NT-proBNP Concentrations in the Umbilical Cord and Serum of Term Neonates: A Systematic Review and Meta-Analysis
Abstract
1. Introduction
2. Methods
2.1. Search Strategy
2.2. Inclusion and Exclusion Criteria
2.3. Data Extraction
2.4. Study Quality
2.5. Risk of Bias
2.6. Statistical Analysis
3. Results
3.1. Participants
3.2. Umbilical Cord NT-proBNP in Term Neonates
3.3. Serum NT-proBNP in Term Neonates
3.4. Quality of Studies
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Sudoh, T.; Kangawa, K.; Minamino, N.; Matsuo, H. A new natriuretic peptide in porcine brain. Nature 1988, 332, 78–81. [Google Scholar] [CrossRef] [PubMed]
- Gupta, K.; Wang, J. Natriuretic Peptides and Cardiometabolic Health. Circ. J. 2015, 79, 1647–1655. [Google Scholar] [CrossRef] [PubMed]
- Rodriguez-Blanco, S.; Oulego-Erroz, I.; Gautreaux-Minaya, S.; Perez-Muñuzuri, A.; Luz Couce-Pico, M. Early NT-proBNP levels as a screening tool for the detection of hemodynamically significant patent ductus arteriosus during the first week of life in very low birth weight infants. J. Perinatol. 2018, 38, 881–888. [Google Scholar] [CrossRef] [PubMed]
- Iliodromiti, Z.; Christou, E.; Vrachnis, N.; Sokou, R.; Vrachnis, D.; Mihopoulou, G.; Boutsikou, T.; Iacovidou, N. Serum and Urinary N-Terminal Pro-brain Natriuretic Peptides as Biomarkers for Bronchopulmonary Dysplasia of Preterm Neonates. Front. Pediatr. 2020, 8, 588738. [Google Scholar] [CrossRef]
- Bührer, C.; Erdeve, Ö.; Van Kaam, A.; Berger, A.; Lechner, E.; Bar-Oz, B.; Allegaert, K.; Stiris, T.; Çelik, İ.H.; Berrington, J. N-terminal B-type natriuretic peptide urinary concentrations and retinopathy of prematurity. Pediatr. Res. 2017, 82, 958–963. [Google Scholar] [CrossRef]
- Dionne, A.; Dahdah, N. A Decade of NT-proBNP in Acute Kawasaki Disease, from Physiological Response to Clinical Relevance. Children 2018, 5, 141. [Google Scholar] [CrossRef]
- Zhao, Y.; Patel, J.; Huang, Y.; Yin, L.; Tang, L. Cardiac markers of multisystem inflammatory syndrome in children (MIS-C) in COVID-19 patients: A meta-analysis. Am. J. Emerg. Med. 2021, 49, 62–70. [Google Scholar] [CrossRef]
- Page, M.J.; McKenzie, J.E.; Bossuyt, P.M.; Boutron, I.; Hoffmann, T.C.; Mulrow, C.D.; Shamseer, L.; Tetzlaff, J.M.; Akl, E.A.; Brennan, S.E.; et al. The PRISMA 2020 statement: An updated guideline for reporting systematic reviews. BMJ 2021, 372, n71. [Google Scholar] [CrossRef]
- Wirsching, J.; Graßmann, S.; Eichelmann, F.; Harms, L.M.; Schenk, M.; Barth, E.; Berndzen, A.; Olalekan, M.; Sarmini, L.; Zuberer, H.; et al. Development and reliability assessment of a new quality appraisal tool for cross-sectional studies using biomarker data (BIOCROSS). BMC Med. Res. Methodol. 2018, 18, 122. [Google Scholar] [CrossRef]
- R Foundation. The R Project for Statistical Computing. 2021. Available online: https://www.rproject.org/ (accessed on 15 January 2022).
- Schwarzer, G. Meta: An R package for meta-analysis. R. News 2007, 7, 40–45. [Google Scholar]
- Balduzzi, S.; Rücker, G.; Schwarzer, G. How to perform a meta-analysis with R: A practical tutorial. Evid. Based Ment. Health 2019, 22, 153–160. [Google Scholar] [CrossRef]
- Hozo, S.P.; Djulbegovic, B.; Hozo, I. Estimating the mean and variance from the median, range, and the size of a sample. BMC Med. Res. Methodol. 2005, 5, 13. [Google Scholar] [CrossRef]
- Bland, M. Estimating Mean and Standard Deviation from the Sample Size, Three Quartiles, Minimum, and Maximum. Int. J. Stat. Med. Res. 2014, 4, 57–64. [Google Scholar] [CrossRef]
- Deepanshu, S.; Surya, U.; Vinay, S.; Sakshi, P.; Ravi, R.K.N. Deep Meta Tool: GUI Tool to Obtain Mean and Standard Deviation (SD) from Median and Interquartile Range (IQR). Research Square 2021. Available online: https://www.researchsquare.com/article/rs-828102/v1 (accessed on 20 January 2022).
- Seong, W.J.; Yoon, D.H.; Chong, G.O.; Hong, D.G.; Koo, T.B.; Lee, T.H.; Chun, S.S. Umbilical cord blood amino-terminal pro-brain natriuretic peptide levels according to the mode of delivery. Arch. Gynecol. Obstet. 2010, 281, 907–912. [Google Scholar] [CrossRef]
- Cardo, L.; Álvarez, E.; García-García, M.; Álvarez, F.V. Amino-terminal proB-type natriuretic peptide reference values in umbilical cord blood. Clin. Chem. Lab. Med. 2020, 58, e179–e181. [Google Scholar] [CrossRef]
- Kocylowski, R.D.; Dubiel, M.; Gudmundsson, S.; Sieg, I.; Fritzer, E.; Alkasi, O.; Breborowicz, G.H.; von Kaisenberg, C.S. Biochemical tissue-specific injury markers of the heart and brain in postpartum cord blood. Am. J. Obstet. Gynecol. 2009, 200, e1–e273. [Google Scholar] [CrossRef]
- Fortunato, G.; Carandente Giarrusso, P.; Martinelli, P.; Sglavo, G.; Vassallo, M.; Tomeo, L.; Rea, M.; Paladini, D. Cardiac troponin T and amino-terminal pro-natriuretic peptide concentrations in fetuses in the second trimester and in healthy neonates. Clin. Chem. Lab. Med. 2006, 44, 834–836. [Google Scholar] [CrossRef]
- Girsen, A.; Ala-Kopsala, M.; Mäkikallio, K.; Vuolteenaho, O.; Räsänen, J. Cardiovascular hemodynamics and umbilical artery N-terminal peptide of proB-type natriuretic peptide in human fetuses with growth restriction. Ultrasound Obs. Gynecol. 2007, 29, 296–303. [Google Scholar] [CrossRef]
- Bar-Oz, B.; Lev-Sagie, A.; Arad, I.; Salpeter, L.; Nir, A. N-terminal pro-B-type natriuretic peptide concentrations in mothers just before delivery, in cord blood, and in newborns. Clin. Chem. 2005, 51, 926–927. [Google Scholar] [CrossRef]
- Hammerer-Lercher, A.; Mair, J.; Tews, G.; Puschendorf, B.; Sommer, R. N-terminal pro-B-type natriuretic peptide concentrations are markedly higher in the umbilical cord blood of newborns than in their mothers. Clin. Chem. 2005, 51, 913–915. [Google Scholar] [CrossRef]
- Bakker, J.; Gies, I.; Slavenburg, B.; Bekers, O.; Delhaas, T.; Van Dieijen-Visser, M. Reference values for N-terminal pro-B-type natriuretic peptide in umbilical cord blood. Clin. Chem. 2004, 50, 2465. [Google Scholar] [CrossRef] [PubMed]
- Lechner, E.; Wiesinger-Eidenberger, G.; Wagner, O.; Weissensteiner, M.; Schreier-Lechner, E.; Leibetseder, D.; Arzt, W.; Tulzer, G. Amino terminal pro B-type natriuretic peptide levels are elevated in the cord blood of neonates with congenital heart defect. Pediatr. Res. 2009, 66, 466–469. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.S.; Laux, R.; Hellwege, H.H.; Liedke, B.; Heinze, C.; von Buelow, H.; Läer, S.; Weil, J. Plasma concentrations of aminoterminal pro atrial natriuretic peptide and aminoterminal pro brain natriuretic peptide in healthy neonates: Marked and rapid increase after birth. Pediatrics 2003, 112, 896–899. [Google Scholar] [CrossRef] [PubMed]
- Schwachtgen, L.; Herrmann, M.; Georg, T.; Schwarz, P.; Marx, N.; Lindinger, A. Reference values of NT-proBNP serum concentrations in the umbilical cord blood and in healthy neonates and children. Z. Kardiol. 2005, 94, 399–404. [Google Scholar] [CrossRef]
- Irmak, K.; Tüten, N.; Karaoglu, G.; Madazli, R.; Tüten, A.; Malik, Ε.; Güralp, O. Evaluation of cord blood creatine kinase (CK), cardiac troponin T (cTnT), N-terminal-pro-B-type natriuretic peptide (NT-proBNP), and s100B levels in nonreassuring foetal heart rate. J. Matern. Fetal Neonatal Med. 2021, 34, 1249–1254. [Google Scholar] [CrossRef]
- Fleming, S.; O’Gorman, T.; O’Byrne, L.; Grimes, H.; Daly, K.M.; Morrison, J.J. Cardiac Troponin I and N-Terminal Pro-Brain Natriuretic Peptide in Umbilical Artery Blood in Relation to Fetal Heart Rate Abnormalities during Labor. Pediatr. Cardiol. 2001, 22, 393–396. [Google Scholar] [CrossRef]
- Rouatbi, H.; Zigabe, S.; Gkiougki, E.; Vranken, L.; Van Linthout, C.; Seghaye, M.C. Biomarkers of neonatal stress assessment: A prospective study. Early Hum. Dev. 2019, 137, 104826. [Google Scholar] [CrossRef]
- Iacovidou, N.; Briana, D.D.; Boutsikou, M.; Gourgiotis, D.; Baka, S.; Vraila, V.M.; Hassiakos, D.; Malamitsi-Puchner, A. Perinatal changes of circulating N-terminal pro B-type natriuretic peptide (NT-proBNP) in normal and intrauterine-growth-restricted pregnancies. Hypertens Pregnancy 2007, 26, 463–471. [Google Scholar] [CrossRef]
- Halse, K.G.; Lindegaard, M.L.; Goetze, J.P.; Damm, P.; Mathiesen, E.R.; Nielsen, L.B. Increased plasma pro-B-type natriuretic peptide in infants of women with type 1 diabetes. Clin. Chem. 2005, 51, 2296–2302. [Google Scholar] [CrossRef][Green Version]
- Lee, S.M.; Jun, J.K.; Kim, S.A.; Kang, M.J.; Song, S.H.; Lee, J.; Park, C.; Park, J.S. N-terminal pro-B-type natriuretic peptide and cardiac troponin T in non-immune hydrops. J. Obstet. Gynaecol. Res. 2016, 42, 380–384. [Google Scholar] [CrossRef]
- Russell, N.E.; Higgins, M.F.; Amaruso, M.; Foley, M.; McAuliffe, F.M. Troponin T and pro-B-type natriuretic Peptide in fetuses of type 1 diabetic mothers. Diabetes Care 2009, 32, 2050–2055. [Google Scholar] [CrossRef]
- Mert, M.K.; Satar, M.; Özbarlas, N.; Yaman, A.; Özgünen, F.T.; Asker, H.S.; Çekinmez, E.K.; Tetiker, T. Troponin T and NT ProBNP Levels in Gestational, Type 1 and Type 2 Diabetic Mothers and Macrosomic Infants. Pediatr. Cardiol. 2016, 37, 76–83. [Google Scholar] [CrossRef]
- Girsen, A.; Ala-Kopsala, M.; Mäkikallio, K.; Vuolteenaho, O.; Räsänen, J. Increased fetal cardiac natriuretic peptide secretion in type-1 diabetic pregnancies. Acta Obstet. Gynecol. Scand. 2008, 87, 307–312. [Google Scholar] [CrossRef]
- El-Ganzoury, M.; El-Farrash, R.; Ahmed, H.; Mohamed, R. Increased N-terminal pro-brain type natriuretic peptide secretion in infants of diabetic mothers. J. Neonatal-Perinat. Med. 2012, 5, 2. [Google Scholar] [CrossRef]
- Lee-Tannock, A.; Hay, K.; Kumar, S. Differences in biomarkers of cardiac dysfunction in cord blood between normal pregnancies and pregnancies complicated by maternal diabetes. Aust. N. Z. J. Obstet. Gynaecol. 2022, 62, 79–85. [Google Scholar] [CrossRef]
- Blohm, M.E.; Arndt, F.; Fröschle, G.M.; Langenbach, N.; Sandig, J.; Vettorazzi, E.; Mir, T.S.; Hecher, K.; Weil, J.; Kozlik-Feldmann, R.; et al. Cardiovascular Biomarkers in Amniotic Fluid, Umbilical Arterial Blood, Umbilical Venous Blood, and Maternal Blood at Delivery, and Their Reference Values for Full-Term, Singleton, Cesarean Deliveries. Front. Pediatr. 2019, 7, 271. [Google Scholar] [CrossRef]
- Blohm, M.E.; Arndt, F.; Sandig, J.; Diehl, W.; Zeller, T.; Mueller, G.C.; Schlesner, C.; Mir, T.S.; Blankenberg, S.; Hecher, K.; et al. Cardiovascular biomarkers in paired maternal and umbilical cord blood samples at term and near term delivery. Early Hum. Dev. 2016, 94, 7–12. [Google Scholar] [CrossRef]
- Ersoy, A.O.; Ozler, S.; Oztas, E.; Ersoy, E.; Ergin, M.; Erkaya, S.; Uygur, D. The Association between N-terminal Pro-Brain Natriuretic Peptide Levels in the Umbilical Vein and Amniotic Fluid Volume Abnormalities. Rev. Bras. Ginecol. Obstet. 2016, 38, 177–182. [Google Scholar] [CrossRef]
- Rauh, M.; Koch, A. Plasma N-terminal pro-B-type natriuretic peptide concentrations in a control population of infants and children. Clin. Chem. 2003, 49, 1563–1564. [Google Scholar] [CrossRef]
- Nir, A.; Bar-Oz, B.; Perles, Z.; Brooks, R.; Korach, A.; Rein, A.J. N-terminal pro-B-type natriuretic peptide: Reference plasma levels from birth to adolescence. Elevated levels at birth and in infants and children with heart diseases. Acta Paediatr. 2004, 93, 603–607. [Google Scholar] [CrossRef]
- Soldin, S.J.; Soldin, O.P.; Boyajian, A.J.; Taskier, M.S. Pediatric brain natriuretic peptide and N-terminal pro-brain natriuretic peptide reference intervals. Clin. Chim. Acta 2006, 366, 304–308. [Google Scholar] [CrossRef] [PubMed]
- Caselli, C.; Ragusa, R.; Prontera, C.; Cabiati, M.; Cantinotti, M.; Federico, G.; Del Ry, S.; Trivella, M.G.; Clerico, A. Distribution of circulating cardiac biomarkers in healthy children: From birth through adulthood. Biomark. Med. 2016, 10, 357–365. [Google Scholar] [CrossRef] [PubMed]
- Nir, A.; Lindinger, A.; Rauh, M.; Bar-Oz, B.; Laer, S.; Schwachtgen, L.; Koch, A.; Falkenberg, J.; Mir, T.S. NT-pro-B-type natriuretic peptide in infants and children: Reference values based on combined data from four studies. Pediatr. Cardiol. 2009, 30, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Xiao, Z.; Li, L.; Hu, B.; Zhou, Z.; Yi, S.; Luo, J.; Xie, L.; Nie, B.; Mo, L.; et al. Establishment of normal reference values of NT-proBNP and its application in diagnosing acute heart failure in children with severe hand foot and mouth disease. Medicine 2018, 97, e12218, Erratum in Medicine 2020, 99, e19688. [Google Scholar] [CrossRef]
- Norozi, K.; Binder, L.; Brack, C.; Potthoff, L.; Hess, G.; Wessel, A. Intravenous luteinizing hormone-releasing hormone has no effect on serum N-terminal pro-brain natriuretic peptide in children and adolescents. Pediatr. Int. 2009, 51, 283–288. [Google Scholar] [CrossRef]
- Deng, M.; Lin, C.; Tang, W.; Zhu, H.; Zhang, Y. Plasma N-terminal pro-B-type natriuretic peptide: Selecting the optimal heart failure marker in children of age up to 18 years. Int. J. Clin. Exp. Pathol. 2016, 9, 10756–10762. [Google Scholar]
- Zhu, R.; Nie, Z. A Clinical Study of the N-Terminal pro-Brain Natriuretic Peptide in Myocardial Injury after Neonatal Asphyxia. Pediatr. Neonatol. 2016, 57, 133–139. [Google Scholar] [CrossRef]
- Aydemir, O.; Aydemir, C.; Sarikabadayi, Y.U.; Altug, N.; Erdeve, O.; Uras, N.; Oguz, S.S.; Dilmen, U. The role of plasma N-terminal pro-B-type natriuretic peptide in predicting the severity of transient tachypnea of the newborn. Early Hum. Dev. 2012, 88, 315–319. [Google Scholar] [CrossRef]
- Ahmed, A.M.; Mohamed, N.A.E.; Abdelhamid, E.M.; Taha, R.I.; Abo-Hashish, M.M.A. N-terminal pro-brain natriuretic peptide as a biomarker for differentiating cardiac and pulmonary disease in term neonates with respiratory distress. J. Saudi Heart Assoc. 2020, 32, 65–70. [Google Scholar] [CrossRef]
- Markovic-Sovtic, G.; Kosutic, J.; Jankovic, B.; Bojanin, D.; Sovtic, A.; Radojicic, Z.; Rakonjac, M.Z. N-terminal pro-brain natriuretic peptide in the assessment of respiratory distress in term neonates. Pediatr. Int. 2014, 56, 373–377. [Google Scholar] [CrossRef]
- Koch, L.; Dabek, M.T.; Frommhold, D.; Poeschl, J. Stable precursor fragments of vasoactive peptides in umbilical cord blood of term and preterm infants. Horm. Res. Paediatr. 2011, 76, 234–239. [Google Scholar] [CrossRef] [PubMed]
- Mir, T.S.; Marohn, S.; Läer, S.; Eiselt, M.; Grollmus, O.; Weil, J. Plasma concentrations of N-terminal pro-brain natriuretic peptide in control children from the neonatal to adolescent period and in children with congestive heart failure. Pediatrics 2002, 110, e76. [Google Scholar] [CrossRef] [PubMed]
- Miyoshi, T.; Hosoda, H.; Minamino, N. Significance of Atrial and Brain Natriuretic Peptide Measurements in Fetuses with Heart Failure. Front. Physiol. 2021, 12, 654356. [Google Scholar] [CrossRef] [PubMed]
- Walther, T.; Stepan, H.; Pankow, K.; Gembardt, F.; Faber, R.; Schultheiss, H.P.; Siems, W.E. Relation of ANP and BNP to their N-terminal fragments in fetal circulation: Evidence for enhanced neutral endopeptidase activity and resistance of BNP to neutral endopeptidase in the fetus. BJOG 2004, 111, 452–455. [Google Scholar] [CrossRef]
- Suga, S.; Nakao, K.; Hosoda, K.; Mukoyama, M.; Ogawa, Y.; Shirakami, G.; Arai, H.; Saito, Y.; Kambayashi, Y.; Inouye, K.; et al. Receptor selectivity of natriuretic peptide family, atrial natriuretic peptide, brain natriuretic peptide, and C-type natriuretic peptide. Endocrinology 1992, 130, 229–239. [Google Scholar] [CrossRef]
- Merz, W.M.; Leufgen, C.; Fimmers, R.; Stoffel-Wagner, B.; Gembruch, U. Reference intervals for N-terminal pro-B-type natriuretic peptide in amniotic fluid between 10 and 34 weeks of gestation. PLoS ONE 2014, 9, e114416. [Google Scholar] [CrossRef]
- Carvajal, J.A.; Delpiano, A.M.; Cuello, M.A.; Poblete, J.A.; Casanello, P.C.; Sobrevia, L.A.; Weiner, C.P. Brain natriuretic peptide (BNP) produced by the human chorioamnion may mediate pregnancy myometrial quiescence. Reprod. Sci. 2009, 16, 32–42. [Google Scholar] [CrossRef]
- Carvajal, J.A.; Ferrer, F.A.; Araya, F.I.; Delpiano, A.M. Normal amino-terminal pro-brain natriuretic peptide (NT-proBNP) values in amniotic fluid. Clin. Biochem. 2017, 50, 23–26. [Google Scholar] [CrossRef]
- Miyoshi, T.; Hosoda, H.; Umekawa, T.; Asada, T.; Fujiwara, A.; Kurosaki, K.I.; Shiraishi, I.; Nakai, M.; Nishimura, K.; Miyazato, M.; et al. Amniotic Fluid Natriuretic Peptide Levels in Fetuses with Congenital Heart Defects or Arrhythmias. Circ. J. 2018, 82, 2619–2626, Erratum in Circ. J. 2018, 82, 3112. [Google Scholar] [CrossRef]
- Clerico, A.; Masotti, S.; Musetti, V.; Passino, C. Pathophysiological mechanisms determining sex differences in circulating levels of cardiac natriuretic peptides and cardiac troponins. J. Lab. Precis. Med. 2019, 4. [Google Scholar] [CrossRef]
- Lam, C.S.; Cheng, S.; Choong, K.; Larson, M.G.; Murabito, J.M.; Newton-Cheh, C.; Bhasin, S.; McCabe, E.L.; Miller, K.K.; Redfield, M.M.; et al. Influence of sex and hormone status on circulating natriuretic peptides. J. Am. Coll. Cardiol. 2011, 58, 618–626. [Google Scholar] [CrossRef]
- Kerkelä, R.; Ulvila, J.; Magga, J. Natriuretic Peptides in the Regulation of Cardiovascular Physiology and Metabolic Events. J. Am. Heart Assoc. 2015, 4, e002423. [Google Scholar] [CrossRef]
- Krüger, C.; Rauh, M.; Dörr, H.G. Immunoreactive renin concentrations in healthy children from birth to adolescence. Clin. Chim. Acta 1998, 274, 15–27. [Google Scholar] [CrossRef]
- Albers, S.; Mir, T.S.; Haddad, M.; Läer, S. N-Terminal pro-brain natriuretic peptide: Normal ranges in the pediatric population including method comparison and interlaboratory variability. Clin. Chem. Lab. Med. 2006, 44, 80–85. [Google Scholar] [CrossRef]
- Johns, M.C.; Stephenson, C. Amino-Terminal Pro–B-Type Natriuretic Peptide Testing in Neonatal and Pediatric Patients. Am. J. Cardiol. 2008, 101, S76–S81. [Google Scholar] [CrossRef]
- O’Brien, F.; Walker, I.A. Fluid homeostasis in the neonate. Paediatr. Anaesth. 2014, 24, 49–59. [Google Scholar] [CrossRef]
- Finnemore, A.; Groves, A. Physiology of the fetal and transitional circulation. Semin. Fetal Neonatal Med. 2015, 20, 210–216. [Google Scholar] [CrossRef]
- Carbajal, R. Neonatal Pain. In Emerging Topics and Controversies in Neonatology; Boyle, E., Cusack, J., Eds.; Springer: Cham, Switzerland, 2020. [Google Scholar] [CrossRef]
- Collin-Chavagnac, D.; Dehoux, M.; Schellenberg, F.; Cauliez, B.; Maupas-Schwalm, F.; Lefevre, G. Société Française de Biologie Clinique Cardiac Markers Working Group. Head-to-head comparison of 10 natriuretic peptide assays. Clin. Chem. Lab. Med. 2015, 53, 1825–1837. [Google Scholar] [CrossRef]
- Miller, W.L.; Phelps, M.A.; Wood, C.M.; Schellenberger, U.; Van Le, A.; Perichon, R.; Jaffe, A.S. Comparison of mass spectrometry and clinical assay measurements of circulating fragments of B-type natriuretic peptide in patients with chronic heart failure. Circ. Heart Fail 2011, 4, 355–360. [Google Scholar] [CrossRef]
- Prontera, C.; Zucchelli, G.C.; Vittorini, S.; Storti, S.; Emdin, M.; Clerico, A. Comparison between analytical performances of polyclonal and monoclonal electrochemiluminescence immunoassays for NTproBNP. Clin. Chim. Acta 2009, 400, 70–73. [Google Scholar] [CrossRef]

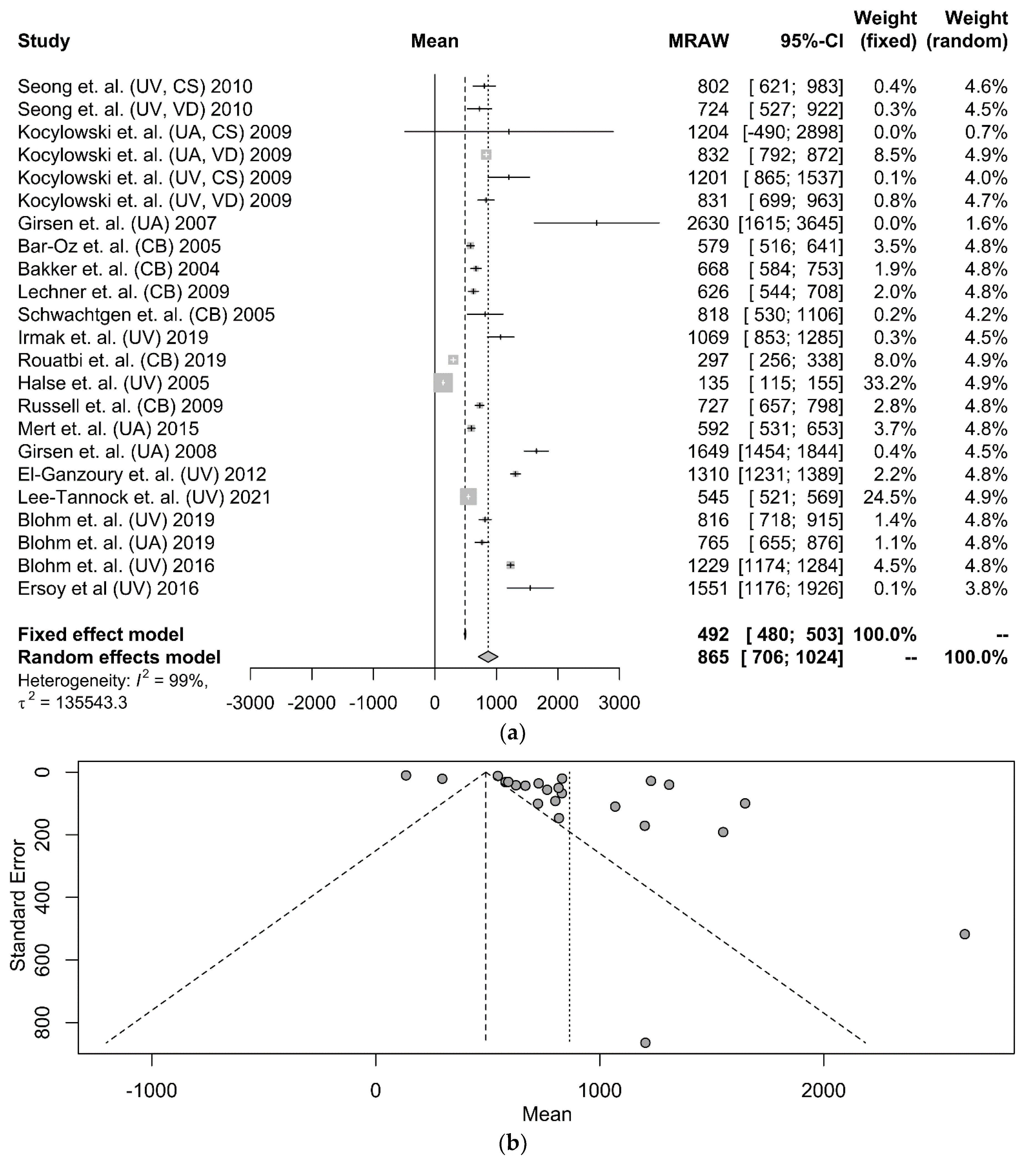
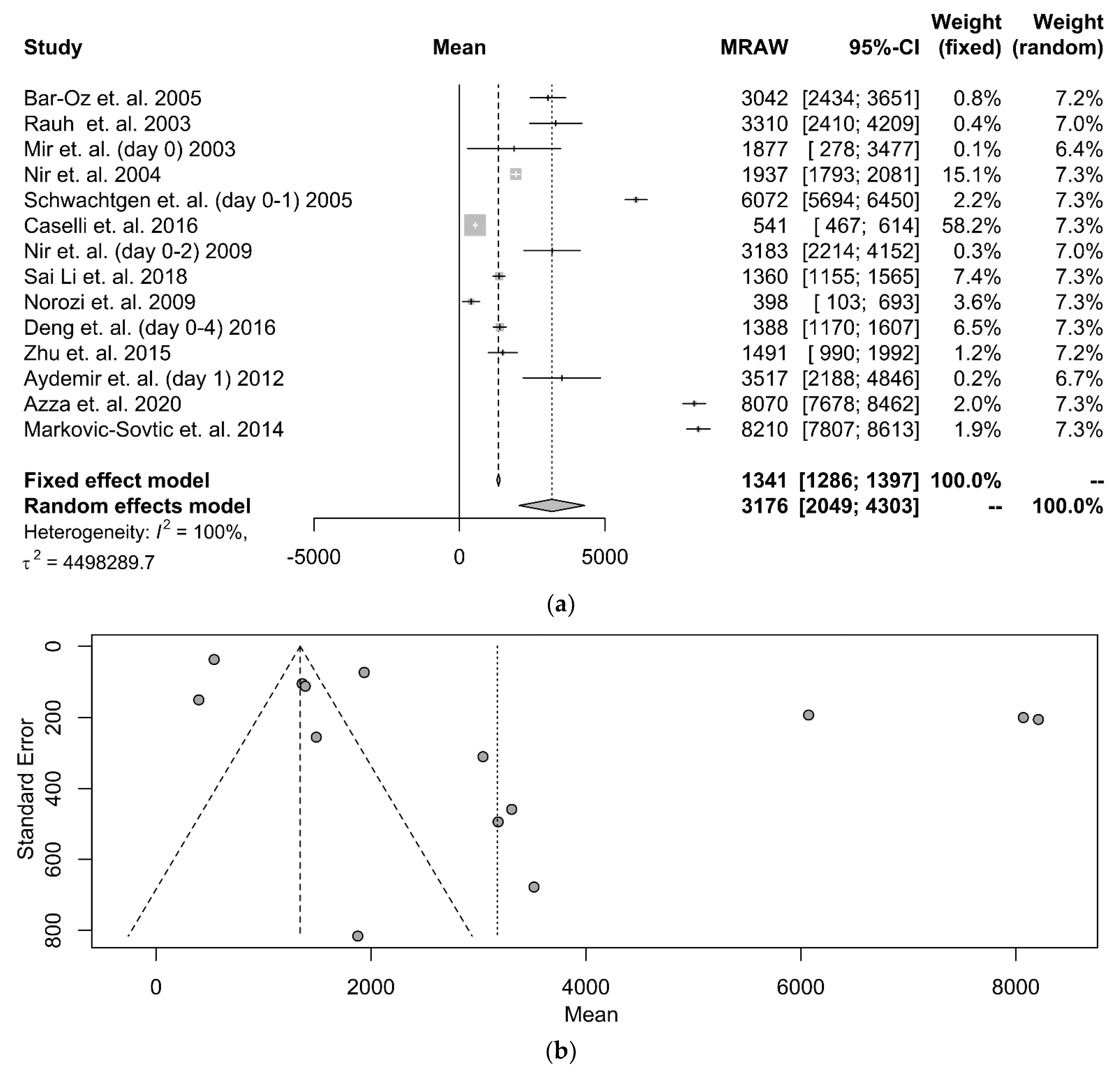
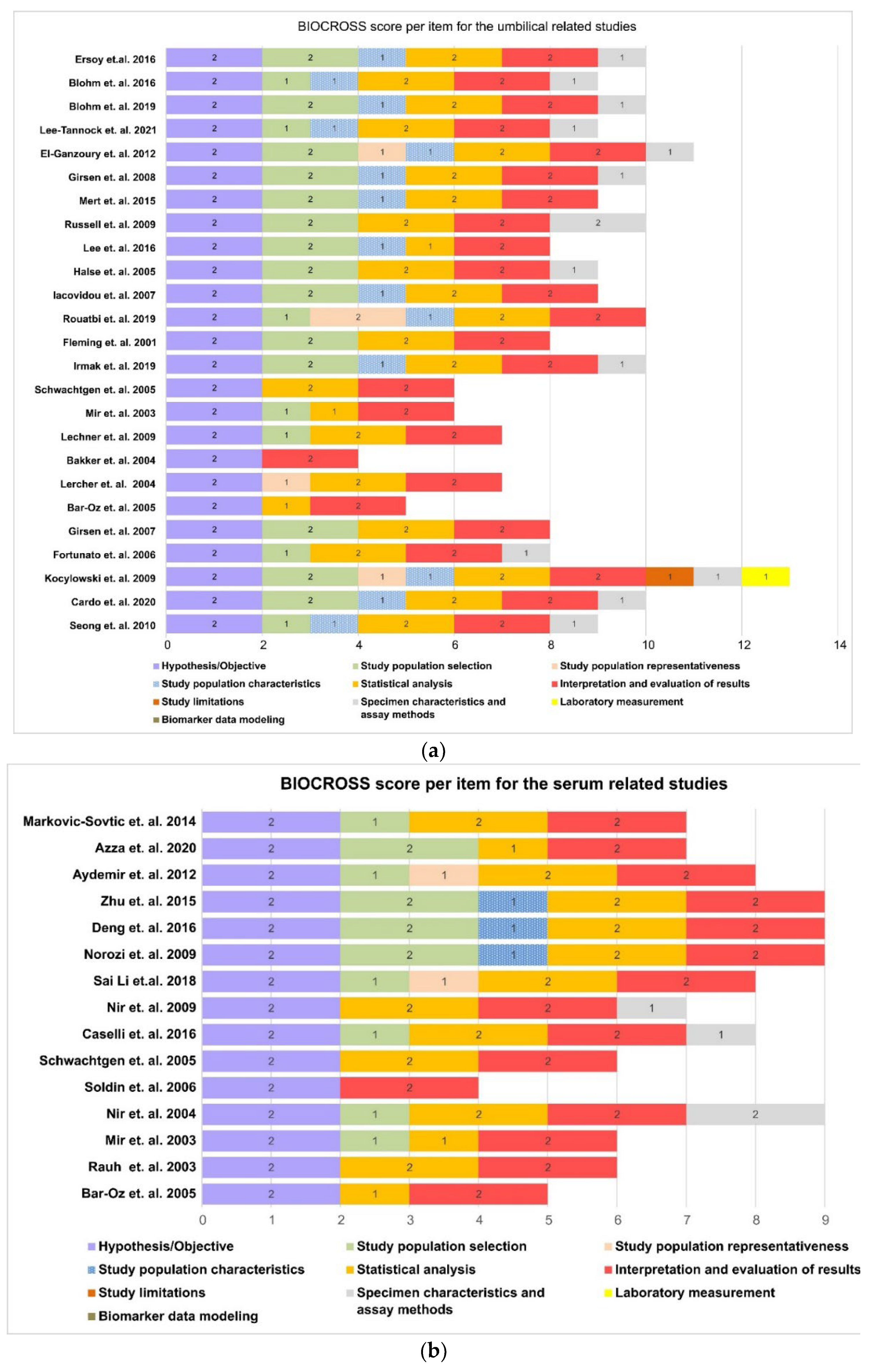
| First Author | Year | Source | Mode of Delivery | N | Kit | NT-proBNP (pg/mL) |
|---|---|---|---|---|---|---|
| Won Joon Seong [16] | 2010 | Umbilical vein | CS | 34 | Modular Analytics | Mean: 801.9 SD: ±537.7 |
| Umbilical vein | VD | 29 | Mean: 724.3 SD: ±542.4 | |||
| Leire Cardo [17] | 2020 | Cord blood | CS | 92 | Cobas 6000 | Mean: 662 SD: - |
| VD | 177 | Mean: 524 SD: - | ||||
| Rafal Kocylowski [18] | 2009 | Umbilical artery | CS | 2 | Elecsys 2010 | Mean: 1204 SD: ±1222.6 |
| Umbilical artery | VD | 154 | Mean: 832 SD: ±253.1 | |||
| Umbilical vein | CS | 51 | Mean: 1201 SD: ±1222.6 | |||
| Umbilical vein | VD | 229 | Mean: 831 SD: ±1022.4 | |||
| Giuliana Fortunato [19] | 2006 | Cord blood | N/A | 87 | Elecsys 2010 | Mean: 548.2 SD: - |
| Anna Girsen [20] | 2007 | Umbilical artery | N/A | 49 | N/A | Median: 2630.1 Range: 1319–15814 |
| Benjamin Bar-Oz [21] | 2005 | Cord blood | N/A | 122 | Elecsys 2010 | Mean: 578.8 SD: ±351.3 |
| Angelika Lercher [22] | 2005 | Umbilical vein | N/A | 42 | Elecsys 2010 | Median: 553.4 SD:- |
| Jaap Bakker [23] | 2004 | Cord blood | N/A | 71 | Elecsys 2010 | Μean: 668.1 SD: ±363.5 |
| Evelyn Lechner [24] | 2009 | Cord blood | N/A | 200 | Elecsys 2010 | Median: 626 Range: 153–2518 |
| Thomas Mir [25] | 2003 | Umbilical vein | VD | 37 | Biomedica | Mean: 1691 SD: - |
| Lynn Schwachtgen [26] | 2005 | Cord blood | N/A | 62 | Elecsys 2010 | Mean: 818 SD: ±281–2595 |
| Kübra Irmak [27] | 2019 | Umbilical vein | CS | 43 | CK-E10219 | Mean: 1069 SD: ±721 |
| Sean Martin Fleming [28] | 2001 | Umbilical artery | N/A | 16 | Biomedica | Mean: 1885.5 SD: - |
| Hatem Rouatbi [29] | 2019 | Cord blood | N/A | 169 | Elecsys 2010 | Mean: 296.87 SD: ±273.8 |
| Nicoletta Iacovidou [30] | 2007 | Cord blood | N/A | 20 | Biomedica | Mean: 8032.6 SD: - |
| Karen Halse [31] | 2005 | Umbilical vein | N/A | 22 | N/A | Median: 135,2 Range: 76.1–270.6 |
| Seung Lee [32] | 2016 | Umbilical vein | N/A | 23 | N/A | Median: 633.2 SD:- |
| Noirin Russell [33] | 2009 | Cord blood | N/A | 39 | Elecsys 2010 | Median: 727.3 Range: 50.7–1403.8 |
| Mustafa Mert [34] | 2015 | Umbilical artery | N/A | 58 | ECLIA | Mean: 592 SD: ±236.8 |
| Anna Girsen [35] | 2008 | Umbilical artery | N/A | 60 | N/A | Median: 1649,1 Range: 414.3–3501.1 |
| Mona El-Ganzoury [36] | 2012 | Umbilical vein | N/A | 30 | R & D Systems | Median: 1310.5 Range: 870.9–1750.2 |
| Alison Lee-Tannock [37] | 2021 | Umbilical vein | N/A | 78 | Vitros 5600 | Median: 545 Range: 368–793 |
| Martin Blohm [38] | 2019 | Umbilical vein | N/A | 86 | Elecsys 2010 | Median: 816.45 5th–95th Percentile: 369.62–2233.15 |
| Umbilical artery | N/A | 48 | Median: 765.48 5th–95th Percentile: 259.84–1819.5 | |||
| Martin Blohm [39] | 2016 | Umbilical vein | N/A | 66 | Elecsys 2010 | Mean: 1228.94 25th–75th Percentile: 708–1617.5 |
| Ali Ersoy [40] | 2016 | Umbilical vein | N/A | 36 | Elisa kit (USCNK, Wuhan, Hubei) | Mean: 1551.18 SD: ±1148.9 |
| First Author | Year | Age Range | N | Kit | NT-proBNP (pg/mL) |
|---|---|---|---|---|---|
| Benjamin Bar-Oz [21] | 2005 | Day 1–4 | 33 | Elecsys 2010 | Mean: 3042.4 SD: ±1783.2 |
| Manfred Rauh [41] | 2003 | Day 0–12 | 13 | Elecsys 2010 | Range: 1121–7740 |
| Thomas Mir [25] | 2003 | Day 0 | 14 | Biomedica | Mean: 1877.1 SD: ±490.4–6586.8 |
| Day 1 | 18 | Mean: 5419,9 SD: ±2147.6–10,755.3 | |||
| Day 2 | 12 | Mean: 2703 SD: - | |||
| Day 3 | 16 | Mean: 2064.8 SD: | |||
| Day 4 | 18 | Mean: 2083.6 SD: - | |||
| Day 5–7 | 16 | Mean: 1724.9 SD: - | |||
| Day 8–14 | 10 | Mean: 1437.4 SD:- | |||
| Day 15–30 | 12 | Mean: 1830 SD: - | |||
| Amiram Nir [42] | 2004 | Day 1–5 | 20 | Elecsys 2010 | Mean: 1937 SD: ±328 |
| Steven Soldin [43] | 2006 | Day 0–30 | 99 | Dade R X L | 97th: 31832.5 |
| Lynn Schwachtgen [26] | 2005 | Day 0–1 | 8 | Elecsys 2010 | Mean: 6072 SD: ±546 |
| Day 2–3 | 40 | Mean: 2972 SD: ±1808 | |||
| Day 4–8 | 11 | Mean: 1731 SD: ±1236 | |||
| Chiara Caselli [44] | 2016 | Day 0–30 | 24 | Elecsys 2010 | Median: 504.3 Percentiles 25th–75th: 211.1–942.7 |
| Amiram Nir [45] | 2009 | Day 0–2 | 43 | Elecsys 2010 | Median: 3183 Range: 260–13,224 |
| Day 3–11 | 84 | Median: 2210 Range: 28–7.250 | |||
| Sai Li [46] | 2018 | Day 0–30 | 80 | VIDAS | Median: 1360 Range: 250–3987 |
| Kambiz Norozi [47] | 2009 | Day 0–30 | 8 | Elecsys 2010 | Mean: 398 SD: ±425 |
| Menghong Deng [48] | 2016 | Day 0–4 | N/A | YZB/CAN 1794-2008 | Median: 1388.5 Range: 750–4615 |
| Day 5–15 | Ν/A | Median = 640.8 Range 515–850 | |||
| Day 16–28 | N/A | Median = 412.7 Range 341–462 | |||
| Rui Zhu [49] | 2015 | Day 0–3 | 63 | Elecsys 2010 | Median: 1491 Percentiles 5th–95th: 499.5–8607.3 |
| Ozger Aydemir [50] | 2012 | 6th hour of life | 33 | Immulite 2000 | Median: 3509 Min–Max: 1174–35,000 |
| Day 1 | 33 | Median: 3517 Min–Max: 875–16,452 | |||
| Day 3 | 33 | Median: 1211 Min–Max: 377–2774 | |||
| Day 5 | 33 | Median: 808 Min–Max: 440–2284 | |||
| Azza Ahmed [51] | 2020 | Day 0–30 | 26 | Abnova | Mean: 8070 SD: ±1020 |
| Gordana Marcovic [52] | 2014 | Day 1–28 | 28 | Elecsys 2010 | Mean: 8210 Range: 6.110–10.460 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Christou, E.; Iliodromiti, Z.; Pouliakis, A.; Sokou, R.; Zantiotou, M.; Petropoulou, C.; Boutsikou, T.; Iacovidou, N. NT-proBNP Concentrations in the Umbilical Cord and Serum of Term Neonates: A Systematic Review and Meta-Analysis. Diagnostics 2022, 12, 1416. https://doi.org/10.3390/diagnostics12061416
Christou E, Iliodromiti Z, Pouliakis A, Sokou R, Zantiotou M, Petropoulou C, Boutsikou T, Iacovidou N. NT-proBNP Concentrations in the Umbilical Cord and Serum of Term Neonates: A Systematic Review and Meta-Analysis. Diagnostics. 2022; 12(6):1416. https://doi.org/10.3390/diagnostics12061416
Chicago/Turabian StyleChristou, Evangelos, Zoe Iliodromiti, Abraham Pouliakis, Rozeta Sokou, Matina Zantiotou, Chrisa Petropoulou, Theodora Boutsikou, and Nicoletta Iacovidou. 2022. "NT-proBNP Concentrations in the Umbilical Cord and Serum of Term Neonates: A Systematic Review and Meta-Analysis" Diagnostics 12, no. 6: 1416. https://doi.org/10.3390/diagnostics12061416
APA StyleChristou, E., Iliodromiti, Z., Pouliakis, A., Sokou, R., Zantiotou, M., Petropoulou, C., Boutsikou, T., & Iacovidou, N. (2022). NT-proBNP Concentrations in the Umbilical Cord and Serum of Term Neonates: A Systematic Review and Meta-Analysis. Diagnostics, 12(6), 1416. https://doi.org/10.3390/diagnostics12061416








