Spontaneous Uterine Rupture and Adenomyosis, a Rare but Possible Correlation: Case Report and Literature Review
Abstract
1. Introduction
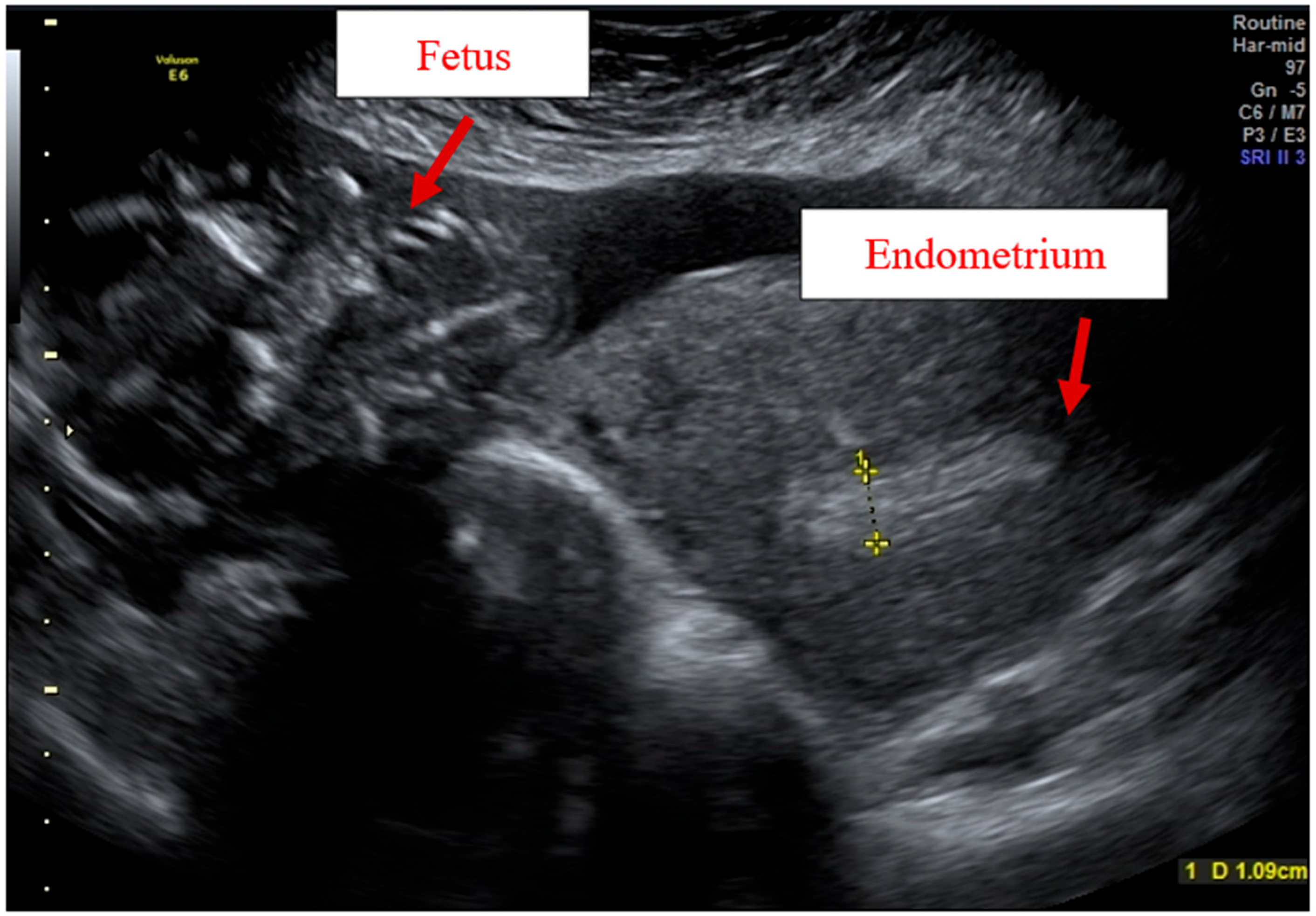
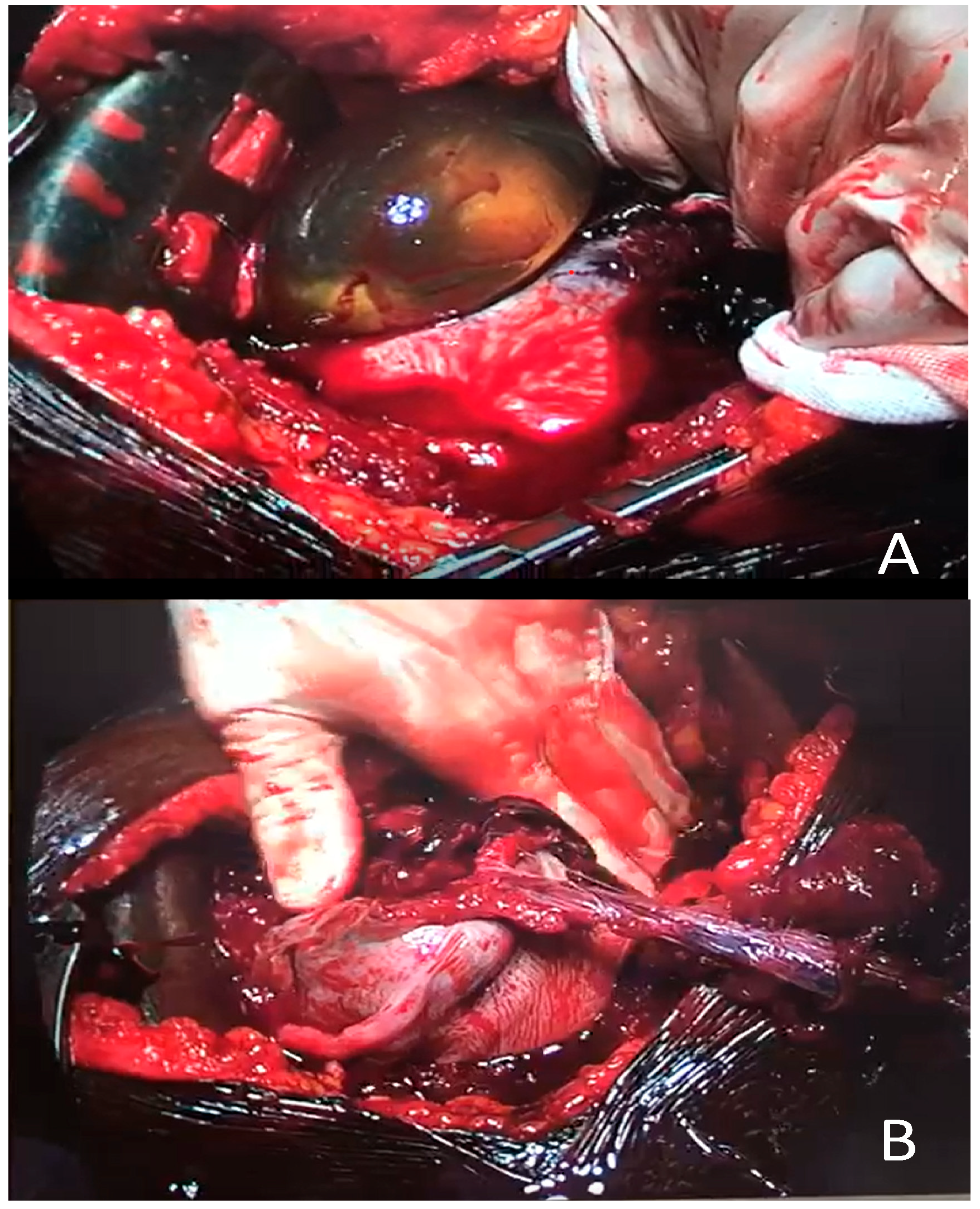
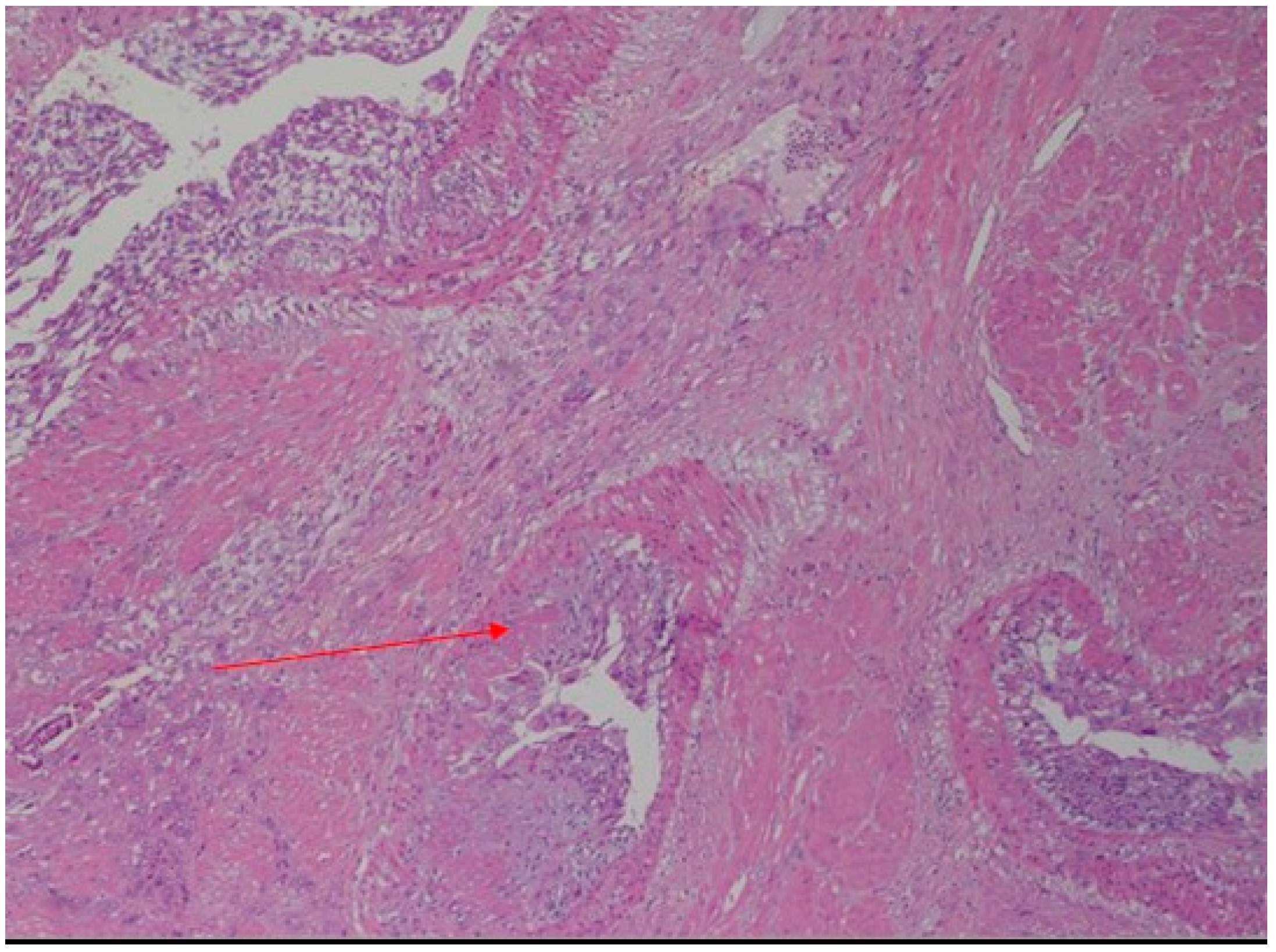
Case Report
2. Discussion
Adenomyosis Ultrasound Diagnosis and Classification
3. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Agostinho, L.; Cruz, R.; Osório, F.; Alves, J.; Setúbal, A.; Guerra, A. MRI for adenomyosis: A pictorial review. Insights Imaging 2017, 8, 549–556. [Google Scholar] [CrossRef]
- Nelsen, L.M.; Lenderking, W.R.; Pokrzywinski, R.; Balantzc, Z.; Black, L.; Pokras, S.; Enslin, M.B.; Cooper, M.; Lukes, A.S. Experi- ence of symptoms and disease impact in patients with adenomyosis. Patient 2018, 11, 319–328. [Google Scholar] [CrossRef]
- Aleksandrovych, V.; Basta, P.; Gil, K. Current facts constituting an understanding of the nature of adenomyosis. Adv. Clin. Exp. Med. 2018, 28, 839–846. [Google Scholar] [CrossRef]
- Kishi, Y.; Suginami, H.; Kuramori, R.; Yabuta, M.; Suginami, R.; Taniguchi, F. Four subtypes of adenomyosis assessed by magnetic resonance imaging and their specification. Am. J. Obstet. Gynecol. 2012, 207, 114.e1–114.e7. [Google Scholar] [CrossRef]
- Azziz, R. Adenomyosis in pregnancy. A review. J. Reprod. Med. 1986, 31, 224–227. [Google Scholar]
- Bensaid, F.; Kettani, F.; el Fehri, S.; Chraibi, C.; Alaoui, M.T. Les complications obstétricales de l’adénomyose. Revue de la littérature, à propos de 2 observations [Obstetrical complications of adenomyosis. Literature review and two case reports. J. Gynecol. Obstet. Biol. Reprod. 1996, 25, 416–418. [Google Scholar]
- Mueller, M.D.; Saile, G.; Brühwiler, H. Spontane Uterusruptur in der 18. Schwangerschaftswoche bei einer Primigravida mit einer ausgedehnten Adenomyose [Spontaneous uterine rupture in the 18th week of pregnancy in a primigravida patient with adenomyosis]. Zentralbl. Gynakol. 1996, 118, 42–44. [Google Scholar]
- Pafumi, C.; Farina, M.; Pernicone, G.; Russo, A.; Bandiera, S.; Giardina, P.; Cianci, A. Adenomyosis and uterus rupture during labor. Chin. Med. J. 2001, 64, 244–246. [Google Scholar]
- Villa, G.; Mabrouk, M.; Guerrini, M.; Mignemi, G.; Colleoni, G.G.; Venturoli, S.; Seracchioli, R. Uterine Rupture in a Primigravida with Adenomyosis Recently Subjected to Laparoscopic Resection of Rectovaginal Endometriosis: Case Report. J. Minim. Invasive Gynecol. 2008, 15, 360–361. [Google Scholar] [CrossRef]
- Miller, D.A.; Goodwin, T.M.; Gherman, R.B.; Paul, R.H. Intrapartum rupture of the unscarred uterus. Obstet. Gynecol. 1997, 89, 671. [Google Scholar] [CrossRef]
- Jovanovic, B.; Petrovic, A.; Petrovic, B. Decidual transformation in adenomyosis during pregnancy as an indication for hysterectomy. Med. Pregl. 2009, 62, 185–188. [Google Scholar] [CrossRef]
- Nikolaou, M.; Kourea, H.P.; Antonopoulos, K.; Geronatsiou, K.; Adonakis, G.; Decavalas, G. Spontaneous uterine rupture in a primigravid woman in the early third trimester attributed to adenomyosis: A case report and review of the literature. J. Obstet. Gynaecol. Res. 2013, 39, 727. [Google Scholar] [CrossRef]
- Indraccolo, U.; Iannicco, A.; Micucci, G. A novel case of an adenomyosis-related uterine rupture in pregnancy. Clin. Exp. Obstet. Gynecol. 2015, 42, 810–811. [Google Scholar] [CrossRef]
- Li, X.; Li, C.; Sun, M.; Li, H.; Cao, Y.; Wei, Z. Spontaneous unscarred uterine rupture in a twin pregnancy complicated by adenomyosis. Medicine 2021, 100, e24048. [Google Scholar] [CrossRef]
- Sun, H.D.; Su, W.H.; Chang, W.H.; Wen, L.; Huang, B.S.; Wang, P.H. Rupture of a pregnant unscarred uterus in an early secondary trimester: A case report and brief review. J. Obstet. Gynaecol. Res. 2012, 38, 442. [Google Scholar] [CrossRef]
- Chen, L.H.; Tan, K.H.; Yeo, G.S. A ten-year review of uterine rupture in modern obstetric practice. Ann. Acad. Med. Singap. 1995, 24, 830–835. [Google Scholar]
- Wu, X.; Jiang, W.; Xu, H.; Ye, X.; Xu, C. Characteristics of uterine rupture after laparoscopic surgery of the uterus: Clinical analysis of 10 cases and literature review. J. Int. Med. Res. 2018, 46, 3630–3639. [Google Scholar] [CrossRef]
- Signorile, P.G.; Cassano, M.; Viceconte, R.; Spyrou, M.; Marcattilj, V.; Baldi, A. Endometriosis: A Retrospective Analysis on Diagnostic Data in a Cohort of 4,401 Patients. Vivo 2021, 36, 430–438. [Google Scholar] [CrossRef]
- Van den Bosch, T.; de Bruijn, A.M.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Bourne, T.; Timmerman, D.; Huirne, J.A.F. Sonographic classification and reporting system for diagnosing adenomyosis. Ultrasound Obstet. Gynecol. 2019, 53, 576–582. [Google Scholar] [CrossRef]
- Harmsen, M.J.; Bosch, T.V.D.; de Leeuw, R.A.; Dueholm, M.; Exacoustos, C.; Valentin, L.; Hehenkamp, W.; Groenman, F.; De Bruyn, C.; Rasmussen, C.; et al. Consensus on revised definitions of morphological uterus sonographic assessment (MUSA) features of adenomyosis: Results of a modified Delphi procedure. Ultrasound Obstet. Gynecol. 2021. [Google Scholar] [CrossRef]
- Vimercati, A.; Dellino, M.; Crupano, F.M.; Gargano, G.; Cicinelli, E. Ultrasonic assessment of cesarean section scar to vesicovaginal fold distance: An instrument to estimate pre-labor uterine rupture risk. J. Matern. Fetal Neonatal Med. 2021, 1–5. [Google Scholar] [CrossRef]
- Buonomo, B.; Massarotti, C.; Dellino, M.; Anserini, P.; Ferrari, A.; Campanella, M.; Magnotti, M.; De Stefano, C.; Peccatori, F.A.; Lambertini, M. Reproductive issues in carriers of germline pathogenic variants in the BRCA1/2 genes: An expert meeting. BMC Med. 2021, 19, 205. [Google Scholar] [CrossRef]
- Dellino, M.; Silvestris, E.; Loizzi, V.; Paradiso, A.; Loiacono, R.; Minoia, C.; Daniele, A.; Cormio, G. Germinal ovarian tumors in reproductive age women: Fertility-sparing and outcome. Medicine 2020, 99, e22146. [Google Scholar] [CrossRef]
- Silvestris, E.; Cormio, G.; Skrypets, T.; Dellino, M.; Paradiso, A.V.; Guarini, A.; Minoia, C. Novel aspects on gonadotoxicity and fertility preservation in lymphoproliferative neoplasms. Crit. Rev. Oncol. 2020, 151, 102981. [Google Scholar] [CrossRef]
- Silvestris, E.; Dellino, M.; Cafforio, P.; Paradiso, A.V.; Cormio, G.; D’Oronzo, S. Breast cancer: An update on treatment-related infertility. J. Cancer Res. Clin. Oncol. 2020, 146, 647–657. [Google Scholar] [CrossRef]
- Silvestris, E.; D’Oronzo, S.; Cafforio, P.; Kardhashi, A.; Dellino, M.; Cormio, G. In Vitro Generation of Oocytes from Ovarian Stem Cells (OSCs): In Search of Major Evidence. Int. J. Mol. Sci. 2019, 20, 6225. [Google Scholar] [CrossRef]
- Dellino, M.; Carriero, C.; Silvestris, E.; Capursi, T.; Paradiso, A.; Cormio, G. Primary Vaginal Carcinoma Arising on Cystocele Mimicking Vulvar Cancer. J. Obstet Gynaecol Can. 2020, 42, 1543–1545. [Google Scholar] [CrossRef]
- Dellino, M.; Minoia, C.; Paradiso, A.V.; De Palo, R.; Silvestris, E. Fertility Preservation in Cancer Patients During the Coronavirus (COVID-19) Pandemic. Front. Oncol. 2020, 10, 1009. [Google Scholar] [CrossRef]
- Date, S.; Murthy, B.; Magdum, A. Post B-Lynch Uterine Rupture: Case Report and Review of Literature. J. Obstet. Gynecol. India 2013, 64, 362–363. [Google Scholar] [CrossRef][Green Version]
- Dellino, M.; Crupano, F.M.; Rossi, A.C.; Xuemin, H.; Tinelli, R.; Cicinelli, E.; Vimercati, A. Relationship between prelabour uterine rupture and previous placenta previa diagnosis: Case reports and review of literature. Ital. J. Gynaecol. Obstet. 2022, 34, 48–53. [Google Scholar] [CrossRef]
| Author | N° | Age | Gravida/Para | Dysmenorrhea | Endometriosis /Adenomyosis | Infertility | Gestational Age | Labor | FHR | Fetus | Apgar | Transfusion | Hysterectomy | Prognosis |
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Azziz (1986) [5] | 1 | 41 | NA/P 10 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA |
| 1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 1 | 25 | NA/P0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 1 | 38 | NA/P1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 1 | 33 | NA/P0 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 1 | 25 | NA/P1 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| 1 | 36 | NA/P3 | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | NA | |
| Bensaid et al. (1996) [6] | 1 | 22 | G1/P1 | NA | NA | No | Term | Yes | Severe bradycardia | 3000 g | 0/2 | NA | No | Newborn demise at 3 days |
| Mueller et al. (1996) [7] | 1 | 30 | G1/P0 | NA | No | Yes | 18 | No | NA | NA | NA | NA | Total hysterectomy | Good |
| Pafumi et al. (2001) [8] | 1 | 30 | G3/P2 | NA | No | No | 37 | Yes | NA | 2750 | 8/10 | NA | Total hysterectomy | Good |
| Villa et al. (2008) [9] | 1 | 30 | G1/P1 | Yes | Rectovaginal septum endometriosis | NA | 37 w + 5 d | Yes | Normal | 2600 | Alive | NA | Rupture 6h postpartum, total hysterectomy | Good |
| Nikolaou et al. (2013) [12] | 1 | 33 | G1/P1 | Yes | Ovarian endometriosis | Yes | 28 | No | Decelerations | 1310 g | 6/6 | 6RBC e 3FFP | Subtotal hysterectomy | Good |
| Indraccolo et al. (2015) [13] | 1 | 37 | G2/P0 | Yes | Laparoscopy with adhesiolysis for chronic pelvic pain- nodule of the uterine posterior wall and diagnosing an adenomyosis | NA | 36 | No | Decelerations | NA | 9/10 | No | No | Good |
| Li et al. (2021) [14] | 1 | 32 | G1/P0- twin pregnancy | Yes | Yes | Yes | 29 | No | NA | Fetus 1—1440 g Fetus 2—1310 g | Fetus 1:3 /8 5/7 | 2RBC | No | Good |
| Present case | 1 | 27 | G0/P0 | Yes | Yes | No | 21 w | No | Severe bradycardia | 460 g | Alive | 2RBC | Total hysterectomy | Good |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vimercati, A.; Dellino, M.; Suma, C.; Damiani, G.R.; Malvasi, A.; Cazzato, G.; Cascardi, E.; Resta, L.; Cicinelli, E. Spontaneous Uterine Rupture and Adenomyosis, a Rare but Possible Correlation: Case Report and Literature Review. Diagnostics 2022, 12, 1574. https://doi.org/10.3390/diagnostics12071574
Vimercati A, Dellino M, Suma C, Damiani GR, Malvasi A, Cazzato G, Cascardi E, Resta L, Cicinelli E. Spontaneous Uterine Rupture and Adenomyosis, a Rare but Possible Correlation: Case Report and Literature Review. Diagnostics. 2022; 12(7):1574. https://doi.org/10.3390/diagnostics12071574
Chicago/Turabian StyleVimercati, Antonella, Miriam Dellino, Cosimina Suma, Gianluca Raffaello Damiani, Antonio Malvasi, Gerardo Cazzato, Eliano Cascardi, Leonardo Resta, and Ettore Cicinelli. 2022. "Spontaneous Uterine Rupture and Adenomyosis, a Rare but Possible Correlation: Case Report and Literature Review" Diagnostics 12, no. 7: 1574. https://doi.org/10.3390/diagnostics12071574
APA StyleVimercati, A., Dellino, M., Suma, C., Damiani, G. R., Malvasi, A., Cazzato, G., Cascardi, E., Resta, L., & Cicinelli, E. (2022). Spontaneous Uterine Rupture and Adenomyosis, a Rare but Possible Correlation: Case Report and Literature Review. Diagnostics, 12(7), 1574. https://doi.org/10.3390/diagnostics12071574











