Advances in OCT Imaging in Myopia and Pathologic Myopia
Abstract
:1. Introduction
2. Advances in OCT/OCTA Technology
2.1. Time Domain and Spectral Domain OCT
2.2. Swept Source OCT
2.3. OCT Angiography
2.4. Widefield OCT
2.5. Polarization Sensitive OCT
3. OCT and OCTA for the Assessment of Ocular Structures in Myopes
3.1. Anterior Segment
3.2. Vitreous
3.3. Retina
3.4. Choroid
4. OCT and OCTA for the Assessment of Pathology in High Myopes
4.1. Myopic Maculopathy
4.1.1. Atrophic Myopic Maculopathy
4.1.2. Myopic Traction Maculopathy (MTM)
4.1.3. Myopic Choroidal Neovascularization (mCNV)
4.2. Dome-Shaped Macula (DSM)
4.3. Optic Nerve
4.4. Sclera and Posterior Staphyloma
5. Artificial Intelligence in OCT/OCTA in Myopia
6. Conclusions and Future Perspectives
7. Methods of Literature Search
Author Contributions
Funding
Institutional Review Board Statement
Conflicts of Interest
References
- Baird, P.N.; Saw, S.M.; Lanca, C.; Guggenheim, J.A.; Smith, E.L., III; Zhou, X.; Matsui, K.O.; Wu, P.C.; Sankaridurg, P.; Chia, A.; et al. Myopia. Nat. Rev. Dis. Primers 2020, 6, 99. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Wu, P.C.; Yamashiro, K.; Vutipongsatorn, K.; Fang, Y.; Cheung, C.M.G.; Lai, T.Y.Y.; Ikuno, Y.; Cohen, S.Y.; Gaudric, A.; et al. IMI Pathologic Myopia. Investig. Ophthalmol. Vis. Sci. 2021, 62, 5. [Google Scholar] [CrossRef]
- Wong, T.Y.; Ferreira, A.; Hughes, R.; Carter, G.; Mitchell, P. Epidemiology and disease burden of pathologic myopia and myopic choroidal neovascularization: An evidence-based systematic review. Am. J. Ophthalmol. 2014, 157, 9–25.e12. [Google Scholar] [CrossRef]
- Hsu, W.M.; Cheng, C.Y.; Liu, J.H.; Tsai, S.Y.; Chou, P. Prevalence and causes of visual impairment in an elderly Chinese population in Taiwan: The Shihpai Eye Study. Ophthalmology 2004, 111, 62–69. [Google Scholar] [CrossRef]
- Iwase, A.; Araie, M.; Tomidokoro, A.; Yamamoto, T.; Shimizu, H.; Kitazawa, Y.; Tajimi Study Group. Prevalence and causes of low vision and blindness in a Japanese adult population: The Tajimi Study. Ophthalmology 2006, 113, 1354–1362. [Google Scholar] [CrossRef]
- Xu, L.; Wang, Y.; Li, Y.; Wang, Y.; Cui, T.; Li, J.; Jonas, J.B. Causes of blindness and visual impairment in urban and rural areas in Beijing: The Beijing Eye Study. Ophthalmology 2006, 113, 1134.e1–1134.e11. [Google Scholar] [CrossRef]
- Modjtahedi, B.S.; Ferris, F.L.; Hunter, D.G., 3rd; Fong, D.S. Public Health Burden and Potential Interventions for Myopia. Ophthalmology 2018, 125, 628–630. [Google Scholar] [CrossRef] [Green Version]
- Ang, M.; Wong, C.W.; Hoang, Q.V.; Cheung, G.C.M.; Lee, S.Y.; Chia, A.; Saw, S.M.; Ohno-Matsui, K.; Schmetterer, L. Imaging in myopia: Potential biomarkers, current challenges and future developments. Br. J. Ophthalmol. 2019, 103, 855–862. [Google Scholar] [CrossRef]
- Ng, D.S.; Cheung, C.Y.; Luk, F.O.; Mohamed, S.; Brelen, M.E.; Yam, J.C.; Tsang, C.W.; Lai, T.Y. Advances of optical coherence tomography in myopia and pathologic myopia. Eye 2016, 30, 901–916. [Google Scholar] [CrossRef] [Green Version]
- Suwan, Y.; Fard, M.A.; Geyman, L.S.; Tantraworasin, A.; Chui, T.Y.; Rosen, R.B.; Ritch, R. Association of Myopia with Peripapillary Perfused Capillary Density in Patients with Glaucoma: An Optical Coherence Tomography Angiography Study. JAMA Ophthalmol. 2018, 136, 507–513. [Google Scholar] [CrossRef] [Green Version]
- Querques, G.; Corvi, F.; Querques, L.; Souied, E.H.; Bandello, F. Optical Coherence Tomography Angiography of Choroidal Neovascularization Secondary to Pathologic Myopia. Dev. Ophthalmol. 2016, 56, 101–106. [Google Scholar] [PubMed]
- Foo, L.L.; Ng, W.Y.; Lim, G.Y.S.; Tan, T.E.; Ang, M.; Ting, D.S.W. Artificial intelligence in myopia: Current and future trends. Curr. Opin. Ophthalmol. 2021, 32, 413–424. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Foo, L.L.; Wong, C.W.; Li, J.; Hoang, Q.V.; Schmetterer, L.; Ting, D.S.W.; Ang, M. Pathologic myopia: Advances in imaging and the potential role of artificial intelligence. Br. J. Ophthalmol. 2022. [Google Scholar] [CrossRef] [PubMed]
- Ting, D.S.W.; Pasquale, L.R.; Peng, L.; Campbell, J.P.; Lee, A.Y.; Raman, R.; Tan, G.S.W.; Schmetterer, L.; Keane, P.A.; Wong, T.Y. Artificial intelligence and deep learning in ophthalmology. Br. J. Ophthalmol. 2019, 103, 167–175. [Google Scholar] [CrossRef] [Green Version]
- Huang, D.; Swanson, E.A.; Lin, C.P.; Schuman, J.S.; Stinson, W.G.; Chang, W.; Hee, M.R.; Flotte, T.; Gregory, K.; Puliafito, C.A.; et al. Optical Coherence Tomography. Science 1991, 254, 1178–1181. [Google Scholar] [CrossRef] [Green Version]
- Swanson, E.A.; Izatt, J.A.; Hee, M.R.; Huang, D.; Lin, C.; Schuman, J.; Puliafito, C.; Fujimoto, J.G. In vivo retinal imaging by optical coherence tomography. Opt. Lett. 1993, 18, 1864–1866. [Google Scholar] [CrossRef]
- Drexler, W.; Morgner, U.; Ghanta, R.K.; Kärtner, F.X.; Schuman, J.S.; Fujimoto, J.G. Ultrahigh-resolution ophthalmic optical coherence tomography. Nat. Med. 2001, 7, 502–507. [Google Scholar] [CrossRef]
- Ko, T.H.; Fujimoto, J.G.; Schuman, J.S.; Paunescu, L.A.; Kowalevicz, A.M.; Hartl, I.; Drexler, W.; Wollstein, G.; Ishikawa, H.; Duker, J.S. Comparison of ultrahigh-and standard-resolution optical coherence tomography for imaging macular pathology. Ophthalmology 2005, 112, 1922.e1–1922.e15. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F.; Koizumi, H.; Pozonni, M.C. Enhanced depth imaging spectral-domain optical coherence tomography. Am. J. Ophthalmol. 2008, 146, 496–500. [Google Scholar] [CrossRef]
- Jia, Y.; Bailey, S.T.; Wilson, D.J.; Tan, O.; Klein, M.L.; Flaxel, C.J.; Potsaid, B.; Liu, J.J.; Lu, C.D.; Kraus, M.F. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 2014, 121, 1435–1444. [Google Scholar] [CrossRef] [Green Version]
- Lim, L.; Cheung, G.; Lee, S. Comparison of spectral domain and swept-source optical coherence tomography in pathological myopia. Eye 2014, 28, 488–491. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Makita, S.; Hong, Y.; Yamanari, M.; Yatagai, T.; Yasuno, Y. Optical coherence angiography. Opt. Express 2006, 14, 7821–7840. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Laíns, I.; Wang, J.C.; Cui, Y.; Katz, R.; Vingopoulos, F.; Staurenghi, G.; Vavvas, D.G.; Miller, J.W.; Miller, J.B. Retinal applications of swept source optical coherence tomography (OCT) and optical coherence tomography angiography (OCTA). Prog. Retin. Eye Res. 2021, 84, 100951. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Alam, M.N.; Le, D.; Toslak, D. Quantitative optical coherence tomography angiography: A review. Exp. Biol. Med. 2020, 245, 301–312. [Google Scholar] [CrossRef]
- Kolb, J.P.; Klein, T.; Kufner, C.L.; Wieser, W.; Neubauer, A.S.; Huber, R. Ultra-widefield retinal MHz-OCT imaging with up to 100 degrees viewing angle. Biomed. Opt. Express 2015, 6, 1534–1552. [Google Scholar] [CrossRef] [Green Version]
- Saito, R.; Shinohara, K.; Tanaka, N.; Takahashi, H.; Yoshida, T.; Ohno-Matsui, K. Association between dome-shaped macula and posterior staphyloma in highly myopic eyes investigated by ultra-widefield optical coherence tomography. Retina 2021, 41, 646–652. [Google Scholar] [CrossRef]
- Tan, B.; McNabb, R.P.; Zheng, F.; Sim, Y.C.; Yao, X.; Chua, J.; Ang, M.; Hoang, Q.V.; Kuo, A.N.; Schmetterer, L. Ultrawide field, distortion-corrected ocular shape estimation with MHz optical coherence tomography (OCT). Biomed. Opt. Express 2021, 12, 5770–5781. [Google Scholar] [CrossRef]
- Kalra, G.; Pichi, F.; Kumar Menia, N.; Shroff, D.; Phasukkijwatana, N.; Aggarwal, K.; Agarwal, A. Recent advances in wide field and ultrawide field optical coherence tomography angiography in retinochoroidal pathologies. Expert Rev. Med. Devices 2021, 18, 375–386. [Google Scholar] [CrossRef]
- De Boer, J.F.; Hitzenberger, C.K.; Yasuno, Y. Polarization sensitive optical coherence tomography–A review. Biomed. Opt. Express 2017, 8, 1838–1873. [Google Scholar] [CrossRef] [Green Version]
- Willemse, J.; Gräfe, M.G.O.; Verbraak, F.D.; de Boer, J.F. In Vivo 3D Determination of Peripapillary Scleral and Retinal Layer Architecture Using Polarization-Sensitive Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2020, 9, 21. [Google Scholar] [CrossRef]
- Harimoto, A.; Obata, R.; Yamamoto, M.; Aoki, N.; Yamanari, M.; Sugiyama, S.; Kitano, M.; Fujita, A.; Minami, T.; Ueda, K. Retinal pigment epithelium melanin distribution estimated by polarisation entropy and its association with retinal sensitivity in patients with high myopia. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Mitsukawa, T.; Suzuki, Y.; Momota, Y.; Suzuki, S.; Yamada, M. Effects of 0.01% Atropine Instillation Assessed Using Swept-Source Anterior Segment Optical Coherence Tomography. J. Clin. Med. 2021, 10, 4384. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Dos Santos, V.A.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Li, Y.; Liu, Q.; Xu, Z.; Gu, J.; Li, A.; Wang, Y.; Lin, K.; Xia, J.; Chen, S. Corneal Vertical and Horizontal Thickness Profiles Generated by UHR-OCT for Suspected and Subclinical Keratoconus Diagnosis. J. Refract. Surg. 2021, 37, 438–445. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Xu, Z.; Liu, Q.; Wang, Y.; Lin, K.; Xia, J.; Chen, S.; Hu, L. Relationship between corneal biomechanical parameters and corneal sublayer thickness measured by Corvis ST and UHR-OCT in keratoconus and normal eyes. Eye Vis. 2021, 8, 2. [Google Scholar] [CrossRef]
- Salaroli, C.H.R.; Li, Y.; Huang, D. High-resolution optical coherence tomography visualization of LASIK flap displacement. J. Cataract. Refract. Surg. 2009, 35, 1640–1642. [Google Scholar] [CrossRef] [Green Version]
- Chan, T.C.; Biswas, S.; Yu, M.; Jhanji, V. Longitudinal evaluation of cornea with swept-source optical coherence tomography and Scheimpflug imaging before and after lasik. Medicine 2015, 94, e1219. [Google Scholar] [CrossRef]
- Chandapura, R.S.; Shetty, R.; Shroff, R.; Shilpy, N.; Francis, M.; Sinha Roy, A. OCT layered tomography of the cornea provides new insights on remodeling after photorefractive keratectomy. J. Biophotonics 2018, 11, e201700027. [Google Scholar] [CrossRef]
- Bachtalia, K.; Plakitsi, A.; Charonis, A.; Charonis, G.; Kyroudis, D.; Palioura, S. Compensatory Corneal Epithelial Thickness Changes after Myopic Photorefractive Keratectomy Imaged with Ultra High Resolution Anterior Segment Optical Coherence Tomography. J. Clin. Exp. Ophthalmol. S 2021, 12. [Google Scholar] [CrossRef]
- Zhang, X.-F.; Li, M.; Shi, Y.; Wan, X.-H.; Wang, H.-Z. Repeatability and agreement of two anterior segment OCT in myopic patients before implantable collamer lenses implantation. Int. J. Ophthalmol. 2020, 13, 625. [Google Scholar] [CrossRef]
- Nakamura, T.; Isogai, N.; Kojima, T.; Yoshida, Y.; Sugiyama, Y. Optimization of implantable collamer lens sizing based on swept-source anterior segment optical coherence tomography. J. Cataract. Refract. Surg. 2020, 46, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Packer, M. Meta-analysis and review: Effectiveness, safety, and central port design of the intraocular collamer lens. Clin. Ophthalmol. 2016, 10, 1059. [Google Scholar] [CrossRef] [Green Version]
- Manito, S.C.; Trancón, A.S.; Sierra, O.T.; Baptista, A.M.; Serra, P.M. Inter-eye vault differences of implantable collamer lens measured using anterior segment optical coherence tomography. Clin. Ophthalmol. 2020, 14, 3563. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Jin, G.; Zhang, J.; Chen, X.; Tan, X.; Wang, W.; Ruan, X.; Gu, X.; He, M.; Liu, Z. Clinically Significant Intraocular Lens Decentration and Tilt in Highly Myopic Eyes: A Swept-Source Optical Coherence Tomography Study. Am. J. Ophthalmol. 2022, 235, 46–55. [Google Scholar] [CrossRef] [PubMed]
- Binotti, W.W.; Mills, H.; Nosé, R.M.; Wu, H.K.; Duker, J.S.; Hamrah, P. Anterior segment optical coherence tomography angiography in the assessment of ocular surface lesions. Ocul. Surf. 2021, 22, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Park, J.W.; Lee, J.E.; Pak, K.Y. Posterior vitreous structures evaluated by swept-source optical coherence tomography with en face imaging. Korean J. Ophthalmol. 2018, 32, 376–381. [Google Scholar] [CrossRef] [PubMed]
- Ohno-Matsui, K.; Takahashi, H.; Mao, Z.; Nakao, N. Determining posterior vitreous structure by analysis of images obtained by AI-based 3D segmentation and ultrawidefield optical coherence tomography. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef]
- Kishi, S.; Shimizu, K. Posterior precortical vitreous pocket. Arch. Ophthalmol. 1990, 108, 979–982. [Google Scholar] [CrossRef]
- Itakura, H.; Kishi, S.; Li, D.; Akiyama, H. Observation of Posterior Precortical Vitreous Pocket Using Swept-Source Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2013, 54, 3102–3107. [Google Scholar] [CrossRef] [Green Version]
- Takahashi, H.; Tanaka, N.; Shinohara, K.; Yokoi, T.; Yoshida, T.; Uramoto, K.; Ohno-Matsui, K. Ultra-widefield optical coherence tomographic imaging of posterior vitreous in eyes with high myopia. Am. J. Ophthalmol. 2019, 206, 102–112. [Google Scholar] [CrossRef]
- Nguyen, J.H.; Nguyen-Cuu, J.; Mamou, J.; Routledge, B.; Yee, K.M.; Sebag, J. Vitreous structure and visual function in myopic vitreopathy causing vision-degrading myodesopsia. Am. J. Ophthalmol. 2021, 224, 246–253. [Google Scholar] [CrossRef]
- Saw, S.M.; Gazzard, G.; Shih-Yen, E.C.; Chua, W.H. Myopia and associated pathological complications. Ophthalmic Physiol. Opt. 2005, 25, 381–391. [Google Scholar] [CrossRef]
- AttaAllah, H.R.; Omar, I.A.N.; Abdelhalim, A.S. Evaluation of optic nerve head parameters and retinal nerve fiber layer thickness in axial myopia using SD OCT. Ophthalmol. Ther. 2017, 6, 335–341. [Google Scholar] [CrossRef] [Green Version]
- Song, A.-P.; Wu, X.-Y.; Wang, J.-R.; Liu, W.; Sun, Y.; Yu, T. Measurement of retinal thickness in macular region of high myopic eyes using spectral domain OCT. Int. J. Ophthalmol. 2014, 7, 122. [Google Scholar]
- Fang, Y.; Jonas, J.B.; Yokoi, T.; Cao, K.; Shinohara, K.; Ohno-Matsui, K. Macular Bruch’s membrane defect and dome-shaped macula in high myopia. PLoS ONE 2017, 12, e0178998. [Google Scholar] [CrossRef] [Green Version]
- Jonas, J.B.; Ohno-Matsui, K.; Spaide, R.F.; Holbach, L.; Panda-Jonas, S. Macular Bruch’s membrane defects and axial length: Association with gamma zone and delta zone in peripapillary region. Investig. Ophthalmol. Vis. Sci. 2013, 54, 1295–1302. [Google Scholar] [CrossRef] [Green Version]
- Patel, S.N.; Shi, A.; Wibbelsman, T.D.; Klufas, M.A. Ultra-widefield retinal imaging: An update on recent advances. Ther. Adv. Ophthalmol. 2020, 12, 2515841419899495. [Google Scholar] [CrossRef] [Green Version]
- Choudhry, N.; Golding, J.; Manry, M.W.; Rao, R.C. Ultra-widefield steering-based spectral-domain optical coherence tomography imaging of the retinal periphery. Ophthalmology 2016, 123, 1368–1374. [Google Scholar] [CrossRef]
- Ludwig, C.A.; Moon, J.; Garg, I.; Miller, J.B. Ultra-widefield imaging for evaluation of the myopic eye. In Seminars in Ophthalmology; Taylor & Francis: Abingdon, UK, 2021; pp. 185–190. [Google Scholar]
- Dai, Y.; Xin, C.; Zhang, Q.; Chu, Z.; Zhou, H.; Zhou, X.; Qiao, L.; Wang, R.K. Impact of ocular magnification on retinal and choriocapillaris blood flow quantification in myopia with swept-source optical coherence tomography angiography. Quant. Imaging Med. Surg. 2020, 11, 948–956. [Google Scholar] [CrossRef]
- Sayanagi, K.; Ikuno, Y.; Uematsu, S.; Nishida, K. Features of the choriocapillaris in myopic maculopathy identified by optical coherence tomography angiography. Br. J. Ophthalmol. 2017, 101, 1524–1529. [Google Scholar] [CrossRef]
- Sun, J.; Wang, J.; You, R.; Wang, Y. Is the Retinal Vasculature Related to beta-Peripapillary Atrophy in Nonpathological High Myopia? An Optical Coherence Tomography Angiography Study in Chinese Adults. J. Ophthalmol. 2018, 2018, 7895238. [Google Scholar] [PubMed] [Green Version]
- Su, L.; Ji, Y.S.; Tong, N.; Sarraf, D.; He, X.; Sun, X.; Xu, X.; Sadda, S.R. Quantitative assessment of the retinal microvasculature and choriocapillaris in myopic patients using swept-source optical coherence tomography angiography. Graefes. Arch. Clin. Exp. Ophthalmol. 2020, 258, 1173–1180. [Google Scholar] [CrossRef] [PubMed]
- Moon, J.Y.; Garg, I.; Cui, Y.; Katz, R.; Zhu, Y.; Le, R.; Lu, Y.; Lu, E.S.; Ludwig, C.A.; Elze, T. Wide-field swept-source optical coherence tomography angiography in the assessment of retinal microvasculature and choroidal thickness in patients with myopia. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Wallman, J.; Wildsoet, C.; Xu, A.; Gottlieb, M.D.; Nickla, D.L.; Marran, L.; Krebs, W.; Christensen, A.M. Moving the retina: Choroidal modulation of refractive state. Vis. Res. 1995, 35, 37–50. [Google Scholar] [CrossRef] [Green Version]
- Yi, X.; Ogata, N.; Komada, M.; Yamamoto, C.; Takahashi, K.; Omori, K.; Uyama, M. Vascular endothelial growth factor expression in choroidal neovascularization in rats. Graefes. Arch. Clin. Exp. Ophthalmol. 1997, 235, 313–319. [Google Scholar] [CrossRef] [PubMed]
- Imamura, Y.; Iida, T.; Maruko, I.; Zweifel, S.A.; Spaide, R.F. Enhanced depth imaging optical coherence tomography of the sclera in dome-shaped macula. Am. J. Ophthalmol. 2011, 151, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Flores-Moreno, I.; Ruiz-Medrano, J.; Duker, J.S.; Ruiz-Moreno, J.M. The relationship between retinal and choroidal thickness and visual acuity in highly myopic eyes. Br. J. Ophthalmol. 2013, 97, 1010–1013. [Google Scholar] [CrossRef]
- Zhang, L.; Buitendijk, G.H.; Lee, K.; Sonka, M.; Springelkamp, H.; Hofman, A.; Vingerling, J.R.; Mullins, R.F.; Klaver, C.C.; Abramoff, M.D. Validity of Automated Choroidal Segmentation in SS-OCT and SD-OCT. Investig. Ophthalmol. Vis. Sci. 2015, 56, 3202–3211. [Google Scholar] [CrossRef] [Green Version]
- Jin, P.; Zou, H.; Xu, X.; Chang, T.C.; Zhu, J.; Deng, J.; Lv, M.; Jin, J.; Sun, S.; Wang, L.; et al. Longitudinal Changes in Choroidal and Retinal Thicknesses in Children with Myopic Shift. Retina 2019, 39, 1091–1099. [Google Scholar] [CrossRef]
- Wong, C.W.; Phua, V.; Lee, S.Y.; Wong, T.Y.; Cheung, C.M. Is Choroidal or Scleral Thickness Related to Myopic Macular Degeneration? Investig. Ophthalmol. Vis. Sci. 2017, 58, 907–913. [Google Scholar] [CrossRef] [Green Version]
- Bartol-Puyal, F.A.; Isanta, C.; Ruiz-Moreno, O.; Abadia, B.; Calvo, P.; Pablo, L. Distribution of Choroidal Thinning in High Myopia, Diabetes Mellitus, and Aging: A Swept-Source OCT Study. J. Ophthalmol. 2019, 2019, 3567813. [Google Scholar] [CrossRef] [Green Version]
- Spaide, R.F.; Akiba, M.; Ohno-Matsui, K. Evaluation of peripapillary intrachoroidal cavitation with swept source and enhanced depth imaging optical coherence tomography. Retina 2012, 32, 1037–1044. [Google Scholar] [CrossRef]
- Shimada, N.; Ohno-Matsui, K.; Yoshida, T.; Yasuzumi, K.; Kojima, A.; Kobayashi, K.; Futagami, S.; Tokoro, T.; Mochizuki, M. Characteristics of peripapillary detachment in pathologic myopia. Arch. Ophthalmol. 2006, 124, 46–52. [Google Scholar] [CrossRef] [Green Version]
- Mastropasqua, R.; Viggiano, P.; Borrelli, E.; Evangelista, F.; Libertini, D.; Di Antonio, L.; Toto, L. In Vivo Mapping of the Choriocapillaris in High myopia: A Widefield Swept Source Optical Coherence Tomography Angiography. Sci. Rep. 2019, 9, 18932. [Google Scholar] [CrossRef] [Green Version]
- Devarajan, K.; Sim, R.; Chua, J.; Wong, C.W.; Matsumura, S.; Htoon, H.M.; Schmetterer, L.; Saw, S.M.; Ang, M. Optical coherence tomography angiography for the assessment of choroidal vasculature in high myopia. Br. J. Ophthalmol. 2020, 104, 917–923. [Google Scholar] [CrossRef]
- Wong, C.W.; Teo, Y.C.K.; Tsai, S.T.A.; Ting, S.W.D.; Yeo, Y.S.I.; Wong, W.K.D.; Lee, S.Y.; Wong, T.Y.; Cheung, C.M.G. Characterization of the choroidal vasculature in myopic maculopathy with optical coherence tomographic angiography. Retina 2019, 39, 1742–1750. [Google Scholar] [CrossRef]
- Li, M.; Yang, Y.; Jiang, H.; Gregori, G.; Roisman, L.; Zheng, F.; Ke, B.; Qu, D.; Wang, J. Retinal microvascular network and microcirculation assessments in high myopia. Am. J. Ophthalmol. 2017, 174, 56–67. [Google Scholar] [CrossRef] [Green Version]
- Zheng, F.; Chua, J.; Ke, M.; Tan, B.; Yu, M.; Hu, Q.; Cheung, C.M.G.; Ang, M.; Lee, S.Y.; Wong, T.Y. Quantitative OCT angiography of the retinal microvasculature and choriocapillaris in highly myopic eyes with myopic macular degeneration. Br. J. Ophthalmol. 2022, 106, 681–688. [Google Scholar] [CrossRef]
- Zheng, F.; Chua, J.; Sim, Y.C.; Tan, B.; Yu, M.; Wong, Q.Y.; Dan, Y.S.; Chong, R.S.; Cheung, C.M.G.; Ang, M. Macular Sensitivity and Capillary Perfusion in Highly Myopic Eyes with Myopic Macular Degeneration. Retina 2022, 42, 529–539. [Google Scholar] [CrossRef]
- Ruiz-Medrano, J.; Montero, J.A.; Flores-Moreno, I.; Arias, L.; García-Layana, A.; Ruiz-Moreno, J.M. Myopic maculopathy: Current status and proposal for a new classification and grading system (ATN). Prog. Retin. Eye Res. 2019, 69, 80–115. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Kawasaki, R.; Jonas, J.B.; Cheung, C.M.G.; Saw, S.-M.; Verhoeven, V.J.; Klaver, C.C.; Moriyama, M.; Shinohara, K.; Kawasaki, Y. International photographic classification and grading system for myopic maculopathy. Am. J. Ophthalmol. 2015, 159, 877–883.e7. [Google Scholar] [CrossRef]
- Fang, Y.; Du, R.; Nagaoka, N.; Yokoi, T.; Shinohara, K.; Xu, X.; Takahashi, H.; Onishi, Y.; Yoshida, T.; Ohno-Matsui, K. OCT-based diagnostic criteria for different stages of myopic maculopathy. Ophthalmology 2019, 126, 1018–1032. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Z.; Qi, Y.; Wei, W.; Jin, Z.-B.; Wang, W.; Duan, A.; Liu, W. Investigation of Macular Choroidal Thickness and Blood Flow Change by Optical Coherence Tomography Angiography After Posterior Scleral Reinforcement. Front. Med. 2021, 453. [Google Scholar] [CrossRef]
- Parolini, B.; Palmieri, M.; Finzi, A.; Besozzi, G.; Lucente, A.; Nava, U.; Pinackatt, S.; Adelman, R.; Frisina, R. The new myopic traction maculopathy staging system. Eur. J. Ophthalmol. 2021, 31, 1299–1312. [Google Scholar] [CrossRef]
- Xiao, W.; Zhu, Z.; Odouard, C.; Xiao, O.; Guo, X.; He, M. Wide-Field En Face Swept-Source Optical Coherence Tomography Features of Extrafoveal Retinoschisis in Highly Myopic Eyes. Investig. Ophthalmol. Vis. Sci. 2017, 58, 1037–1044. [Google Scholar] [CrossRef] [Green Version]
- Parolini, B.; Palmieri, M.; Finzi, A.; Besozzi, G.; Frisina, R. Myopic traction maculopathy: A new perspective on classification and management. Asia-Pac. J. Ophthalmol. 2021, 10, 49–59. [Google Scholar] [CrossRef]
- Wang, S.-W.; Hung, K.-C.; Tsai, C.-Y.; Chen, M.-S.; Ho, T.-C. Myopic traction maculopathy biomarkers on optical coherence tomography angiography—An overlooked mechanism of visual acuity correction in myopic eyes. Eye 2019, 33, 1305–1313. [Google Scholar] [CrossRef]
- Cheung, C.M.G.; Arnold, J.J.; Holz, F.G.; Park, K.H.; Lai, T.Y.Y.; Larsen, M.; Mitchell, P.; Ohno-Matsui, K.; Chen, S.J.; Wolf, S.; et al. Myopic Choroidal Neovascularization: Review, Guidance, and Consensus Statement on Management. Ophthalmology 2017, 124, 1690–1711. [Google Scholar] [CrossRef] [Green Version]
- Battaglia Parodi, M.; Iacono, P.; Romano, F.; Bandello, F. Fluorescein Leakage and Optical Coherence Tomography Features of Choroidal Neovascularization Secondary to Pathologic Myopia. Investig. Ophthalmol. Vis. Sci. 2018, 59, 3175–3180. [Google Scholar] [CrossRef] [Green Version]
- Ding, X.; Zhan, Z.; Sun, L.; Yang, Y.; Li, S.; Zhang, A.; Luo, X.; Lu, L. Retinal pigmental epithelium elevation and external limiting membrane interruption in myopic choroidal neovascularization: Correlation with activity. Graefe's Arch. Clin. Exp. Ophthalmol. 2018, 256, 1831–1837. [Google Scholar] [CrossRef]
- Bruyère, E.; Caillaux, V.; Cohen, S.Y.; Martiano, D.; Ores, R.; Puche, N.; Souied, E.H. Spectral-domain optical coherence tomography of subretinal hyperreflective exudation in myopic choroidal neovascularization. Am. J. Ophthalmol. 2015, 160, 749–758.e1. [Google Scholar] [CrossRef] [PubMed]
- Bruyere, E.; Miere, A.; Cohen, S.Y.; Martiano, D.; Sikorav, A.; Popeanga, A.; Semoun, O.; Querques, G.; Souied, E.H. Neovascularization Secondary to High Myopia Imaged by Optical Coherence Tomography Angiography. Retina 2017, 37, 2095–2101. [Google Scholar] [CrossRef] [PubMed]
- Bagchi, A.; Schwartz, R.; Hykin, P.; Sivaprasad, S. Diagnostic algorithm utilising multimodal imaging including optical coherence tomography angiography for the detection of myopic choroidal neovascularisation. Eye 2019, 33, 1111–1118. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Sun, L.; Zhao, X.; Huang, S.; Luo, X.; Zhang, A.; Chen, C.; Wang, Z.; Liu, C.; Ding, X. Assessing the activity of myopic choroidal neovascularization: Comparison between optical coherence tomography angiography and dye angiography. Retina 2020, 40, 1757. [Google Scholar] [CrossRef] [PubMed]
- Ohayon, A.; Sacconi, R.; Semoun, O.; Corbelli, E.; Souied, E.H.; Querques, G. Choroidal neovascular area and vessel density comparison between two swept-source optical coherence tomography angiography devices. Retina 2020, 40, 521–528. [Google Scholar] [CrossRef]
- Hosoda, Y.; Miyata, M.; Uji, A.; Ooto, S.; Yamashiro, K.; Tamura, H.; Oishi, A.; Ueda-Arakawa, N.; Miyake, M.; Hata, M.; et al. Novel Predictors of Visual Outcome in Anti-VEGF Therapy for Myopic Choroidal Neovascularization Derived Using OCT Angiography. Ophthalmol. Retina 2018, 2, 1118–1124. [Google Scholar] [CrossRef]
- Cheng, Y.; Li, Y.; Huang, X.; Qu, Y. Application of optical coherence tomography angiography to assess anti–vascular endothelial growth factor therapy in myopic choroidal neovascularization. Retina 2019, 39, 712–718. [Google Scholar] [CrossRef]
- Ueda-Consolvo, T.; Shibuya, N.; Oiwake, T.; Abe, S.; Numata, A.; Honda, Y.; Yanagisawa, S.; Hayashi, A. Using optical coherence tomography angiography to guide myopic choroidal neovascularization treatment: A 3-year follow-up study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 3295–3303. [Google Scholar] [CrossRef]
- Wang, Y.; Hu, Z.; Zhu, T.; Su, Z.; Fang, X.; Lin, J.; Chen, Z.; Su, Z.; Ye, P.; Ma, J. Optical coherence tomography angiography-based quantitative assessment of morphologic changes in active myopic choroidal neovascularization during anti-vascular endothelial growth factor therapy. Front. Med. 2021, 8, 586. [Google Scholar] [CrossRef]
- Spaide, R.F.; Fujimoto, J.G.; Waheed, N.K. Image artifacts in optical coherence tomography angiography. Retina 2015, 35, 2163–2180. [Google Scholar] [CrossRef]
- Ishida, T.; Watanabe, T.; Yokoi, T.; Shinohara, K.; Ohno-Matsui, K. Possible connection of short posterior ciliary arteries to choroidal neovascularisations in eyes with pathologic myopia. Br. J. Ophthalmol. 2019, 103, 457–462. [Google Scholar] [CrossRef] [PubMed]
- Kumar, V.; Verma, S.; Azad, S.V.; Chawla, R.; Bhayana, A.A.; Surve, A.; Vohra, R.; Venkatesh, P. Dome-shaped macula—Review of literature. Surv. Ophthalmol. 2021, 66, 560–571. [Google Scholar] [CrossRef] [PubMed]
- Caillaux, V.; Gaucher, D.; Gualino, V.; Massin, P.; Tadayoni, R.; Gaudric, A. Morphologic characterization of dome-shaped macula in myopic eyes with serous macular detachment. Am. J. Ophthalmol. 2013, 156, 958–967.e1. [Google Scholar] [CrossRef] [PubMed]
- Dai, F.; Li, S.; Wang, Y.; Li, S.; Han, J.; Li, M.; Zhang, Z.; Jin, X.; Dou, S. Correlation between posterior staphyloma and dome-shaped macula in high myopic eyes. Retina 2020, 40, 2119–2126. [Google Scholar] [CrossRef]
- Ohno-Matsui, K. Proposed classification of posterior staphylomas based on analyses of eye shape by three-dimensional magnetic resonance imaging and wide-field fundus imaging. Ophthalmology 2014, 121, 1798–1809. [Google Scholar] [CrossRef]
- Lee, K.M.; Choung, H.-K.; Kim, M.; Oh, S.; Kim, S.H. Change of β-Zone Parapapillary Atrophy During Axial Elongation: Boramae Myopia Cohort Study Report 3. Investig. Ophthalmol. Vis. Sci. 2018, 59, 4020–4030. [Google Scholar] [CrossRef] [Green Version]
- Fan, Y.Y.; Jonas, J.B.; Wang, Y.X.; Chen, C.X.; Wei, W.B. Horizontal and vertical optic disc rotation. The Beijing Eye Study. PLoS ONE 2017, 12, e0175749. [Google Scholar] [CrossRef]
- Sung, M.S.; Lee, T.H.; Heo, H.; Park, S.W. Association between optic nerve head deformation and retinal microvasculature in high myopia. Am. J. Ophthalmol. 2018, 188, 81–90. [Google Scholar] [CrossRef]
- Akagi, T.; Hangai, M.; Kimura, Y.; Ikeda, H.O.; Nonaka, A.; Matsumoto, A.; Akiba, M.; Yoshimura, N. Peripapillary scleral deformation and retinal nerve fiber damage in high myopia assessed with swept-source optical coherence tomography. Am. J. Ophthalmol. 2013, 155, 927–936.e1. [Google Scholar] [CrossRef]
- Nagaoka, N.; Jonas, J.B.; Morohoshi, K.; Moriyama, M.; Shimada, N.; Yoshida, T.; Ohno-Matsui, K. Glaucomatous-type optic discs in high myopia. PLoS ONE 2015, 10, e0138825. [Google Scholar] [CrossRef] [Green Version]
- Zemborain, Z.Z.; Jarukasetphon, R.; Tsamis, E.; De Moraes, C.G.; Ritch, R.; Hood, D.C. Optical Coherence Tomography Can Be Used to Assess Glaucomatous Optic Nerve Damage in Most Eyes with High Myopia. J. Glaucoma 2020, 29, 833–845. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Ikuno, Y.; Akiba, M.; Kikawa, T.; Usui, S.; Nishida, K. Analysis of Peripapillary Geometric Characters in High Myopia Using Swept-Source Optical Coherence Tomography. Investig. Ophthalmol. Vis. Sci. 2016, 57, 137–144. [Google Scholar] [CrossRef] [Green Version]
- Balaji, J.J.; Lakshminarayanan, V. ODTiD: Optic Nerve Head SD-OCT Image Dataset. Clin. Ophthalmol. 2021, 15, 4239. [Google Scholar] [CrossRef] [PubMed]
- He, J.; Chen, Q.; Yin, Y.; Zhou, H.; Fan, Y.; Zhu, J.; Zou, H.; Xu, X. Association between retinal microvasculature and optic disc alterations in high myopia. Eye 2019, 33, 1494–1503. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Zhang, F.; Wang, Y.; Xie, Z.; Wu, W.; Wang, Q.; Zheng, M.; Lu, F.; Mao, X. Associations between optic disc characteristics and macular choroidal microvasculature in young patients with high myopia. Clin. Exp. Ophthalmol. 2021, 49, 560–569. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.; Maeng, K.J.; Kim, J.Y.; Yang, H.; Choi, W.; Lee, S.Y.; Seong, G.J.; Kim, C.Y.; Bae, H.W. Diagnostic ability of vessel density measured by spectral-domain optical coherence tomography angiography for glaucoma in patients with high myopia. Sci. Rep. 2020, 10, 3027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, F.; Li, F.; Gao, K.; He, W.; Zeng, J.; Chen, Y.; Chen, M.; Cheng, W.; Song, Y.; Peng, Y. Longitudinal changes in macular optical coherence tomography angiography metrics in primary open-angle glaucoma with high myopia: A prospective study. Investig. Ophthalmol. Vis. Sci. 2021, 62, 30. [Google Scholar] [CrossRef]
- Hu, X.; Shang, K.; Chen, X.; Sun, X.; Dai, Y. Clinical features of microvasculature in subzones of parapapillary atrophy in myopic eyes: An OCT-angiography study. Eye 2021, 35, 455–463. [Google Scholar] [CrossRef]
- Na, H.-M.; Lee, E.J.; Lee, S.H.; Kim, T.-W. Evaluation of peripapillary choroidal microvasculature to detect glaucomatous damage in eyes with high myopia. J. Glaucoma 2020, 29, 39–45. [Google Scholar] [CrossRef]
- Kim, Y.J.; Na, K.I.; Lim, H.W.; Seong, M.; Lee, W.J. Combined wide-field optical coherence tomography angiography density map for high myopic glaucoma detection. Sci. Rep. 2021, 11, 22034. [Google Scholar] [CrossRef]
- Ohno-Matsui, K.; Akiba, M.; Modegi, T.; Tomita, M.; Ishibashi, T.; Tokoro, T.; Moriyama, M. Association between shape of sclera and myopic retinochoroidal lesions in patients with pathologic myopia. Investig. Ophthalmol. Vis. Sci. 2012, 53, 6046–6061. [Google Scholar] [CrossRef] [Green Version]
- Deng, J.; Jin, J.; Lv, M.; Jiang, W.; Sun, S.; Yao, C.; Zhu, J.; Zou, H.; Wang, L.; He, X. Distribution of scleral thickness and associated factors in 810 Chinese children and adolescents: A swept-source optical coherence tomography study. Acta Ophthalmol. 2019, 97, e410–e418. [Google Scholar] [CrossRef] [Green Version]
- Curtin, B.J.; Teng, C.C. Scleral changes in pathological myopia. Trans. Am. Acad. Ophthalmol. Otolaryngol. 1958, 62, 777–788. [Google Scholar] [PubMed]
- Ohsugi, H.; Ikuno, Y.; Oshima, K.; Yamauchi, T.; Tabuchi, H. Morphologic characteristics of macular complications of a dome-shaped macula determined by swept-source optical coherence tomography. Am. J. Ophthalmol. 2014, 158, 162–170.e1. [Google Scholar] [CrossRef]
- Ellabban, A.A.; Tsujikawa, A.; Matsumoto, A.; Yamashiro, K.; Oishi, A.; Ooto, S.; Nakata, I.; Akagi-Kurashige, Y.; Miyake, M.; Elnahas, H.S. Three-dimensional tomographic features of dome-shaped macula by swept-source optical coherence tomography. Am. J. Ophthalmol. 2013, 155, 320–328.e2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shinohara, K.; Shimada, N.; Moriyama, M.; Yoshida, T.; Jonas, J.B.; Yoshimura, N.; Ohno-Matsui, K. Posterior staphylomas in pathologic myopia imaged by widefield optical coherence tomography. Investig. Ophthalmol. Vis. Sci. 2017, 58, 3750–3758. [Google Scholar] [CrossRef] [PubMed]
- Zheng, F.; Wong, C.W.; Sabanayagam, C.; Cheung, Y.B.; Matsumura, S.; Chua, J.; Man, R.E.K.; Ohno-Matsui, K.; Wong, T.Y.; Cheng, C.Y. Prevalence, risk factors and impact of posterior staphyloma diagnosed from wide-field optical coherence tomography in Singapore adults with high myopia. Acta Ophthalmol. 2021, 99, e144–e153. [Google Scholar] [CrossRef]
- Shinohara, K.; Tanaka, N.; Jonas, J.B.; Shimada, N.; Moriyama, M.; Yoshida, T.; Ohno-Matsui, K. Ultrawide-Field OCT to Investigate Relationships between Myopic Macular Retinoschisis and Posterior Staphyloma. Ophthalmology 2018, 125, 1575–1586. [Google Scholar] [CrossRef]
- Yoo, T.K.; Ryu, I.H.; Kim, J.K.; Lee, I.S. Deep learning for predicting uncorrected refractive error using posterior segment optical coherence tomography images. Eye 2021. [Google Scholar] [CrossRef]
- Li, Y.; Feng, W.; Zhao, X.; Liu, B.; Zhang, Y.; Chi, W.; Lu, M.; Lin, J.; Wei, Y.; Li, J.; et al. Development and validation of a deep learning system to screen vision-threatening conditions in high myopia using optical coherence tomography images. Br. J. Ophthalmol. 2022, 106, 633–639. [Google Scholar] [CrossRef]
- Sogawa, T.; Tabuchi, H.; Nagasato, D.; Masumoto, H.; Ikuno, Y.; Ohsugi, H.; Ishitobi, N.; Mitamura, Y. Accuracy of a deep convolutional neural network in the detection of myopic macular diseases using swept-source optical coherence tomography. PLoS ONE 2020, 15, e0227240. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ye, X.; Wang, J.; Chen, Y.; Lv, Z.; He, S.; Mao, J.; Xu, J.; Shen, L. Automatic Screening and Identifying Myopic Maculopathy on Optical Coherence Tomography Images Using Deep Learning. Transl. Vis. Sci. Technol. 2021, 10. [Google Scholar] [CrossRef]
- Ran, A.R.; Cheung, C.Y.; Wang, X.; Chen, H.; Luo, L.-Y.; Chan, P.P.; Wong, M.O.; Chang, R.T.; Mannil, S.S.; Young, A.L. Detection of glaucomatous optic neuropathy with spectral-domain optical coherence tomography: A retrospective training and validation deep-learning analysis. Lancet Digit. Health 2019, 1, e172–e182. [Google Scholar] [CrossRef] [Green Version]
- Cahyo, D.A.; Wong, D.W.; Yow, A.P.; Saw, S.-M.; Schmetterer, L. Volumetric Choroidal Segmentation Using Sequential Deep Learning Approach in High Myopia Subjects. In Proceedings of the 2020 42nd Annual International Conference of the IEEE Engineering in Medicine & Biology Society (EMBC), Montreal, QC, Canada, 20–24 July 2020; pp. 1286–1289. [Google Scholar]
- Li, J.; Zhu, L.; Zhu, R.; Lu, Y.; Rong, X.; Zhang, Y.; Gu, X.; Wang, Y.; Zhang, Z.; Ren, Q. Automated Analysis of Choroidal Sublayer Morphologic Features in Myopic Children Using EDI-OCT by Deep Learning. Transl. Vis. Sci. Technol. 2021, 10, 12. [Google Scholar] [CrossRef]
- Kamiya, K.; Ryu, I.H.; Yoo, T.K.; Kim, J.S.; Lee, I.S.; Kim, J.K.; Ando, W.; Shoji, N.; Yamauchi, T.; Tabuchi, H. Prediction of Phakic Intraocular Lens Vault Using Machine Learning of Anterior Segment Optical Coherence Tomography Metrics. Am. J. Ophthalmol. 2021, 226, 90–99. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, Z.; Qiu, B.; Meng, X.; You, Y.; Liu, X.; Liu, G.; Zhou, C.; Yang, K.; Maier, A. Comparative study of deep learning models for optical coherence tomography angiography. Biomed. Opt. Express 2020, 11, 1580–1597. [Google Scholar] [CrossRef]
- Jiang, Z.; Huang, Z.; Qiu, B.; Meng, X.; You, Y.; Liu, X.; Geng, M.; Liu, G.; Zhou, C.; Yang, K. Weakly Supervised Deep Learning-Based Optical Coherence Tomography Angiography. IEEE Trans. Med. Imaging 2020, 40, 688–698. [Google Scholar] [CrossRef]
- Sawai, Y.; Miyata, M.; Uji, A.; Ooto, S.; Tamura, H.; Ueda-Arakawa, N.; Muraoka, Y.; Miyake, M.; Takahashi, A.; Kawashima, Y. Usefulness of Denoising process to Depict Myopic choroidal neovascularisation Using a Single optical coherence tomography Angiography image. Sci. Rep. 2020, 10, 6172. [Google Scholar] [CrossRef] [Green Version]
- Choi, K.J.; Choi, J.E.; Roh, H.C.; Eun, J.S.; Kim, J.M.; Shin, Y.K.; Kang, M.C.; Chung, J.K.; Lee, C.; Lee, D. Deep learning models for screening of high myopia using optical coherence tomography. Sci. Rep. 2021, 11, 21663. [Google Scholar] [CrossRef]
- Wei, L.; He, W.; Wang, J.; Zhang, K.; Du, Y.; Qi, J.; Meng, J.; Qiu, X.; Cai, L.; Fan, Q. An Optical Coherence Tomography-Based Deep Learning Algorithm for Visual Acuity Prediction of Highly Myopic Eyes After Cataract Surgery. Front. Cell Dev. Biol. 2021, 9, 1195. [Google Scholar] [CrossRef]
- Du, R.; Xie, S.; Fang, Y.; Hagino, S.; Yamamoto, S.; Moriyama, M.; Yoshida, T.; Igarashi-Yokoi, T.; Takahashi, H.; Nagaoka, N. Validation of Soft Labels in Developing Deep Learning Algorithms for Detecting Lesions of Myopic Maculopathy From Optical Coherence Tomographic Images. Asia-Pac. J. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
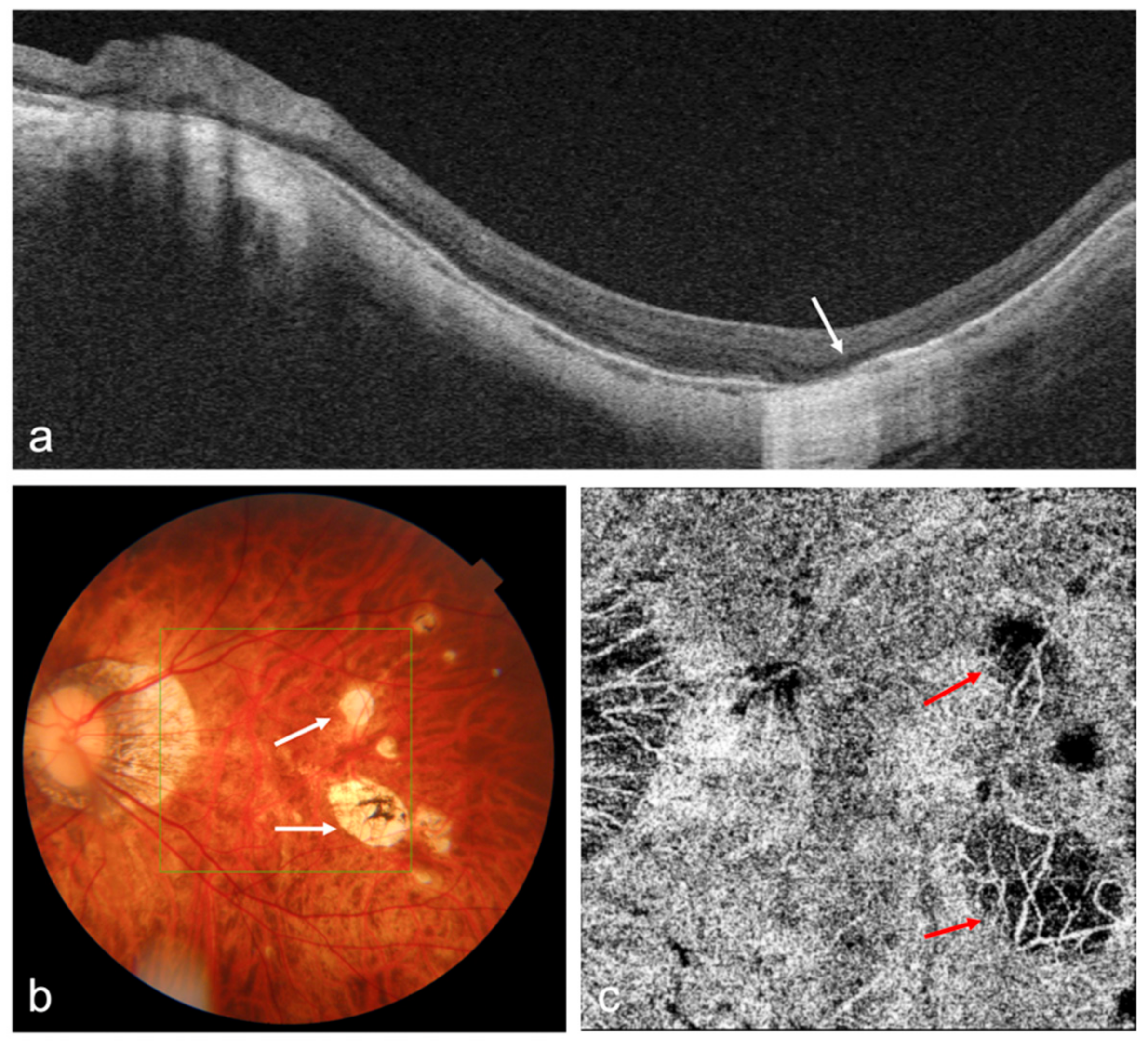
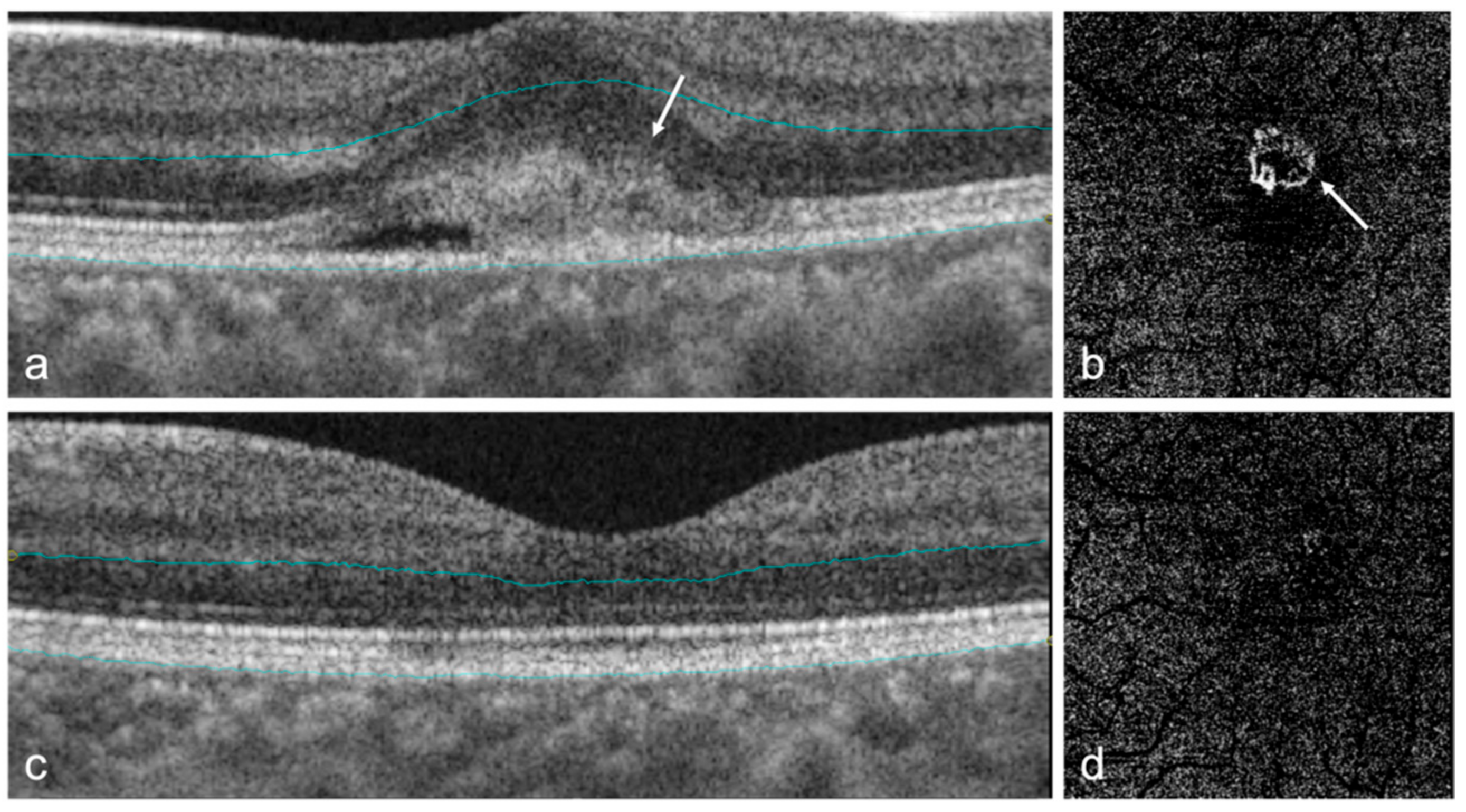
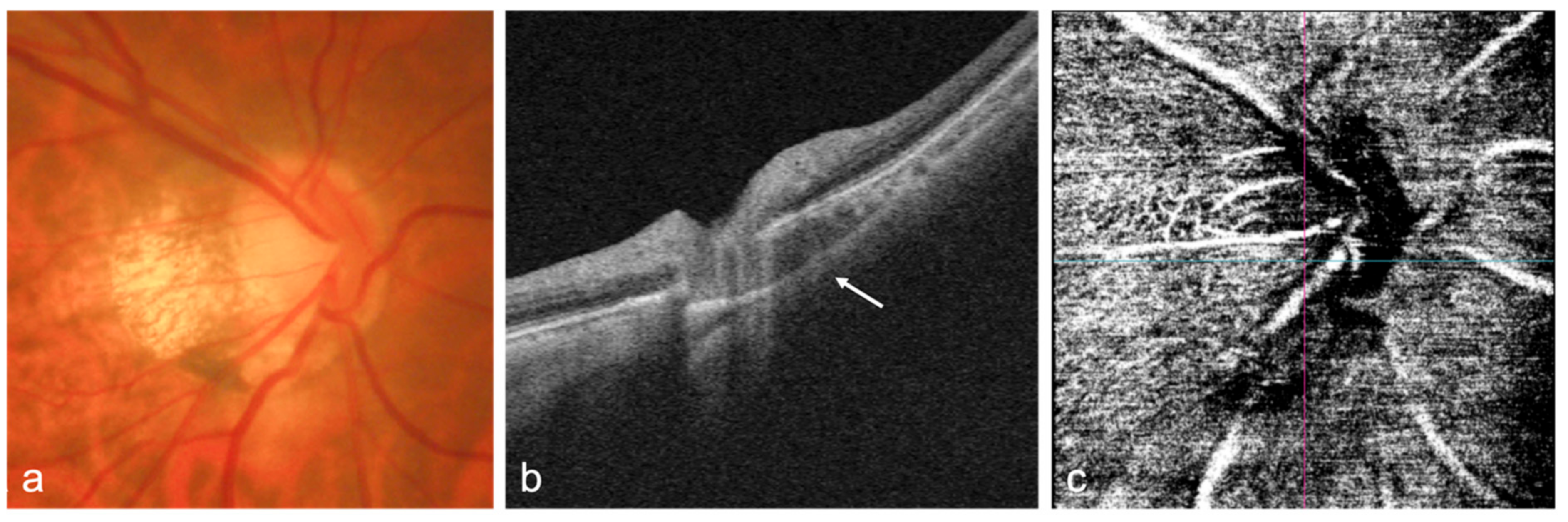
| Author | Title (Year) | Study Design | Population Based | Total Sample Size | OCT/OCTA Used | Main Results |
|---|---|---|---|---|---|---|
| Wong, CW., et al. | Characterization of the choroidal vasculature in myopic maculopathy with optical coherence tomographic angiography (2019) [77] | Cross-sectional study | Clinic-based | 42 eyes with high myopia | SS-OCT (Topcon DRI OCT Triton; Topcon) | Choriocapillaris flow impairment was observed and worsened with increasing severity of myopic maculopathy |
| Wong, CW., et al. | Is Choroidal or Scleral Thickness Related to Myopic Macular Degeneration? (2016) [71] | Prospective study | Clinic-based | 62 eyes with high myopia | SS-OCT (Topcon Medical Systems, Paramus, NJ, USA) | Significant thinning of the choroid with increasing MMD severity |
| Zheng F, et al. | Quantitative OCT angiography of the retinal microvasculature and choriocapillaris in highly myopic eyes with myopic macular degeneration (2020) [79] | Prospective study | Clinic-based | 162 eyes with high myopia | PLEX Elite 9000 SS-OCTA (Carl Zeiss Meditec V.1.7) | Significant OCTA alterations in the retina and choriocapillaris in high myopic eyes with varying severities of MMD |
| Zheng, F., et al. | Macular Sensitivity and Capillary Perfusion in Highly Myopic Eyes with Myopic Macular Degeneration (2022) [80] | Prospective study | Clinic-based | 138 eyes with high myopia | PLEX Elite 9000 SS-OCTA (Carl Zeiss Meditec V.1.7) | There was a strong correlation between reduced macular sensitivity and increasing MMD severity |
| Zhang, Z., et al. | Investigation of Macular Choroidal Thickness and Blood Flow Change by Optical Coherence Tomography Angiography After Posterior Scleral Reinforcement (2021) [84] | Prospective study | Hospital-based | 25 eyes with high myopia | VG200 SS-OCTA | Choroidal thickness and choroidal blood flow increased significantly in patients with high myopia; choroidal thickness and choroidal perfusion area were independently associated with MMD |
| Author | Title (Year) | Study Design | Population Based | Total Sample Size | OCT/OCTA Used | Main Results |
|---|---|---|---|---|---|---|
| Wang, Yao., et al. | Optical Coherence Tomography Angiography-Based Quantitative Assessment of Morphologic Changes in Active Myopic Choroidal Neovascularization During Anti-vascular Endothelial Growth Factor Therapy (2021) [100] | Retrospective study | Hospital-based | 31 eyes | SD-OCT system (RTVue-XR; Optovue, Inc., Freemont, CA, USA) | OCTA-based analysis could promote new insights into the therapeutic response assessment in mCNV patients |
| Bagchi, Akanksha., et al. | Diagnostic algorithm utilising multimodal imaging including optical coherence tomography angiography for the detection of myopic choroidal neovascularisation (2019) [94] | Retrospective study | Hospital-based | 27 eyes | SD-OCT Spectralis system (Spectralis; Heidelberg Engineering, Heidelberg, Germany); OCTA AngioPlex (Carl Zeiss Meditec, Inc., Dublin, CA, USA) | When combined, OCTA and SD-OCT or SD-OCT and FFA showed similar higher sensitivities than each modality alone |
| Li, Songshan., et al. | Assessing the Activity of Myopic Choroidal Neovascularizaiton: Comparison between Optical Coherence Tomography Angiography and Dye Angiography (2020) [95] | Retrospective study | Hospital-based | 82 patients | RTVue AngioVue System, XR Avanti SD-OCT device (Optovue, Inc, Fremont, CA, USA) | In mCNV, the acquisition rate of clear OCTA images was 75.9% |
| Hosoda, Yoshikatsu., et al. | Novel Predictors of Visual Outcome in Anti-VEGF Therapy for Myopic Choroidal Neovascularization Derived Using OCT Angiography (2018) [97] | Prospective study | Hospital-based | 28 eyes | OCTA (RTVue XR Avanti with AngioVue; Optovue, Inc., Fremont, CA, USA) | Exuberant mCNV, characterized by high VLD and FD derived using OCTA, is a predictor of poor visual outcomes |
| Cheng, Ying., et al. | Application of Optical Coherence Tomography Angiography to Assess Anti-Vascular Endothelial Growth Factor Therapy in Myopic Choroid Neovascularization (2019) [98] | Prospective study | Hospital-based | 13 eyes | OCTA (RTVue XR Avanti with AngioVue; Optovue, Inc., Fremont, CA, USA) | OCTA could provide sensitive and intuitive images and quantitative analysis for monitoring and evaluating the therapeutic effect |
| Ueda-Consolvo, Tomoko., et al. | Using optical coherence tomography angiography to guide myopic choroidal neovascularization treatment: a 3-year follow-up study (2021) [99] | Retrospective study | Hospital-based | 11 eyes | RTVue XR spectral domain OCT device (Optovue Inc., Freemont, CA, USA) | Regular examination and prompt treatments against recurrences are critical to prevent enlargement of mCNV |
| Ding, Xiaoyan., et al. | Retinal pigmental epithelium elevation and external limiting membrane interruption in myopic choroidal neovascularization: correlation with activity (2018) [91] | Prospective study | Hospital-based | 54 eyes | SD-OCT Spectralis HRA (Heidelberg Engineering, Heidelberg, Germany) | Provided a simple, fast, accurate alternative to evaluate the mCNV activity based on non-invasive OCT |
| Ishida, Tomok., et al. | Possible connection of short posterior ciliary arteries to choroidal neovascularisations in eyes with pathologic myopia (2019) [102] | Retrospective study | Hospital-based | 124 eyes | Swept-source OCT (DRI-OCT; Topcon, Tokyo, Japan) | Swept-source OCT showed that some of the mCNV were continuous with scleral vessels mainly the short posterior ciliary arteries |
| Battaglia Parodi, Maurizio., et al. | Fluorescein Leakage and Optical Coherence Tomography Features of Choroidal Neovascularization Secondary to Pathologic Myopia (2018) [90] | Prospective study | Hospital-based | 49 patients | SD-OCT Spectralis HRA (Heidelberg Engineering, Heidelberg, Germany) | Different patterns of mCNV may be identified in FA and they correlate with specific SD-OCT alterations |
| Author | Title (Year) | Outcome Measures | Modalities | AI Models | Total Sample Sizes | Performance |
|---|---|---|---|---|---|---|
| Choi, KJ., et al. | Deep learning models for screening of high myopia using optical coherence tomography (2021) [141] | Screening of high myopia | OCT images | DL-CNN | 690 eyes | AUC 0.86–0.99 |
| Yoo, TK., et al. | Deep learning for predicting uncorrected refractive error using posterior segment optical coherence tomography images (2021) [130] | Prediction of uncorrected refractive error | OCT images | DL-CNN | 936 eyes | Detect high myopia: AUC 0.813 accuracy 71.4% |
| Li, Y., et al. | Development and validation of a deep learning system to screen vision-threatening conditions in high myopia using optical coherence tomography images (2020) [131] | Detection of retinoschisis, macular hole, retinal detachment, mCNV | OCT images | DL-CNN | 5505 images | AUC 0.961–0.999; sensitivity and specificity > 90% |
| Sogawa, T., et al. | Accuracy of a deep convolutional neural network in the detection of myopic macular diseases using swept-source optical coherence tomography (2020) [132] | Detection of myopic macular lesions (mCNV, retinoschisis) | Swept-source OCT | DL-CNN | 910 images | AUC 0.970; sensitivity 90.6%; specificity 94.2% |
| Cahyo, DA., et al. | Volumetric Choroidal Segmentation Using Sequential Deep Learning Approach in High Myopia Subjects (2020) [135] | Choroidal volumetric segmentation | OCT images | DL-CNN | 40 eyes | LoU 0.92 |
| Wei, L., et al. | An Optical Coherence Tomography-Based Deep Learning Algorithm for Visual Acuity Prediction of Highly Myopic Eyes After Cataract Surgery (2021) [142] | Prediction of BCVA after cataract surgery | OCT images | DL-CNN | 1415 eyes | MAE 0.1566 logMAR RMSE 0.2433 logMAR |
| Kamiya, K., et al. | Prediction of Phakic Intraocular Lens Vault Using Machine Learning of Anterior Segment Optical Coherence Tomography Metrics (2021) [137] | Prediction of phakic intraocular lens vault | Anterior segment OCT metrics | ML | 1745 eyes | Significantly higher predictability of the ICL vault |
| Li, J., et al. | Automated Analysis of Choroidal Sublayer Morphologic Features in Myopic Children Using EDI-OCT by Deep Learning (2021) [136] | Analysis of choroidal sublayer morphologic features | EDI-OCT | DL-CNN | 92 eyes | Accuracy 0.987 Dice coefficient 0.959 |
| Ye, X., et al. | Automatic Screening and Identifying Myopic Maculopathy on Optical Coherence Tomography Images Using Deep Learning (2021) [133] | Detection of myopic maculopathy | OCT images | DL-CNN | 2342 images | AUC 0.927–0.974 |
| Du, R., et al. | Validation of Soft Labels in Developing Deep Learning Algorithms for Detecting Lesions of Myopic Maculopathy From Optical Coherence Tomographic Images (2021) [143] | Detection of myopic maculopathy | OCT images | DL-CNN | 9176 images | AUC in mCNV, MTM, DSM were 0.985, 0.946, 0.978 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Zheng, F.; Foo, L.L.; Wong, Q.Y.; Ting, D.; Hoang, Q.V.; Chong, R.; Ang, M.; Wong, C.W. Advances in OCT Imaging in Myopia and Pathologic Myopia. Diagnostics 2022, 12, 1418. https://doi.org/10.3390/diagnostics12061418
Li Y, Zheng F, Foo LL, Wong QY, Ting D, Hoang QV, Chong R, Ang M, Wong CW. Advances in OCT Imaging in Myopia and Pathologic Myopia. Diagnostics. 2022; 12(6):1418. https://doi.org/10.3390/diagnostics12061418
Chicago/Turabian StyleLi, Yong, Feihui Zheng, Li Lian Foo, Qiu Ying Wong, Daniel Ting, Quan V. Hoang, Rachel Chong, Marcus Ang, and Chee Wai Wong. 2022. "Advances in OCT Imaging in Myopia and Pathologic Myopia" Diagnostics 12, no. 6: 1418. https://doi.org/10.3390/diagnostics12061418
APA StyleLi, Y., Zheng, F., Foo, L. L., Wong, Q. Y., Ting, D., Hoang, Q. V., Chong, R., Ang, M., & Wong, C. W. (2022). Advances in OCT Imaging in Myopia and Pathologic Myopia. Diagnostics, 12(6), 1418. https://doi.org/10.3390/diagnostics12061418





