Evaluating the Impact of High Intensity Interval Training on Axial Psoriatic Arthritis Based on MR Images
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patient Cohort
2.2. Disease Activity Scores
2.3. MR Image Acquisition
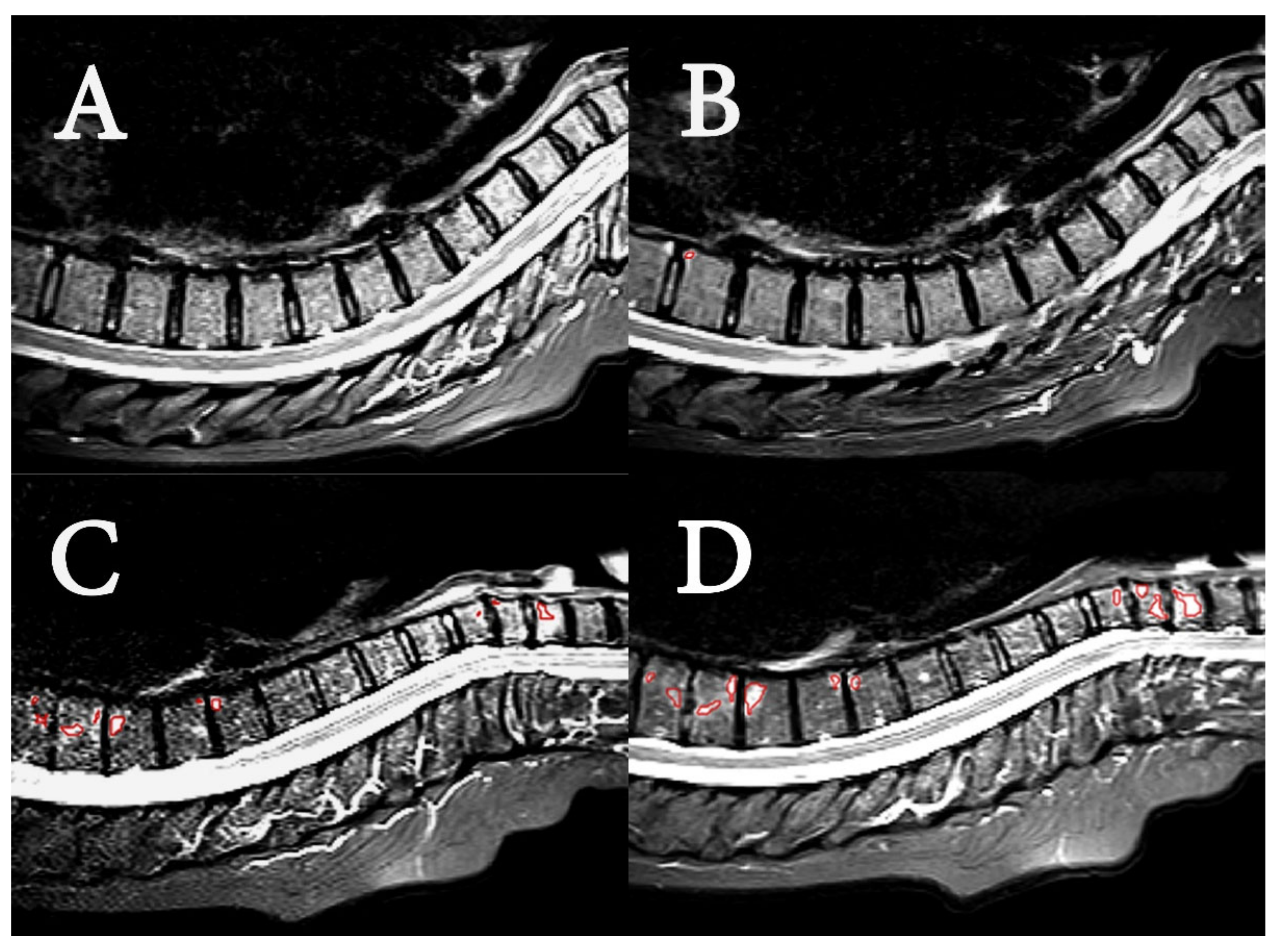
2.4. Image Analysis
- Radiological evaluation
- SPARCC scoring
- Image pre-processing and textural feature extraction
2.5. Statistical Analysis
3. Results
3.1. Patient Cohort
3.2. Image Analysis
- Radiological evaluation
- SPARCC scoring
- Textural features
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Appendix A
| Intensity features | Grey-level intensity value of the central pixel | |
| Mean of grey-level intensity values | ||
| Median of grey-level intensity values | ||
| Standard deviation of grey-level intensity values | ||
| Minimum of grey-level intensity values | ||
| Maximum of grey-level intensity values | ||
| Semi-interquartile range of the grey-level intensity values | ||
| Gradient features | Sum of | |
| Sum of | ||
| Mean of | ||
| Mean of | ||
| Standard deviation of | ||
| Standard deviation of | ||
| Median of | ||
| Minimum of | ||
| Maximum of | ||
| Semi-interquartile range of | ||
| GLCM features | energy | |
| contrast | ||
| correlation | ||
| homogeneity (inverse difference moment) | ||
| GLCM: grey level co-occurrence matrix | ||
References
- Wright, V.; Moll, J.M.H. (Eds.) Psoriatic Arthritis. In Seronegative Polyarthritis; North Holland Publishing Co.: Amsterdam, The Netherlands, 1976; pp. 169–223. [Google Scholar]
- Peters, M.J.; van der Horst-Bruinsma, I.E.; Dijkmans, B.A.; Nurmohamed, M.T. Cardiovascular risk profile of patients with spondylarthropathies, particularly ankylosing spondylitis and psoriatic arthritis. Semin. Arthritis Rheum. 2004, 34, 585–592. [Google Scholar] [CrossRef] [PubMed]
- Boehncke, W.-H.; Boehncke, S.; Tobin, A.-M.; Kirby, B. The ‘psoriatic march’: A concept of how severe psoriasis may drive cardiovascular comorbidity. Exp. Dermatol. 2011, 20, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Hoff, M.; Gulati, A.M.; Romundstad, P.R.; Kavanaugh, A.; Haugeberg, G. Prevalence and incidence rates of psoriatic arthritis in central Norway: Data from the Nord-Trøndelag Health Study (HUNT). Ann. Rheum. Dis. 2015, 74, 60–64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ibrahim, G.; Waxman, R.; Helliwell, P.S. The prevalence of psoriatic arthritis in people with psoriasis. Arthritis Care Res. 2009, 61, 1373–1378. [Google Scholar] [CrossRef]
- Baraliakos, X.; Coates, L.C.; Braun, J. The involvement of the spine in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S31–S35. [Google Scholar]
- Sveaas, S.H.; Smedslund, G.; Hagen, K.B.; Dagfinrud, H. Effect of cardiorespiratory and strength exercises on disease activity in patients with inflammatory rheumatic diseases: A systematic review and meta-analysis. Br. J. Sports Med. 2017, 51, 1065–1072. [Google Scholar] [CrossRef]
- Coates, L.C.; Kavanaugh, A.; Mease, P.J.; Soriano, E.R.; Acosta-Felquer, M.L.; Armstrong, A.W.; Bautista-Molano, W.; Boehncke, W.-H.; Campbell, W.; Cauli, A.; et al. Group for Research and Assessment of Psoriasis and Psoriatic Arthritis 2015 Treatment Recommendations for Psoriatic Arthritis. Arthritis Rheumatol. 2016, 68, 1060–1071. [Google Scholar] [CrossRef] [Green Version]
- Sandstad, J.; Stensvold, D.; Hoff, M.; Nes, B.M.; Arbo, I.; Bye, A. The effects of high intensity interval training in women with rheumatic disease: A pilot study. J. Appl. Physiol. 2015, 115, 2081–2089. [Google Scholar] [CrossRef]
- Ramos, J.; Dalleck, L.C.; Tjonna, A.E.; Beetham, K.; Coombes, J.S. The Impact of High-Intensity Interval Training Versus Moderate-Intensity Continuous Training on Vascular Function: A Systematic Review and Meta-Analysis. Sports Med. 2015, 45, 679–692. [Google Scholar] [CrossRef]
- Weston, K.S.; Wisløff, U.; Coombes, J.S. High-intensity interval training in patients with lifestyle-induced cardiometabolic disease: A systematic review and meta-analysis. Br. J. Sports Med. 2014, 48, 1227–1234. [Google Scholar] [CrossRef]
- Gleeson, M.; Bishop, N.C.; Stensel, D.J.; Lindley, M.R.; Mastana, S.S.; Nimmo, M.A. The anti-inflammatory effects of exercise: Mechanisms and implications for the prevention and treatment of disease. Nat. Rev. Immunol. 2011, 11, 607–615. [Google Scholar] [CrossRef] [PubMed]
- Sveaas, S.H.; Bilberg, A.; Berg, I.J.; Provan, S.A.; Rollefstad, S.; Semb, A.G.; Hagen, K.B.; Johansen, M.W.; Pedersen, E.; Dagfinrud, H. High intensity exercise for 3 months reduces disease activity in axial spondyloarthritis (axSpA): A multicentre randomised trial of 100 patients. Br. J. Sports Med. 2019, 54, 292–297. [Google Scholar] [CrossRef] [PubMed]
- Thomsen, R.S.; Nilsen, T.I.L.; Haugeberg, G.; Bye, A.; Kavanaugh, A.; Hoff, M. Effect of high-intensity interval training on cardiovascular disease risk factors and body composition in psoriatic arthritis: A randomised controlled trial. RMD Open 2018, 4, e000729. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomsen, R.S.; Nilsen, T.I.L.; Haugeberg, G.; Bye, A.; Kavanaugh, A.; Hoff, M. Impact of High-Intensity Interval Training on Disease Activity and Disease in Patients with Psoriatic Arthritis: A Randomized Controlled Trial. Arthritis Care Res. 2019, 71, 530–537. [Google Scholar] [CrossRef]
- Baraliakos, X.; Richter, A.; Feldmann, D.; Ott, A.; Buelow, R.; Schmidt, O.C.; Braun, J. Which factors are associated with bone marrow oedema suspicious of axial spondyloarthritis as detected by MRI in the sacroiliac joints and the spine in the general population? Ann. Rheum. Dis. 2020, 80, 469–474. [Google Scholar] [CrossRef]
- Horga, L.M.; Henckel, J.; Fotiadou, A.; Di Laura, A.; Hirschmann, A.; Hart, A. 3.0 T MRI findings of 104 hips of asymptomatic adults: From non-runners to ultra-distance runners. BMJ Open Sport Exerc. Med. 2021, 7, e000997. [Google Scholar] [CrossRef]
- Simon, M.J.; Barvencik, F.; Luttke, M.; Amling, M.; Mueller-Wohlfahrt, H.-W.; Ueblacker, P. Intravenous bisphosphonates and vitamin D in the treatment of bone marrow oedema in professional athletes. Injury 2014, 45, 981–987. [Google Scholar] [CrossRef]
- Grampp, S.; Henk, C.B.; Mostbeck, G.H. Overuse Edema in the Bone Marrow of the Hand: Demonstration with MRI. J. Comput. Assist. Tomogr. 1998, 22, 25–27. [Google Scholar] [CrossRef]
- Elias, I.; Zoga, A.C.; Raikin, S.M.; Peterson, J.R.; Besser, M.P.; Morrison, W.B.; Schweitzer, E.M. Bone stress injury of the ankle in professional ballet dancers seen on MRI. BMC Musculoskelet. Disord. 2008, 9, 39. [Google Scholar] [CrossRef] [Green Version]
- Debusschere, K.; Cambré, I.; Gracey, E.; Elewaut, D. Born to run: The paradox of biomechanical force in spondyloarthritis from an evolutionary perspective. Best Pr. Res. Clin. Rheumatol. 2017, 31, 887–894. [Google Scholar] [CrossRef]
- Schett, G.; Lories, R.J.; D’Agostino, M.-A.; Elewaut, D.; Kirkham, B.; Soriano, E.R.; McGonagle, D. Enthesitis: From pathophysiology to treatment. Nat. Rev. Rheumatol. 2017, 13, 731–741. [Google Scholar] [CrossRef] [PubMed]
- Poggenborg, R.P.; Sørensen, I.J.; Pedersen, S.J.; Østergaard, M. Magnetic resonance imaging for diagnosing, monitoring and prognostication in psoriatic arthritis. Clin. Exp. Rheumatol. 2015, 33, S66–S69. [Google Scholar] [PubMed]
- Mackay, J.W.; Aboelmagd, S.; Gaffney, J.K. Correlation between clinical and MRI disease activity scores in axial spondyloarthritis. Clin. Rheumatol. 2015, 34, 1633–1638. [Google Scholar] [CrossRef] [PubMed]
- Braun, J.; Baraliakos, X.; Golder, W.; Brandt, J.; Rudwaleit, M.; Listing, J.; Bollow, M.; Sieper, J.; Van Der Heijde, D. Magnetic resonance imaging examinations of the spine in patients with ankylosing spondylitis, before and after successful therapy with infliximab: Evaluation of a new scoring system. Arthritis Care Res. 2003, 48, 1126–1136. [Google Scholar] [CrossRef] [PubMed]
- Maksymowych, W.P.; Inman, R.; Salonen, D.; Dhillon, S.S.; Krishnananthan, R.; Stone, M.; Conner-Spady, B.; Palsat, J.; Lambert, R. Spondyloarthritis research consortium of canada magnetic resonance imaging index for assessment of spinal inflammation in ankylosing spondylitis. Arthritis Care Res. 2005, 53, 502–509. [Google Scholar] [CrossRef] [PubMed]
- Fritz, J.; Henes, J.C.; Thomas, C.; Clasen, S.; Fenchel, M.; Claussen, C.D.; Lewin, J.S.; Pereira, P.L. Diagnostic and Interventional MRI of the Sacroiliac Joints Using a 1.5-T Open-Bore Magnet: A One-Stop-Shopping Approach. Am. J. Roentgenol. 2008, 191, 1717–1724. [Google Scholar] [CrossRef]
- Rudwaleit, M.; Jurik, A.G.; Hermann, K.-G.; Landewé, R.; Van Der Heijde, D.; Baraliakos, X.; Marzo-Ortega, H.; Ostergaard, M.; Braun, J.; Sieper, J. Defining active sacroiliitis on magnetic resonance imaging (MRI) for classification of axial spondyloarthritis: A consensual approach by the ASAS/OMERACT MRI group. Ann. Rheum. Dis. 2009, 68, 1520–1527. [Google Scholar] [CrossRef]
- Tuceryan, M.; Jain, A.K. Texture Analysis. In The Handbook of Pattern Recognition and Computer Vision, 2nd ed.; Chen, C.H., Pau, L.F., Wang, P.S.P., Eds.; World Scientific Publishing Co.: Singapore, 1998; pp. 207–248. [Google Scholar]
- Mayerhoefer, M.E.; Breitenseher, M.J.; Kramer, J.; Aigner, N.; Hofmann, S.; Materka, A. Texture analysis for tissue discrimination on T1-weighted MR images of the knee joint in a multicenter study: Transferability of texture features and comparison of feature selection methods and classifiers. J. Magn. Reson. Imaging 2005, 22, 674–680. [Google Scholar] [CrossRef]
- Mayerhoefer, M.E.; Breitenseher, M.J.; Kramer, J.; Aigner, N.; Norden, C.; Hofmann, S. STIR vs. T1-weighted fat-suppressed gadolinium-enhanced MRI of bone marrow edema of the knee: Computer-assisted quantitative comparison and influence of injected contrast media volume and acquisition parameters. J. Magn. Reson. Imaging 2005, 22, 788–793. [Google Scholar] [CrossRef]
- Heilmeier, U.; Wamba, J.M.; Joseph, G.B.; Darakananda, K.; Callan, J.; Neumann, J.; Link, T.M. Baseline knee joint effusion and medial femoral bone marrow edema, in addition to MRI-based T2 relaxation time and texture measurements of knee cartilage, can help predict incident total knee arthroplasty 4–7 years later: Data from the Osteoarthritis Initiative. Skelet. Radiol. 2019, 48, 89–101. [Google Scholar] [CrossRef]
- Chuah, T.K.; Poh, C.L.; Sheah, K. Quantitative texture analysis of MRI images for detection of cartilage-related bone marrow edema. Conf. Proc. IEEE Eng. Med. Biol. Soc. 2011, 5112–5115. [Google Scholar] [CrossRef]
- Chuah, T.K.; Van Reeth, E.; Sheah, K.; Poh, C.L. Texture analysis of bone marrow in knee MRI for classification of subjects with bone marrow lesion—Data from the Osteoarthritis Initiative. Magn. Reson. Imaging 2013, 31, 930–938. [Google Scholar] [CrossRef] [PubMed]
- Faleiros, M.C.; Nogueira-Barbosa, M.H.; Dalto, V.; Junior, J.R.F.; Tenorio, A.P.M.; Luppino-Assad, R.; Louzada-Junior, P.; Rangayyan, R.M.; De Azevedo-Marques, P.M. Machine learning techniques for computer-aided classification of active inflammatory sacroiliitis in magnetic resonance imaging. Adv. Rheumatol. 2020, 60, 25. [Google Scholar] [CrossRef] [PubMed]
- Sikiö, M.; Harrison, L.C.V.; Nikander, R.; Ryymin, P.; Dastidar, P.; Eskola, H.; Sievänen, H. Influence of exercise loading on magnetic resonance image texture of thigh soft tissues. Clin. Physiol. Funct. Imaging 2014, 34, 370–376. [Google Scholar] [CrossRef]
- Taylor, W.; Gladman, D.; Helliwell, P.; Marchesoni, A.; Mease, P.; Mielants, H.; CASPAR Study Group. Classification criteria for psoriatic arthritis: Development of new criteria from a large international study. Arthritis Care Res. 2006, 54, 2665–2673. [Google Scholar] [CrossRef]
- Chronaiou, I.; Thomsen, R.S.; Huuse, E.M.; Euceda, L.R.; Pedersen, S.J.; Hoff, M.; Sitter, B. Quantifying bone marrow inflammatory edema in the spine and sacroiliac joints with thresholding. BMC Musculoskelet. Disord. 2017, 18, 497. [Google Scholar] [CrossRef] [Green Version]
- Lambert, R.G.; Bakker, P.A.; Van Der Heijde, D.; Weber, U.; Rudwaleit, M.; Hermann, K.-G.; Sieper, J.; Baraliakos, X.; Bennett, A.; Braun, J.; et al. Defining active sacroiliitis on MRI for classification of axial spondyloarthritis: Update by the ASAS MRI working group. Ann. Rheum. Dis. 2016, 75, 1958–1963. [Google Scholar] [CrossRef]
- Maksymowych, W.P.; Lambert, R.G.; Brown, L.S.; Pangan, A.L. Defining the Minimally Important Change for the SpondyloArthritis Research Consortium of Canada Spine and Sacroiliac Joint Magnetic Resonance Imaging Indices for Ankylosing Spondylitis. J. Rheumatol. 2012, 39, 1666–1674. [Google Scholar] [CrossRef] [Green Version]
- Tustison, N.J.; Avants, B.B.; Cook, P.A.; Zheng, Y.; Egan, A.; Yushkevich, P.A.; Gee, J.C. N4ITK: Improved N3 Bias Correction. IEEE Trans. Med. Imaging 2010, 29, 1310–1320. [Google Scholar] [CrossRef] [Green Version]
- Perona, P.; Malik, J. Scale-space and edge detection using anisotropic diffusion. IEEE Trans. Pattern Anal. Mach. Intell. 1990, 12, 629–639. [Google Scholar] [CrossRef] [Green Version]
- De Winter, J.; De Hooge, M.; Van De Sande, M.; De Jong, H.; Van Hoeven, L.; De Koning, A.; Berg, I.J.; Ramonda, R.; Baeten, D.; Van Der Heijde, D.; et al. Magnetic Resonance Imaging of the Sacroiliac Joints Indicating Sacroiliitis According to the Assessment of SpondyloArthritis international Society Definition in Healthy Individuals, Runners, and Women with Postpartum Back Pain. Arthritis Rheumatol. 2018, 70, 1042–1048. [Google Scholar] [CrossRef] [PubMed]
- Lukas, C.; Braun, J.; Van Der Heijde, D.; Hermann, A.K.-G.; Rudwaleit, M.; Østergaard, M.; Oostveen, A.; O’Connor, P.; Maksymowych, W.P.; Lambert, R.G.W.; et al. Scoring inflammatory activity of the spine by magnetic resonance imaging in ankylosing spondylitis: A multireader experiment. J. Rheumatol. 2007, 34, 862–870. [Google Scholar] [PubMed]
- Maksymowych, W.P.; Lambert, R.G.; Østergaard, M.; Pedersen, S.J.; Machado, P.M.; Weber, U.; Bennett, A.N.; Braun, J.; Burgos-Vargas, R.; De Hooge, M.; et al. MRI lesions in the sacroiliac joints of patients with spondyloarthritis: An update of definitions and validation by the ASAS MRI working group. Ann. Rheum. Dis. 2019, 78, 1550–1558. [Google Scholar] [CrossRef] [PubMed]
- Hu, Z.; Xu, M.; Li, Q.; Lin, Z.; Liao, Z.; Cao, S.; Wei, Q.; Zhang, Y.L.; Li, T.-W.; Jin, O.; et al. Adalimumab significantly reduces inflammation and serum DKK-1 level but increases fatty deposition in lumbar spine in active ankylosing spondylitis. Int. J. Rheum. Dis. 2012, 15, 358–365. [Google Scholar] [CrossRef]
- Lambert, R.; Salonen, D.; Rahman, P.; Inman, R.; Wong, R.L.; Einstein, S.G.; Thomson, G.T.D.; Beaulieu, A.; Choquette, D.; Maksymowych, W.P. Adalimumab significantly reduces both spinal and sacroiliac joint inflammation in patients with ankylosing spondylitis: A multicenter, randomized, double-blind, placebo-controlled study. Arthritis Care Res. 2007, 56, 4005–4014. [Google Scholar] [CrossRef]
- Romanos, O.; Solomou, E.; Georgiadis, P.; Kardamakis, D.; Siablis, D. Magnetic resonance imaging and image analysis of post—radiation changes of bone marrow in patients with skeletal metastases. J. BUON 2013, 18, 789. [Google Scholar]
- Mackay, J.W.; Murray, P.J.; Kasmai, B.; Johnson, G.; Donell, S.T.; Toms, A.P. MRI texture analysis of subchondral bone at the tibial plateau. Eur. Radiol. 2016, 26, 3034–3045. [Google Scholar] [CrossRef] [Green Version]
- Mackay, J.W.; Murray, P.J.; Low, S.B.L.; Kasmai, B.; Johnson, G.; Donell, S.T.; Toms, A.P. Quantitative analysis of tibial subchondral bone: Texture analysis outperforms conventional trabecular microarchitecture analysis. J. Magn. Reson. Imaging 2016, 43, 1159–1170. [Google Scholar] [CrossRef]
- Harrison, L.C.; Nikander, R.; Sikiö, M.; Luukkaala, T.; Helminen, M.T.; Ryymin, P.; Soimakallio, S.; Eskola, H.J.; Dastidar, P.; Sievänen, H. MRI texture analysis of femoral neck: Detection of exercise load-associated differences in trabecular bone. J. Magn. Reson. Imaging 2011, 34, 1359–1366. [Google Scholar] [CrossRef]

| HIIT | Controls | |||||
|---|---|---|---|---|---|---|
| Baseline | 11 Weeks | p | Baseline | 11 Weeks | p | |
| Number, n | 19 | - | 20 | - | ||
| Men, n (%) | 4 (21) | - | 8 (40) | - | ||
| Women, n (%) | 15 (79) | - | 12 (60) | - | ||
| Age, median (range) | 52 (39–64) | - | 45 (23–64) | - | ||
| PGA a, median (range) | 50.0 (1.0–95.0) | 43.0 (3.0– 81.0) | 0.465 | 46.0 (6–85) | 35.5 (0–89) | 0.227 |
| hs-CRP b mg/L, median (range) | 1.8 (0.4–24.0) | 2.1 (0.5–10.2) | 0.096 | 2.2 (0.1–28.7) | 2.2 (0.3–22.0) | 0.571 |
| BASDAI c, median (range) | 4.0 (0.4–8.3) | 3.2 (0.5–6.6) | 0.049 | 3.7 (0.3–6.7) | 2.6 (0.2–7.7) | 0.133 |
| DAS44 d, median (range) | 2.3 (0.8–3.3) | 1.9 (0.5–2.4) | 0.001 | 2.3 (0.6–3.1) | 1.7 (0.6–3.0) | 0.007 |
| HIIT | Controls | |||
|---|---|---|---|---|
| Baseline | 11 Weeks | Baseline | 11 Weeks | |
| Number, n | 17 | 20 | ||
| BME detected, n (%) | 9 (53) | 9 (53) | 5 (25) | 5 (25) |
| No change, n (%) | 17 (100) | 17 (85) | ||
| Increased BME, n (%) | 0 (0) | 1 (5) | ||
| Reduced BME, n (%) | 0 (0) | 2 (10) | ||
| HIIT | Controls | |||
|---|---|---|---|---|
| Baseline | 11 Weeks | Baseline | 11 Weeks | |
| Number, n | 17 | 20 | ||
| SPARCC a score > 0, n (%) | 13 (72) | 13 (72) | 13 (65) | 10 (50) |
| SPARCC a score, median (max value) | 4.0 (39) | 5.0 (50) | 4.0 (36) | 0.5 (20) |
| No change in SPARCC a score, n (%) | 14 (82) | 16 (80) | ||
| Increased SPARCC a score, n (%) | 1 (6) | 1 (5) | ||
| Reduced SPARCC a score, n (%) | 2 (12) | 3 (15) | ||
| Voxels with BME (N = 3289) | Healthy Voxels (N = 3289) | |||||
|---|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p-Value | q-Value | |
| i1 | 159.8 | 21.0 | 117.1 | 21.7 | <0.001 | <0.001 |
| i2 | 150.1 | 18.0 | 116.6 | 16.9 | <0.001 | <0.001 |
| i3 | 153.2 | 19.0 | 117.5 | 16.5 | <0.001 | <0.001 |
| i4 | 20.6 | 10.2 | 13.4 | 7.3 | <0.001 | <0.001 |
| i5 | 104.3 | 34.2 | 87.8 | 29.8 | <0.001 | <0.001 |
| i6 | 178.2 | 18.4 | 139.9 | 16.7 | <0.001 | <0.001 |
| i7 | 14.1 | 8.2 | 8.6 | 5.2 | <0.001 | <0.001 |
| g1 | 3219.6 | 1571.4 | 2231.4 | 1188.8 | <0.001 | <0.001 |
| g2 | 2557.3 | 1250.6 | 1777.9 | 948.2 | <0.001 | <0.001 |
| g3 | 128.8 | 62.9 | 89.3 | 47.6 | <0.001 | <0.001 |
| g4 | 102.3 | 50.0 | 71.1 | 38.0 | <0.001 | <0.001 |
| g5 | 78.1 | 43.3 | 51.4 | 31.5 | <0.001 | <0.001 |
| g6 | 61.5 | 34.0 | 40.9 | 25.1 | <0.001 | <0.001 |
| g7 | 115.5 | 59.9 | 80.4 | 43.9 | <0.001 | <0.001 |
| g8 | 20.3 | 17.8 | 15.9 | 12.8 | 0.062 | 1 |
| g9 | 292.2 | 150.5 | 201.0 | 115.1 | <0.001 | <0.001 |
| g10 | 56.3 | 34.4 | 36.2 | 23.6 | <0.001 | <0.001 |
| f1 | 4.52 | 1.7 | 5.31 | 1.91 | <0.001 | <0.001 |
| f2 | 0.52 | 0.23 | 0.32 | 0.22 | <0.001 | <0.001 |
| f3 | 0.13 | 0.09 | 0.09 | 0.03 | <0.001 | <0.001 |
| f4 | 0.56 | 0.08 | 0.51 | 0.06 | <0.001 | <0.001 |
| Intervention (N = 1174) | Control (N = 2115) | ||||
|---|---|---|---|---|---|
| Mean | SD | Mean | SD | p | |
| i1 | 160.6 | 22.2 | 159.3 | 20.4 | 0.56 |
| i2 | 151.9 | 20.1 | 149.2 | 16.6 | 0.71 |
| i3 | 155.4 | 22.2 | 152.0 | 16.8 | 0.74 |
| i4 | 19.4 | 9.4 | 21.2 | 10.6 | 0.84 |
| i5 | 107.6 | 34.0 | 102.5 | 34.1 | 0.65 |
| i6 | 176.4 | 15.8 | 179.2 | 19.6 | 0.73 |
| i7 | 13.2 | 8.4 | 14.7 | 8.0 | 0.92 |
| g1 | 2956.9 | 1439.8 | 3365.4 | 1621.9 | 0.87 |
| g2 | 2346.1 | 1141.0 | 2674.5 | 1293.0 | 0.87 |
| g3 | 118.3 | 57.6 | 134.6 | 64.9 | 0.87 |
| g4 | 93.8 | 45.6 | 107.0 | 51.7 | 0.87 |
| g5 | 77.8 | 42.0 | 78.2 | 44.0 | 0.82 |
| g6 | 61.0 | 32.4 | 61.8 | 34.8 | 0.81 |
| g7 | 103.9 | 57.7 | 121.9 | 60.2 | 0.82 |
| g8 | 15.9 | 15.5 | 22.8 | 18.5 | 0.95 |
| g9 | 283.7 | 141.2 | 297.0 | 155.2 | 0.79 |
| g10 | 55.5 | 33.9 | 56.7 | 34.6 | 0.95 |
| f1 | 4.73 | 1.84 | 4.40 | 1.60 | 0.75 |
| f2 | 0.50 | 0.23 | 0.53 | 0.23 | 0.72 |
| f3 | 0.16 | 0.13 | 0.11 | 0.04 | 0.23 |
| f4 | 0.59 | 0.10 | 0.54 | 0.06 | 0.26 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chronaiou, I.; Giskeødegård, G.F.; Neubert, A.; Hoffmann-Skjøstad, T.V.; Thomsen, R.S.; Hoff, M.; Bathen, T.F.; Sitter, B. Evaluating the Impact of High Intensity Interval Training on Axial Psoriatic Arthritis Based on MR Images. Diagnostics 2022, 12, 1420. https://doi.org/10.3390/diagnostics12061420
Chronaiou I, Giskeødegård GF, Neubert A, Hoffmann-Skjøstad TV, Thomsen RS, Hoff M, Bathen TF, Sitter B. Evaluating the Impact of High Intensity Interval Training on Axial Psoriatic Arthritis Based on MR Images. Diagnostics. 2022; 12(6):1420. https://doi.org/10.3390/diagnostics12061420
Chicago/Turabian StyleChronaiou, Ioanna, Guro Fanneløb Giskeødegård, Ales Neubert, Tamara Viola Hoffmann-Skjøstad, Ruth Stoklund Thomsen, Mari Hoff, Tone Frost Bathen, and Beathe Sitter. 2022. "Evaluating the Impact of High Intensity Interval Training on Axial Psoriatic Arthritis Based on MR Images" Diagnostics 12, no. 6: 1420. https://doi.org/10.3390/diagnostics12061420
APA StyleChronaiou, I., Giskeødegård, G. F., Neubert, A., Hoffmann-Skjøstad, T. V., Thomsen, R. S., Hoff, M., Bathen, T. F., & Sitter, B. (2022). Evaluating the Impact of High Intensity Interval Training on Axial Psoriatic Arthritis Based on MR Images. Diagnostics, 12(6), 1420. https://doi.org/10.3390/diagnostics12061420






