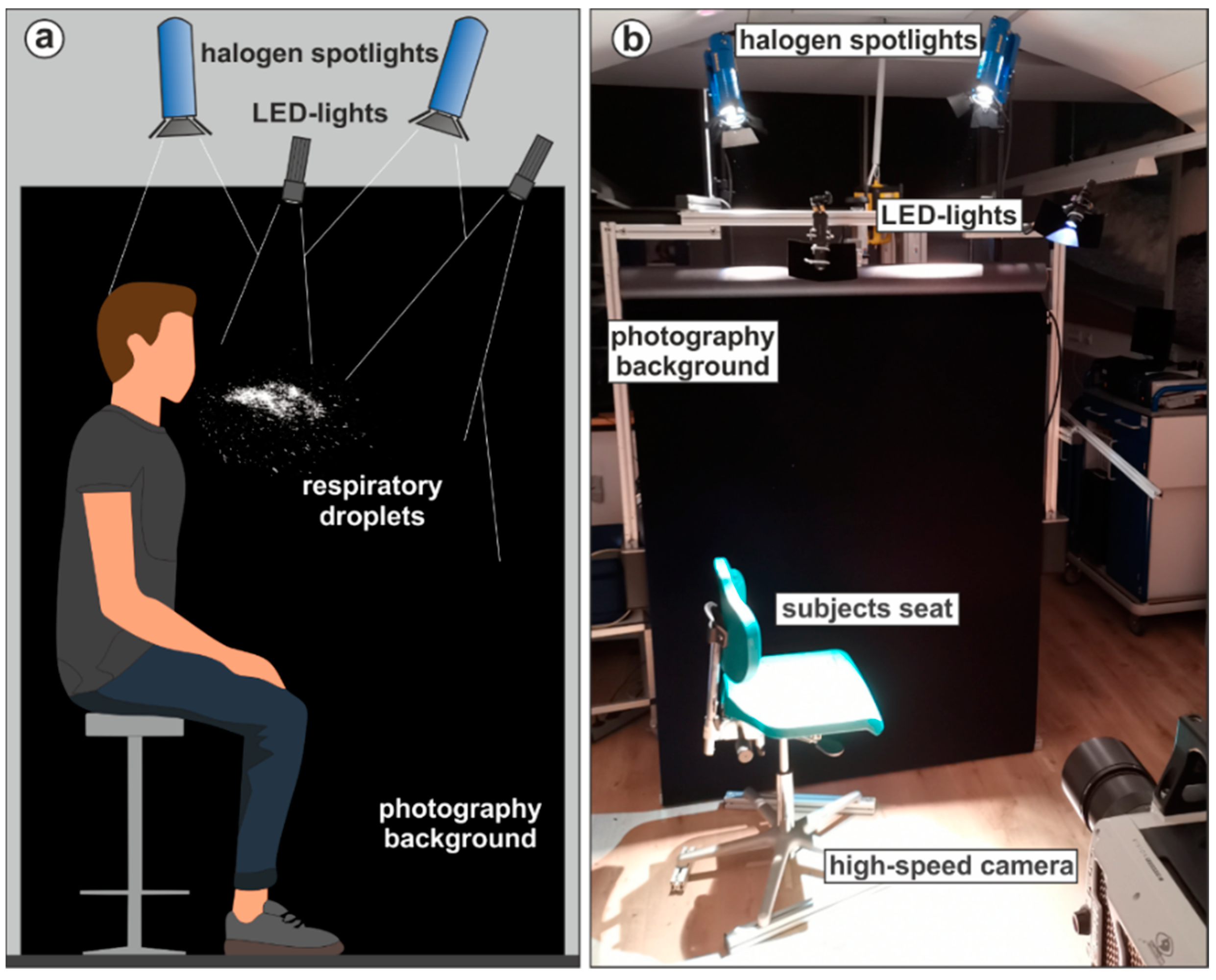
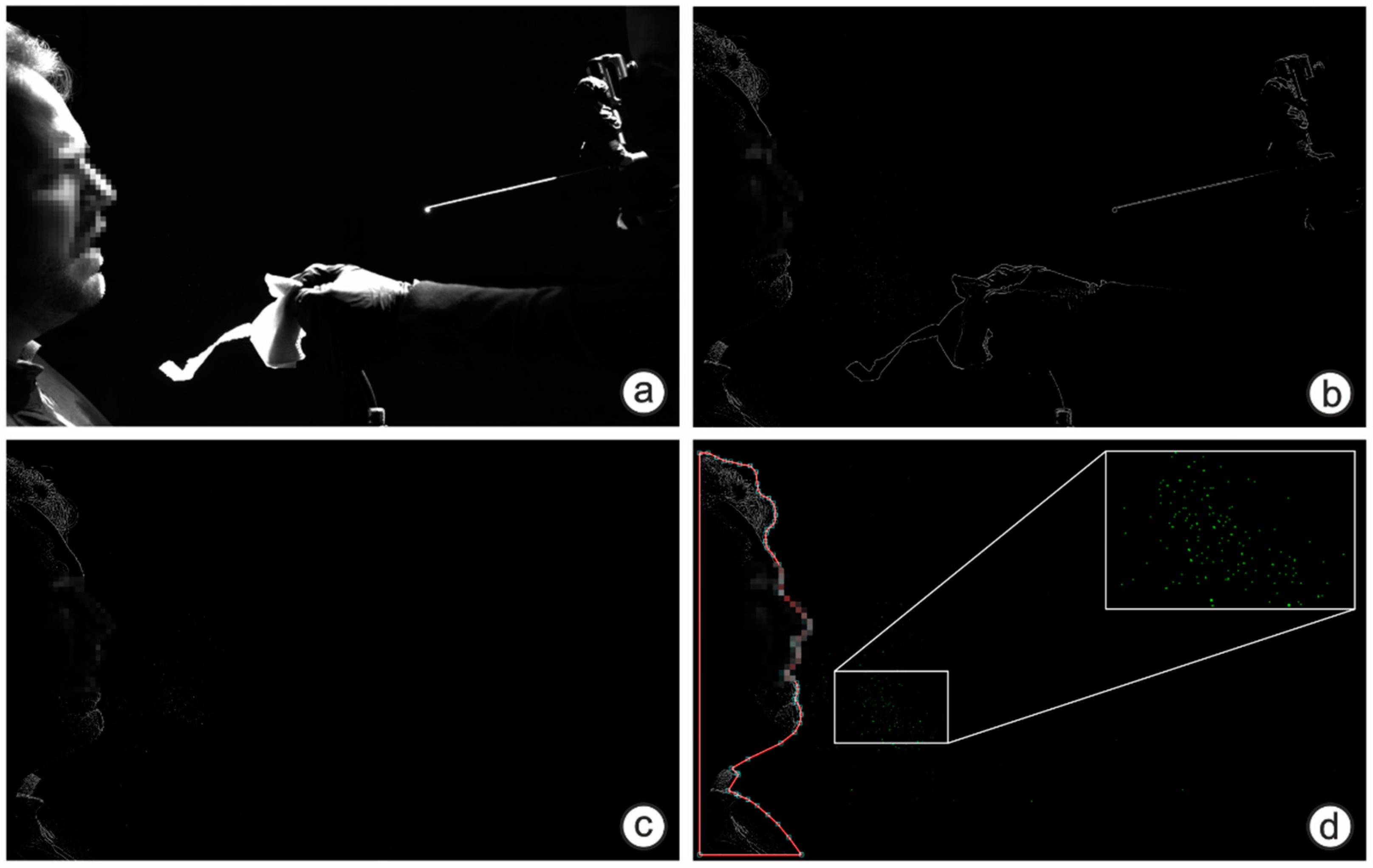
2.3. Large Droplet Measurements
- Patient interview in sedentary position with subjects reading aloud the standardized text “The north wind and the sun” (German version), equivalent to Rainbow passage, with and without a mask of both types (surgical and FFP2) for 6.6 s.
- Pharyngoscopy in sedentary position without a mask, where the subject’s pharynx is inspected through the mouth with a mirror.
- Laryngoscopy in sedentary position, performed orally and nasally. Orally with a rigid endoscope and nasally with a flexible endoscope. During the task with the rigid endoscope, the physician held the subject’s tongue with a paper tissue. The task with the flexible endoscope was additionally performed with an MNC.
- Rhinoscopy in sedentary position with a rigid endoscope, once performed typically without a mask and once with the subject wearing an MNC with a small hole through which the endoscope was passed through.
- Otoscopy in sedentary position with MNC. The physician inspects the subject’s ear with an otoscope. Since it is common for patients to speak or cough during otoscopy and sonography, subjects were saying aloud the phrase “Stay healthy” and afterwards coughed once within a measuring cycle to simulate the worst-case scenario. The phrase was chosen for its benchmark status [14,29]. For the whole tasks, two cycles of “Stay healthy” and coughing were executed by each subject.
- Sonography of the throat in supine position wearing no mask, an MNC or an FFP2 mask. Subjects speaking/coughing pattern was analogue to otoscopy. The physician positioned next to the subject’s head.
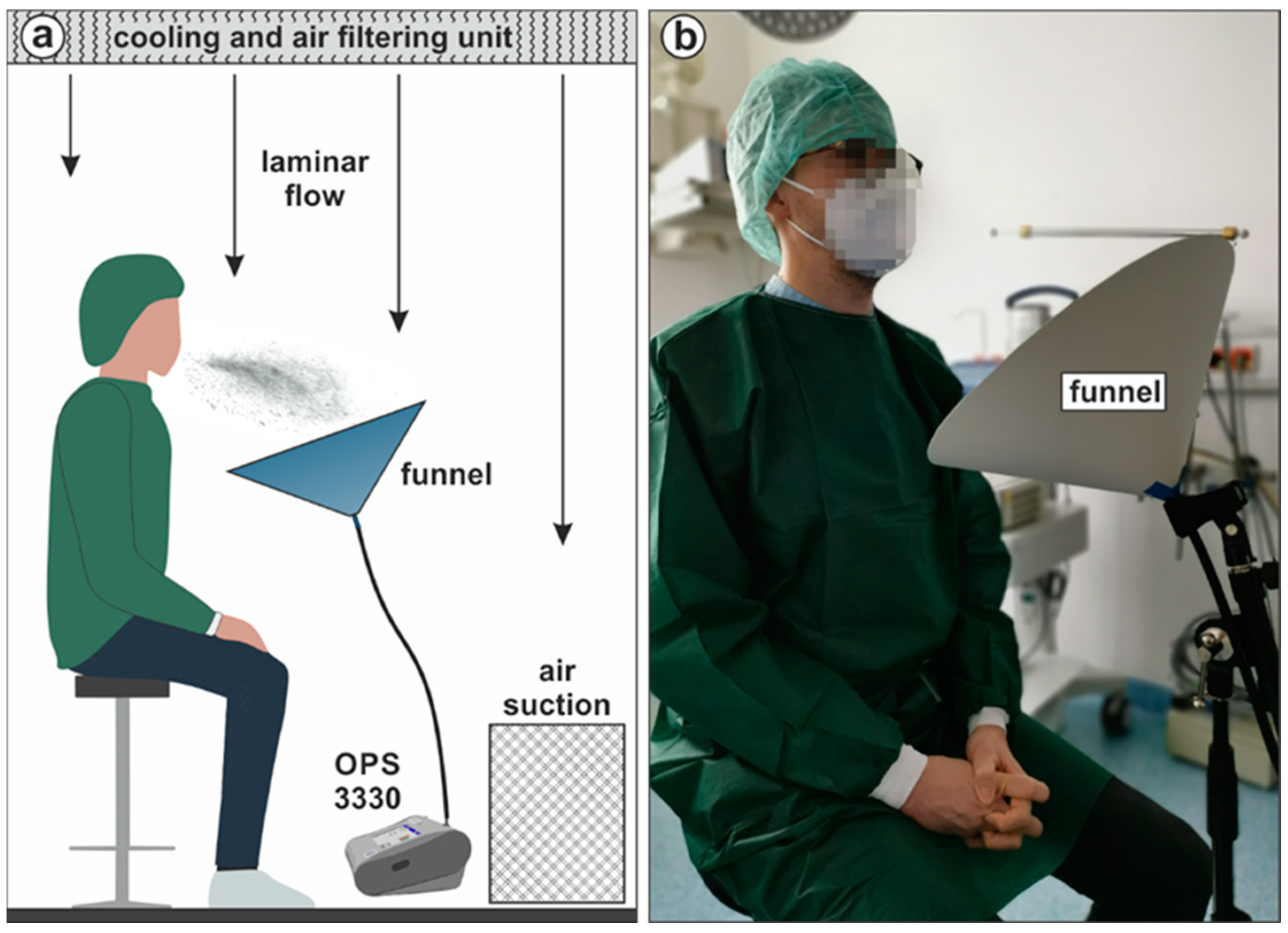
2.4. Aerosol Particle Measurements
3. Results
3.1. Droplet Emission
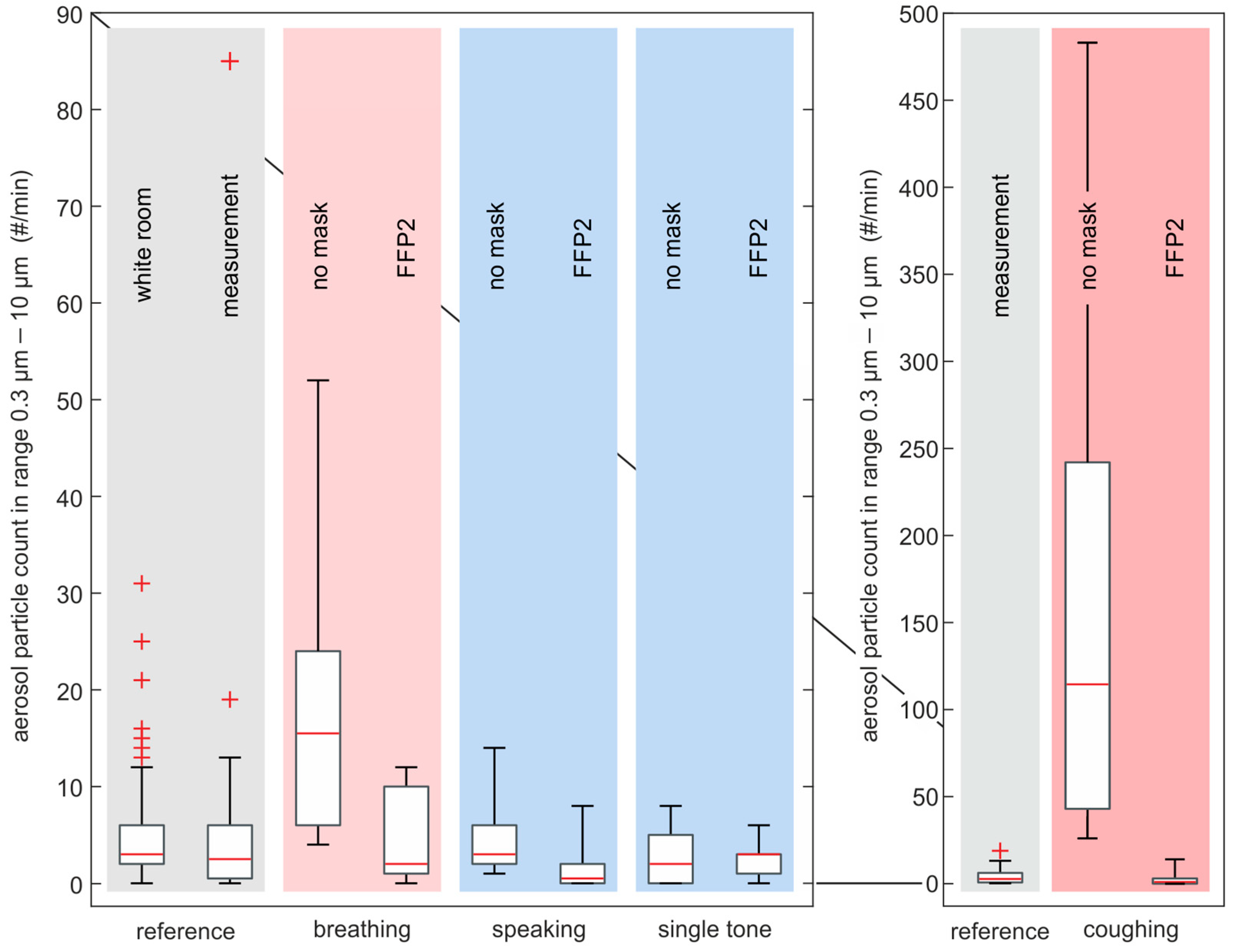
3.2. Aerosol Particle Emission
4. Discussion
Limitations
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Çelik, H.; Yürük Bal, E.; Çetinkaya, Ş. The risks that nurses meet in the global COVID-19 epidemic, the importance of protection. Turk. J. Public Health 2020, 18, 66–77. [Google Scholar] [CrossRef]
- Nguyen, L.H.; Drew, D.A.; Graham, M.S.; Joshi, A.D.; Guo, C.-G.; Ma, W.; Mehta, R.S.; Warner, E.T.; Sikavi, D.R.; Lo, C.-H.; et al. Risk of COVID-19 among front-line health-care workers and the general community: A prospective cohort study. Lancet Public Health 2020, 5, e475–e483. [Google Scholar] [CrossRef]
- Mutambudzi, M.; Niedwiedz, C.; Macdonald, E.B.; Leyland, A.; Mair, F.; Anderson, J.; Celis-Morales, C.; Cleland, J.; Forbes, J.; Gill, J.; et al. Occupation and risk of severe COVID-19: Prospective cohort study of 120 075 UK Biobank participants. Occup. Environ. Med. 2021, 78, 307–314. [Google Scholar] [CrossRef]
- Kulcsar, M.A.; Montenegro, F.L.; Arap, S.S.; Tavares, M.R.; Kowalski, L.P. High Risk of COVID-19 Infection for Head and Neck Surgeons. Int. Arch. Otorhinolaryngol. 2020, 24, e129–e130. [Google Scholar] [CrossRef]
- ECDC. Infection Prevention and Control and Preparedness for COVID-19 in Healthcare Settings; ECDC: Solna, Sweden, 2020. [Google Scholar]
- Jin, Y.-H.; Cai, L.; Cheng, Z.-S.; Cheng, H.; Deng, T.; Fan, Y.-P.; Fang, C.; Huang, D.; Huang, L.-Q.; Huang, Q.; et al. A rapid advice guideline for the diagnosis and treatment of 2019 novel coronavirus (2019-nCoV) infected pneumonia (standard version). Mil. Med. Res. 2020, 7, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Morawska, L.; Johnson, G.R.; Ristovski, Z.D.; Hargreaves, M.; Mengersen, K.; Corbett, S.; Chao, C.; Li, Y.; Katoshevski, D. Size distribution and sites of origin of droplets expelled from the human respiratory tract during expiratory activities. J. Aerosol Sci. 2009, 40, 256–269. [Google Scholar] [CrossRef] [Green Version]
- Edwards, D.A.; Man, J.C.; Brand, P.; Katstra, J.P.; Sommerer, K.; Stone, H.A.; Nardell, E.; Scheuch, G. Inhaling to mitigate exhaled bioaerosols. Proc. Natl. Acad. Sci. USA 2004, 101, 17383–17388. [Google Scholar] [CrossRef] [Green Version]
- Papineni, R.S.; Rosenthal, F.S. The size distribution of droplets in the exhaled breath of healthy human subjects. J. Aerosol Med. 1997, 10, 105–116. [Google Scholar] [CrossRef]
- Schijven, J.; Vermeulen, L.C.; Swart, A.; Meijer, A.; Duizer, E.; de Roda Husman, A.M. Quantitative Microbial Risk Assessment for Airborne Transmission of SARS-CoV-2 via Breathing, Speaking, Singing, Coughing, and Sneezing. Environ. Health Perspect. 2021, 129, 47002. [Google Scholar] [CrossRef]
- Prather, K.A.; Wang, C.C.; Schooley, R.T. Reducing transmission of SARS-CoV-2. Science 2020, 368, 1422–1424. [Google Scholar] [CrossRef]
- Asadi, S.; Bouvier, N.; Wexler, A.S.; Ristenpart, W.D. The coronavirus pandemic and aerosols: Does COVID-19 transmit via expiratory particles? Aerosol Sci. Technol. 2020, 54, 635–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mahjoub Mohammed Merghani, K.; Sagot, B.; Gehin, E.; Da, G.; Motzkus, C. A review on the applied techniques of exhaled airflow and droplets characterization. Indoor Air 2021, 31, 7–25. [Google Scholar] [CrossRef]
- Anfinrud, P.; Stadnytskyi, V.; Bax, C.E.; Bax, A. Visualizing Speech-Generated Oral Fluid Droplets with Laser Light Scattering. N. Engl. J. Med. 2020, 382, 2061–2063. [Google Scholar] [CrossRef] [PubMed]
- Kniesburges, S.; Schlegel, P.; Peters, G.; Westphalen, C.; Jakubaß, B.; Veltrup, R.; Kist, A.M.; Döllinger, M.; Gantner, S.; Kuranova, L.; et al. Effects of surgical masks on aerosol dispersion in professional singing. J. Expo. Sci. Environ. Epidemiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Bandiera, L.; Pavar, G.; Pisetta, G.; Otomo, S.; Mangano, E.; Seckl, J.R.; Digard, P.; Molinari, E.; Menolascina, F.; Viola, I.M. Face coverings and respiratory tract droplet dispersion. R. Soc. Open Sci. 2020, 7, 201663. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, H.; Sakata, S.; Kaga, A. A new methodology for studying dynamics of aerosol particles in sneeze and cough using a digital high-vision, high-speed video system and vector analyses. PLoS ONE 2013, 8, e80244. [Google Scholar] [CrossRef] [PubMed]
- Bourouiba, L. Turbulent Gas Clouds and Respiratory Pathogen Emissions: Potential Implications for Reducing Transmission of COVID-19. JAMA 2020, 323, 1837–1838. [Google Scholar] [CrossRef]
- Scharfman, B.E.; Techet, A.H.; Bush, J.W.M.; Bourouiba, L. Visualization of sneeze ejecta: Steps of fluid fragmentation leading to respiratory droplets. Exp. Fluids 2016, 57, 24. [Google Scholar] [CrossRef]
- Turner, C.E.; Jennison, M.W.; Edgerton, H.E. Public Health Applications of High-Speed Photography. Am. J. Public Health Nations Health 1941, 31, 319–324. [Google Scholar] [CrossRef]
- Han, Z.Y.; Weng, W.G.; Huang, Q.Y. Characterizations of particle size distribution of the droplets exhaled by sneeze. J. R. Soc. Interface 2013, 10, 20130560. [Google Scholar] [CrossRef]
- Zayas, G.; Chiang, M.C.; Wong, E.; MacDonald, F.; Lange, C.F.; Senthilselvan, A.; King, M. Cough aerosol in healthy participants: Fundamental knowledge to optimize droplet-spread infectious respiratory disease management. BMC Pulm. Med. 2012, 12, 11. [Google Scholar] [CrossRef] [Green Version]
- Loudon, R.G.; Roberts, R.M. Droplet expulsion from the respiratory tract. Am. Rev. Respir. Dis. 1967, 95, 435–442. [Google Scholar]
- Duguid, J.P. The Numbers and the Sites of Origin of the Droplets Expelled during Expiratory Activities. Edinb. Med. J. 1945, 52, 385–401. [Google Scholar]
- Xie, X.; Li, Y.; Sun, H.; Liu, L. Exhaled droplets due to talking and coughing. J. R. Soc. Interface 2009, 6 (Suppl. S6), S703–S714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Workman, A.D.; Jafari, A.; Welling, D.B.; Varvares, M.A.; Gray, S.T.; Holbrook, E.H.; Scangas, G.A.; Xiao, R.; Carter, B.S.; Curry, W.T.; et al. Airborne Aerosol Generation During Endonasal Procedures in the Era of COVID-19: Risks and Recommendations. Otolaryngol. Head Neck Surg. 2020, 163, 465–470. [Google Scholar] [CrossRef] [PubMed]
- Good, N.; Fedak, K.M.; Goble, D.; Keisling, A.; L’Orange, C.; Morton, E.; Phillips, R.; Tanner, K.; Volckens, J. Respiratory Aerosol Emissions from Vocalization: Age and Sex Differences Are Explained by Volume and Exhaled CO2. Environ. Sci. Technol. Lett. 2021, 8, 1071–1076. [Google Scholar] [CrossRef]
- Otrisal, P.; Bungau, C.; Obsel, V.; Melicharik, Z.; Tont, G. Selected Respiratory Protective Devices: Respirators and Significance of Some Markings. Sustainability 2021, 13, 4988. [Google Scholar] [CrossRef]
- Mueller, S.K.; Veltrup, R.; Jakubaß, B.; Kniesburges, S.; Huebner, M.J.; Kempfle, J.S.; Dittrich, S.; Iro, H.; Döllinger, M. Clinical characterization of respiratory large droplet production during common airway procedures using high-speed imaging. Sci. Rep. 2021, 11, 10627. [Google Scholar] [CrossRef]
- Andrejko, K.L.; Pry, J.M.; Myers, J.F.; Fukui, N.; DeGuzman, J.L.; Openshaw, J.; Watt, J.P.; Lewnard, J.A.; Jain, S. Effectiveness of Face Mask or Respirator Use in Indoor Public Settings for Prevention of SARS-CoV-2 Infection—California, February–December 2021. Morb. Mortal. Wkly. Rep. 2022, 71, 212–216. [Google Scholar] [CrossRef]
- Mello, V.M.; Eller, C.M.; Salvio, A.L.; Nascimento, F.F.; Figueiredo, C.M.; Silva, E.S.R.F.; Sousa, P.S.F.; Costa, P.F.; Paiva, A.A.P.; Mares-Guias, M.A.M.M.; et al. Effectiveness of face masks in blocking the transmission of SARS-CoV-2: A preliminary evaluation of masks used by SARS-CoV-2-infected individuals. PLoS ONE 2022, 17, e0264389. [Google Scholar] [CrossRef]
- Rossettie, S.; Perry, C.; Pourghaed, M.; Zumwalt, M. Effectiveness of manufactured surgical masks, respirators, and home-made masks in prevention of respiratory infection due to airborne microorganisms. Southwest Respir. Crit. Care Chron. 2020, 8, 11–26. [Google Scholar] [CrossRef]
- Sharma, S.; Pinto, R.; Saha, A.; Chaudhuri, S.; Basu, S. On secondary atomization and blockage of surrogate cough droplets in single- and multilayer face masks. Sci. Adv. 2021, 7, eabf0452. [Google Scholar] [CrossRef] [PubMed]
- Ueki, H.; Furusawa, Y.; Iwatsuki-Horimoto, K.; Imai, M.; Kabata, H.; Nishimura, H.; Kawaoka, Y. Effectiveness of Face Masks in Preventing Airborne Transmission of SARS-CoV-2. mSphere 2020, 5, e00637–20. [Google Scholar] [CrossRef] [PubMed]
- Durbin, R.P. Letter: Acid secretion by gastric mucous membrane. Am. J. Physiol. 1975, 229, 1726. [Google Scholar] [CrossRef] [PubMed]
- Tcharkhtchi, A.; Abbasnezhad, N.; Zarbini Seydani, M.; Zirak, N.; Farzaneh, S.; Shirinbayan, M. An overview of filtration efficiency through the masks: Mechanisms of the aerosols penetration. Bioact. Mater. 2021, 6, 106–122. [Google Scholar] [CrossRef]
- Falk, S.; Mueller, S.K.; Kniesburges, S.; Döllinger, M. Simulation of aerosol dispersion during medical examinations. J. Eng. Sci. Med. Diagn. Ther. 2022, 5, 031105. [Google Scholar] [CrossRef]
- Cappa, C.D.; Asadi, S.; Barreda, S.; Wexler, A.S.; Bouvier, N.M.; Ristenpart, W.D. Expiratory aerosol particle escape from surgical masks due to imperfect sealing. Sci. Rep. 2021, 11, 12110. [Google Scholar] [CrossRef]
- Edwards, D.A.; Ausiello, D.; Langer, R.; Salzman, J.; Devlin, T.; Beddingfield, B.J.; Fears, A.C.; Doyle-Meyers, L.A.; Redmann, R.K.; Killeen, S.Z.; et al. Exhaled aerosol increases with COVID-19 infection, and risk factors of disease symptom severity. medRxiv 2020. [Google Scholar] [CrossRef]
- Jiang, G.; Li, F.; Hu, T. Transport Characteristics and Transmission Risk of Virus-Containing Droplets from Coughing in Outdoor Windy Environment. Toxics 2022, 10, 294. [Google Scholar] [CrossRef]
- Hersen, G.; Moularat, S.; Robine, E.; Géhin, E.; Corbet, S.; Vabret, A.; Freymuth, F. Impact of Health on Particle Size of Exhaled Respiratory Aerosols: Case-control Study. Clean—Soil Air Water (Weinh.) 2008, 36, 572–577. [Google Scholar] [CrossRef]
- van Doremalen, N.; Bushmaker, T.; Morris, D.H.; Holbrook, M.G.; Gamble, A.; Williamson, B.N.; Tamin, A.; Harcourt, J.L.; Thornburg, N.J.; Gerber, S.I.; et al. Aerosol and Surface Stability of SARS-CoV-2 as Compared with SARS-CoV-1. N. Engl. J. Med. 2020, 382, 1564–1567. [Google Scholar] [CrossRef] [PubMed]
- Stabile, L.; Pacitto, A.; Mikszewski, A.; Morawska, L.; Buonanno, G. Ventilation procedures to minimize the airborne transmission of viruses in classrooms. Build. Environ. 2021, 202, 108042. [Google Scholar] [CrossRef] [PubMed]
- Lescanne, E.; van der Mee-Marquet, N.; Juvanon, J.-M.; Abbas, A.; Morel, N.; Klein, J.-M.; Hanau, M.; Couloigner, V. Best practice recommendations: ENT consultations during the COVID-19 pandemic. Eur. Ann. Otorhinolaryngol. Head Neck Dis. 2020, 137, 303–308. [Google Scholar] [CrossRef] [PubMed]


| ENT Procedure | Subject’s Face Mask | Subject’s Position |
|---|---|---|
| Patient interview | No mask|MNC|FFP2 | Sedentary |
| Pharyngoscopy | No mask | Sedentary |
| Laryngoscopy (nasal) | No mask|MNC | Sedentary |
| Laryngoscopy (oral) | No mask | Sedentary |
| Rhinoscopy | No mask|MNC | Sedentary |
| Otoscopy | MNC | Sedentary |
| Sonography | No mask|MNC|FFP2 | Supine |
| Equivalent Task | Subject’s Face Mask | Task Description | Derived From |
|---|---|---|---|
| Patient interview | No mask|FFP2 | 1 min of reading | Patient interview Sonography Otoscopy |
| Pharyngoscopy | No mask|FFP2 | 1 min with up to 25 forced exhalations | Pharyngoscopy Rhinoscopy Laryngoscopy |
| Laryngoscopy (nasal) | No mask|FFP2 | 1 min of coughing (approx. 25 coughs) | Otoscopy Laryngoscopy Rhinoscopy Pharyngoscopy |
| Laryngoscopy (oral) | No mask|FFP2 | 1 min at comfortable pitch and loudness of the single tone “/a/” | Laryngoscopy Patient interview |
| Aerosol Particle Diameter Range (µm) | Breathing (%) | Speaking (%) | Single Tone Vocalization (%) | Coughing (%) | ||||
|---|---|---|---|---|---|---|---|---|
| No Mask | FFP2 Mask | No Mask | FFP2 Mask | No Mask | FFP2 Mask | No Mask | FFP2 Mask | |
| 0.3–0.37 | 15.1 | 10.3 | 20.7 | 3.8 | 17.6 | 21.1 | 29.2 | 20.9 |
| 0.37–0.47 | 23.5 | 10.3 | 24.1 | 11.5 | 23.5 | 15.8 | 23.1 | 18.6 |
| 0.47–0.58 | 21.8 | 10.3 | 20.7 | 7.7 | 11.8 | 0 | 16.6 | 16.3 |
| 0.58–0.72 | 6.7 | 13.8 | 6.9 | 34.6 | 17.6 | 5.3 | 8.2 | 9.3 |
| 0.72–0.9 | 8.4 | 10.3 | 3.4 | 7.7 | 0 | 10.5 | 6.5 | 11.6 |
| 0.90–1.12 | 9.2 | 3.4 | 3.4 | 7.7 | 5.9 | 5.3 | 5.8 | 7 |
| 1.12–1.39 | 3.4 | 0 | 0 | 3.8 | 0 | 15.8 | 3.2 | 4.7 |
| 1.39–1.73 | 0.8 | 3.4 | 0 | 0 | 0 | 5.3 | 1.5 | 2.3 |
| 1.73–2.16 | 2.5 | 6.9 | 3.4 | 3.8 | 5.9 | 0 | 2.8 | 2.3 |
| 2.16–2.69 | 2.5 | 6.9 | 0 | 7.7 | 11.8 | 0 | 1.3 | 0 |
| 2.69–3.34 | 1.7 | 6.9 | 0 | 0 | 0 | 5.3 | 0.7 | 0 |
| 3.34–4.16 | 0 | 3.4 | 0 | 0 | 0 | 0 | 0.5 | 0 |
| 4.16–5.18 | 0.8 | 3.4 | 0 | 3.8 | 5.9 | 0 | 0.2 | 0 |
| 5.18–6.45 | 1.7 | 3.4 | 0 | 3.8 | 0 | 5.3 | 0.1 | 4.7 |
| 6.45–8.03 | 0 | 3.4 | 0 | 0 | 0 | 5.3 | 0.3 | 0 |
| 8.03–10 | 0 | 0 | 0 | 0 | 0 | 0 | 0.1 | 0 |
| <10 | 1.7 | 3.4 | 0 | 3.8 | 0 | 5.3 | 0 | 2.3 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Veltrup, R.; Kniesburges, S.; Döllinger, M.; Falk, S.; Mueller, S.K. Evaluation of Respiratory Particle Emission during Otorhinolaryngological Procedures in the Context of the SARS-CoV-2 Pandemic. Diagnostics 2022, 12, 1603. https://doi.org/10.3390/diagnostics12071603
Veltrup R, Kniesburges S, Döllinger M, Falk S, Mueller SK. Evaluation of Respiratory Particle Emission during Otorhinolaryngological Procedures in the Context of the SARS-CoV-2 Pandemic. Diagnostics. 2022; 12(7):1603. https://doi.org/10.3390/diagnostics12071603
Chicago/Turabian StyleVeltrup, Reinhard, Stefan Kniesburges, Michael Döllinger, Sebastian Falk, and Sarina K. Mueller. 2022. "Evaluation of Respiratory Particle Emission during Otorhinolaryngological Procedures in the Context of the SARS-CoV-2 Pandemic" Diagnostics 12, no. 7: 1603. https://doi.org/10.3390/diagnostics12071603
APA StyleVeltrup, R., Kniesburges, S., Döllinger, M., Falk, S., & Mueller, S. K. (2022). Evaluation of Respiratory Particle Emission during Otorhinolaryngological Procedures in the Context of the SARS-CoV-2 Pandemic. Diagnostics, 12(7), 1603. https://doi.org/10.3390/diagnostics12071603







