Monitoring Protein Denaturation of Egg White Using Passive Microwave Radiometry (MWR)
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Goryanin, I.; Karbainov, S.; Shevelev, O.; Tarakanov, A.; Redpath, K.; Vesnin, S.; Ivanov, Y. Passive microwave radiometry in biomedical studies. Drug Discov. Today 2020, 25, 757–763. [Google Scholar] [CrossRef] [PubMed]
- Shevelev, O.; Petrova, M.; Smolensky, A.; Osmonov, B.; Toimatov, S.; Kharybina, T.; Karbainov, S.; Goryanin, I. Using medical microwave radiometry for brain temperature measurements. Drug Discov. Today 2021, 27, 881–889. [Google Scholar] [CrossRef] [PubMed]
- Levshinskii, V.; Galazis, C.; Losev, A.; Zamechnik, T.; Kharybina, T.; Vesnin, S.; Goryanin, I. Using AI and passive medical radiometry for diagnostics (MWR) of venous diseases. Comput. Methods Programs Biomed. 2022, 215, 106611. [Google Scholar] [CrossRef] [PubMed]
- Vesnin, S.; Turnbull, A.K.; Dixon, J.M.; Goryanin, I. Modern microwave thermometry for breast cancer. J. Mol. Imaging Dyn. 2017, 7, 1000136. [Google Scholar]
- Stauffer, P.R.; Snow, B.W.; Rodrigues, D.B.; Salahi, S.; de Oliveira, T.R.; Reudink, D.; Maccarini, P.F. Non-Invasive Measurement of Brain Temperature with Microwave Radiometry: Demonstration in a Head Phantom and Clinical Case. Neuroradiol. J. 2014, 27, 3–12. [Google Scholar] [CrossRef]
- Hand, W. Monitoring of deep brain temperature in infants using multi-frequency microwave radiometry and thermal modelling. Phys. Med. Biol. 2001, 46, 1885. [Google Scholar] [CrossRef]
- Toutouzas, K.; Synetos, A.; Nikolaou, C.; Stathogiannis, K.; Tsiamis, E.; Stefanadis, C. Microwave radiometry: A new non-invasive method for the detection of vulnerable plaque. Cardiovasc. Diagn. Ther. 2012, 2, 290–297. [Google Scholar]
- Vesnin, S.G.; Sedankin, M.K.; Gudkov, A.G.; Leushin, V.Y.; Sidorov, I.A.; Porokhov, I.O.; Agasieva, S.V.; Vidyakin, S.I. A Printed Antenna with an Infrared Temperature Sensor for a Medical Multichannel Microwave Radiometer. Biomed. Eng. 2020, 54, 235–239. [Google Scholar] [CrossRef]
- Ring, E.F.J.; Ammer, K. Infrared thermal imaging in medicine. Physiol. Meas. 2012, 33, R33–R46. [Google Scholar] [CrossRef]
- Ivanov, Y.; Malsagova, K.; Izotov, A.; Pleshakova, T.; Tatur, V.; Vesnin, S.; Ivanova, N.; Usanov, S.; Archakov, A. Detection of microwave radiation of cytochrome CYP102 A1 solution during the enzyme reaction. Biochem. Biophys. Rep. 2016, 5, 285–289. [Google Scholar] [CrossRef][Green Version]
- Ivanov, Y.; Kozlov, A.; Malsagova, K.A.; Pleshakova, T.; Vesnin, S.; Tatur, V.; Ivanova, N.; Ziborov, V. Monitoring of microwave emission of HRP system during the enzyme functioning. Biochem. Biophys. Rep. 2016, 7, 20–25. [Google Scholar] [CrossRef] [PubMed]
- Ivanov, Y.; Kozlov, A.F.; Galiullin, R.A.; Tatur, V.Y.; Ziborov, V.S.; Ivanova, N.D.; Goryanin, I. Use of microwave radiometry to monitor thermal denaturation of albumin. Front. Physiol. 2018, 9, 956. [Google Scholar] [CrossRef]
- Nishio, H.; Dugaiczyk, A. Complete structure of the human alpha-albumin gene, a new member of the serum albumin multigene family. Proc. Natl. Acad. Sci. USA 1996, 93, 7557–7561. [Google Scholar] [CrossRef]
- Bunking, A.F.; Pershin, S.M. Coherent laser spectroscopy of the processes of hydration of biomolecules and nanoparticles. Proc. Inst. Gen. Phys. 2013, 69, 58–69. [Google Scholar]
- Borzova, V.; Markossian, K.A.; Chebotareva, N.A.; Kleymenov, S.Y.; Poliansky, N.B.; Muranov, K.O.; Stein-Margolina, V.A.; Shubin, V.V.; Markov, D.I.; Kurganov, B.I. Kinetics of Thermal Denaturation and Aggregation of Bovine Serum Albumin. PLoS ONE 2016, 11, e0153495. [Google Scholar] [CrossRef] [PubMed]
- Vetri, V.; Librizzi, F.; Leone, M.; Militello, V. Thermal aggregation of bovine serum albumin at different pH: Comparison with human serum albumin. Eur. Biophys. J. 2007, 36, 717–725. [Google Scholar] [CrossRef]
- Liu, R.; Qin, P.; Wang, L.; Zhao, X.; Liu, Y.; Hao, X. Toxic effects of ethanol on bovine serum albumin. J. Biochem. Mol. Toxicol. 2010, 24, 66–71. [Google Scholar] [CrossRef] [PubMed]
- Pershin, S.M.; Bunkin, A.F.; Anisimov, N.V.; Pirogov, Y.A. Water enrichment by H2O ortho-isomer: Four-photon and NMR spectroscopy. Laser Phys. 2009, 19, 410–413. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Malsagova, K.A.; Tatur, V.Y.; Vesnin, S.G.; Ivanova, N.D.; Ziborov, V.S. Microwave Radiation of Water in Analytical Systems. Pathol. Physiol. Exp. Ther. 2015, 59, 78–81. [Google Scholar]
- Wilson, B.C.; Patterson, M.S. The physics of photodynamic therapy. Phys. Med. Biol. 1986, 31, 327. [Google Scholar] [CrossRef]
- Matthews, Q.; Jirasek, A.; Lum, J.J.; Brolo, A.G. Biochemical signatures of in vitro radiation response in human lung, breast and prostate tumor cells observed with Raman spectroscopy. Phys. Med. Biol. 2011, 56, 6839. [Google Scholar] [CrossRef] [PubMed]
- Mak, P.J.; Luthra, A.; Sligar, S.G.; Kincaid, J.R. Resonance Raman spectroscopy of the oxygenated intermediates of human CYP19A1 implicates a compound I intermediate in the final lyase step. J. Am. Chem. Soc. 2014, 136, 4825–4828. [Google Scholar] [CrossRef] [PubMed]
- Betskii, O.V.; Kislov, V.V.; Lebedeva, N.N. Millimeter Waves and Living Systems; Moscow Science Press: Moscow, Russia, 2004. [Google Scholar]
- Barrett, A.H.; Myers, P.C. Subcutaneous Temperature: A method of Noninvasive Sensing. Science 1975, 190, 669–671. [Google Scholar] [CrossRef]
- Ivanov, Y.D.; Malsagova, K.A.; Pleshakova, T.O.; Vesnin, S.G.; Tatur, V.Y.; Yarygin, K.N. Monitoring of the brightness temperature of a suspension of cells of follicular thyroid carcinoma in the microwave range using radiothermometry. Pathol. Physiol. Exp. Ther. 2016, 60, 174–177. [Google Scholar]
- Al-Alousi, L.M.; Anderson, R.A.; Land, D.V. A noninvasive method for postmortem temperature measurements using a microwave probe. Forensic Sci. Int. 1994, 64, 35–46. [Google Scholar] [CrossRef]
- Cairns, C.J.; Andrews, P.J. Management of hyperthermia in traumatic brain injury. Curr. Opin. Crit. Care 2002, 8, 106–110. [Google Scholar] [CrossRef]
- Greer, D.M.; Funk, S.E.; Reaven, N.L.; Ouzounelli, M.; Uman, G.C. Impact of fever on outcome in patients with stroke and neurologic injury: A comprehensive meta-analysis. Stroke 2008, 39, 3029–3035. [Google Scholar] [CrossRef]
- Fernandez, A.; Schmidt, J.M.; Claassen, J.; Pavlicova, M.; Huddleston, D.; Kreiter, K.T.; Ostapkovich, N.D.; Kowalski, R.G.; Parra, A.; Connolly, E.S.; et al. Fever after subarachnoid hemorrhage: Risk factors and impact on outcome. Neurology 2007, 68, 1013–1019. [Google Scholar] [CrossRef]
- Ginès, P.; Arroyo, V. Is there still a need for albumin infusions to treat patients with liver disease? Gut 2012, 46, 588–590. [Google Scholar] [CrossRef]
- Ginsberg, M.D.; Palesch, Y.Y.; Hill, M.D.; Martin, R.H.; Moy, C.S.; Barsan, W.G.; Waldman, B.D.; Tamariz, D.; Ryckborst, K.J. High-dose albumin treatment for acute ischaemic stroke (ALIAS) Part 2: A randomised, double-blind, phase 3, placebo-controlled trial. Lancet Neurol. 2013, 12, 1049–1058. [Google Scholar] [CrossRef]

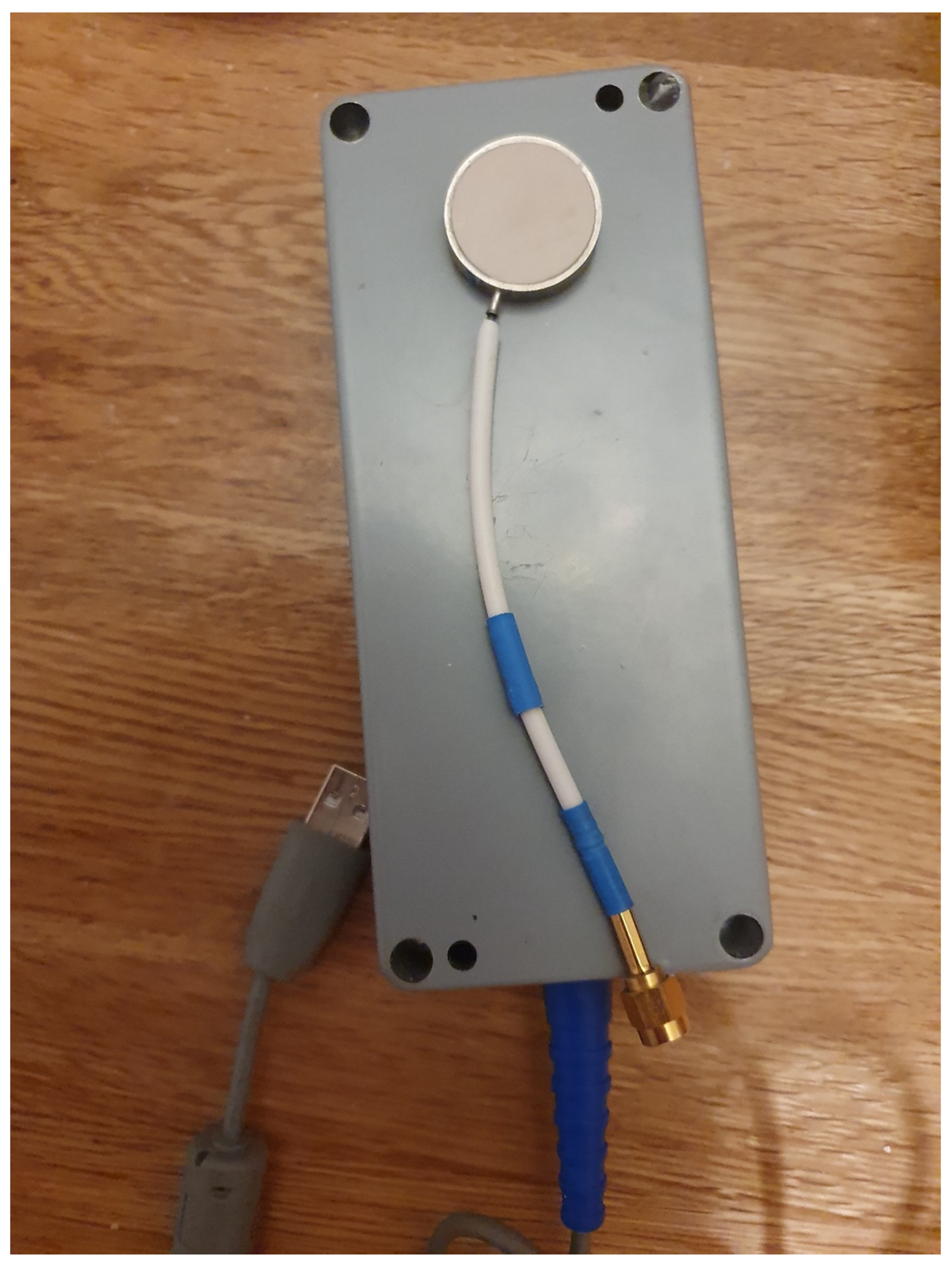





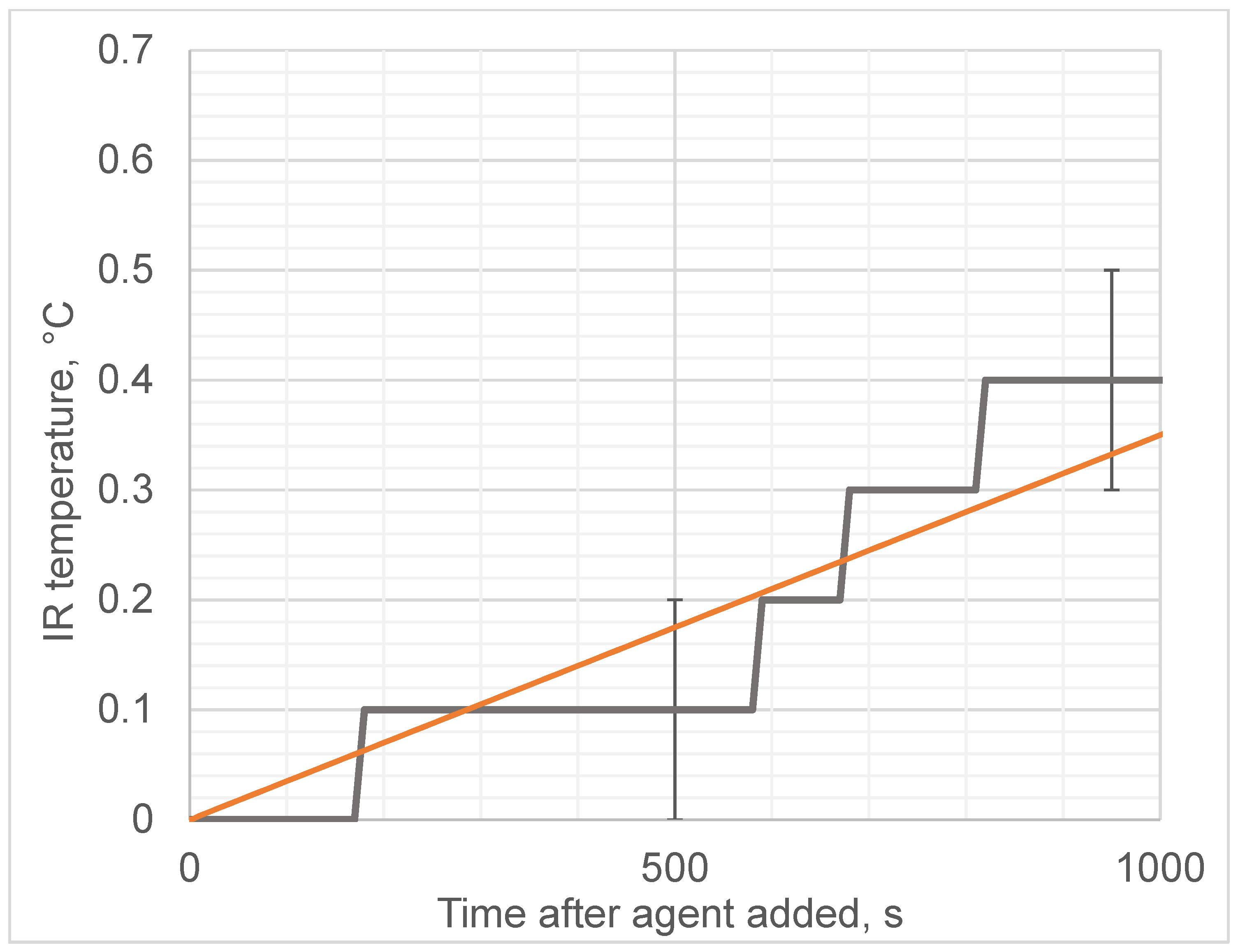
| Experiment | Linear Slope (MWR), K/s | Linear Slope (IR), K/s |
|---|---|---|
| Egg and alcohol | 0.127 ± 0.007 | 3.5 × 10−4 ± 0.7 × 10−4 |
| Egg and water | −7.5 × 10−3 ± 1.5 × 10−3 | 2.5 × 10−3 ± 0.2 × 10−3 |
| Water and alcohol | 0.073 ± 0.01 | 0.015 ± 0.005 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Goryanin, I.; Ovchinnikov, L.; Vesnin, S.; Ivanov, Y. Monitoring Protein Denaturation of Egg White Using Passive Microwave Radiometry (MWR). Diagnostics 2022, 12, 1498. https://doi.org/10.3390/diagnostics12061498
Goryanin I, Ovchinnikov L, Vesnin S, Ivanov Y. Monitoring Protein Denaturation of Egg White Using Passive Microwave Radiometry (MWR). Diagnostics. 2022; 12(6):1498. https://doi.org/10.3390/diagnostics12061498
Chicago/Turabian StyleGoryanin, Igor, Lev Ovchinnikov, Sergey Vesnin, and Yuri Ivanov. 2022. "Monitoring Protein Denaturation of Egg White Using Passive Microwave Radiometry (MWR)" Diagnostics 12, no. 6: 1498. https://doi.org/10.3390/diagnostics12061498
APA StyleGoryanin, I., Ovchinnikov, L., Vesnin, S., & Ivanov, Y. (2022). Monitoring Protein Denaturation of Egg White Using Passive Microwave Radiometry (MWR). Diagnostics, 12(6), 1498. https://doi.org/10.3390/diagnostics12061498






