Posterior Reversible Encephalopathy Syndrome Following Chemotherapy and Immune Checkpoint Inhibitor Combination in a Patient with Small-Cell Lung Cancer
Abstract
:1. Introduction
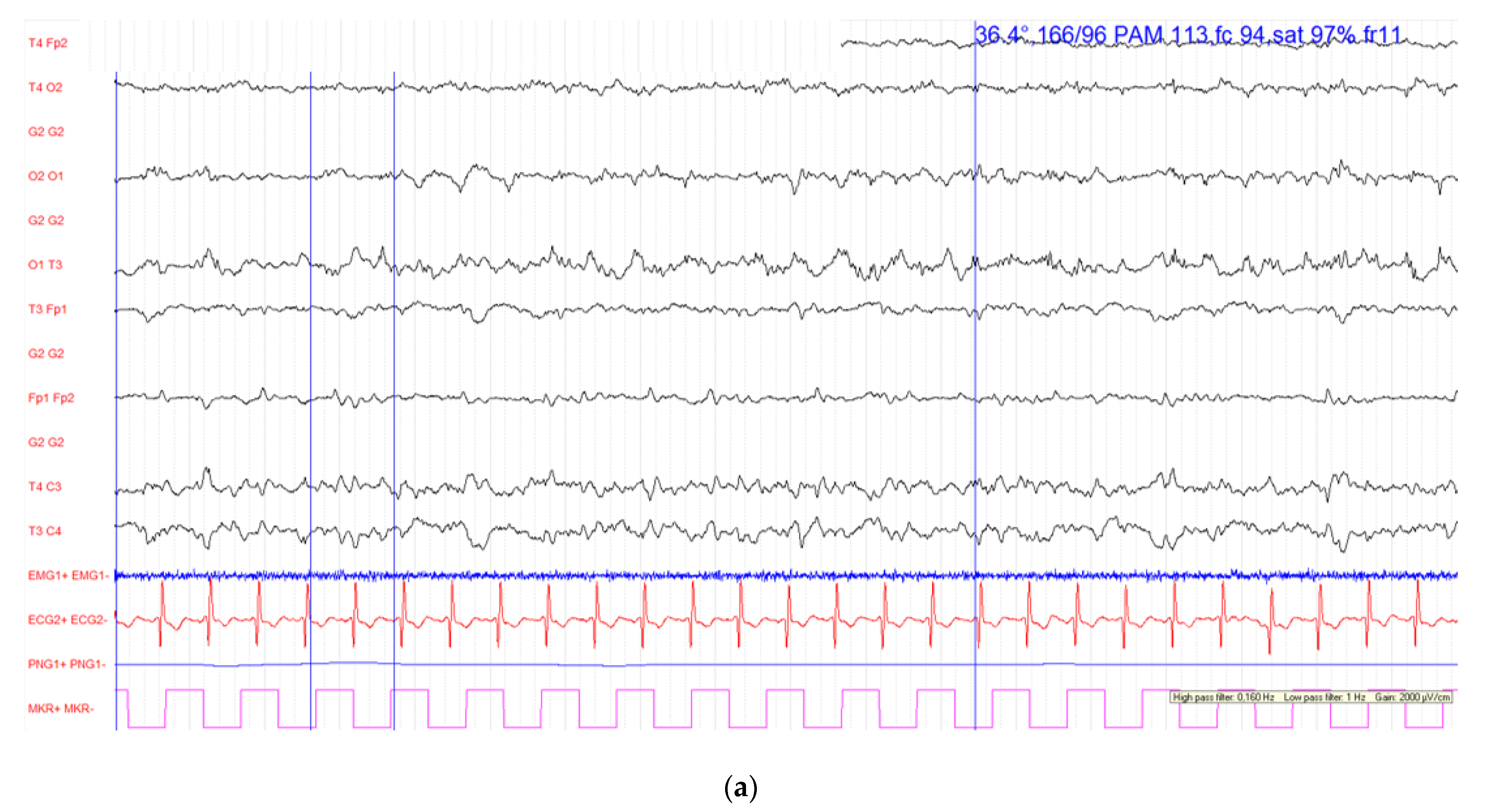
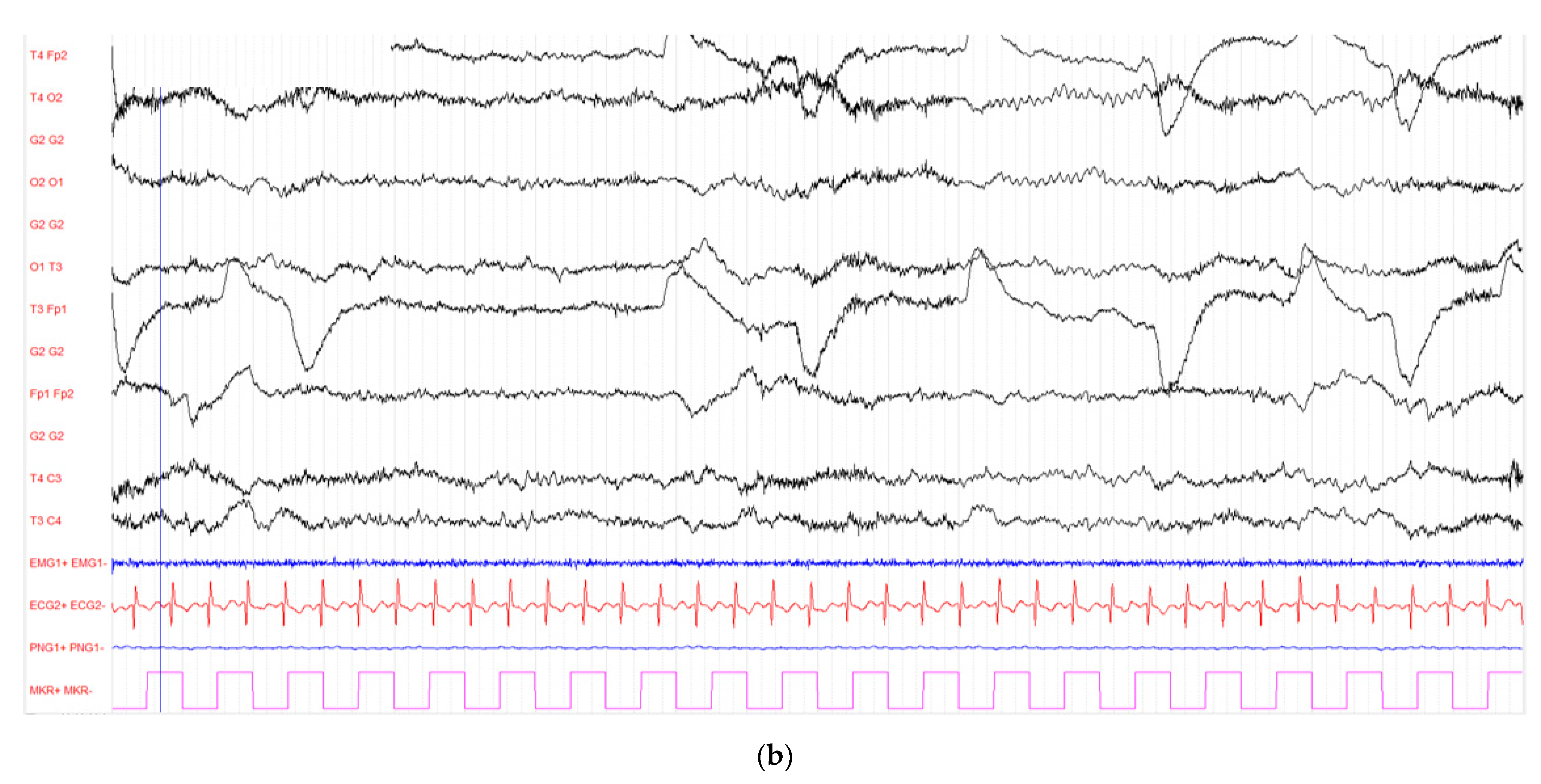
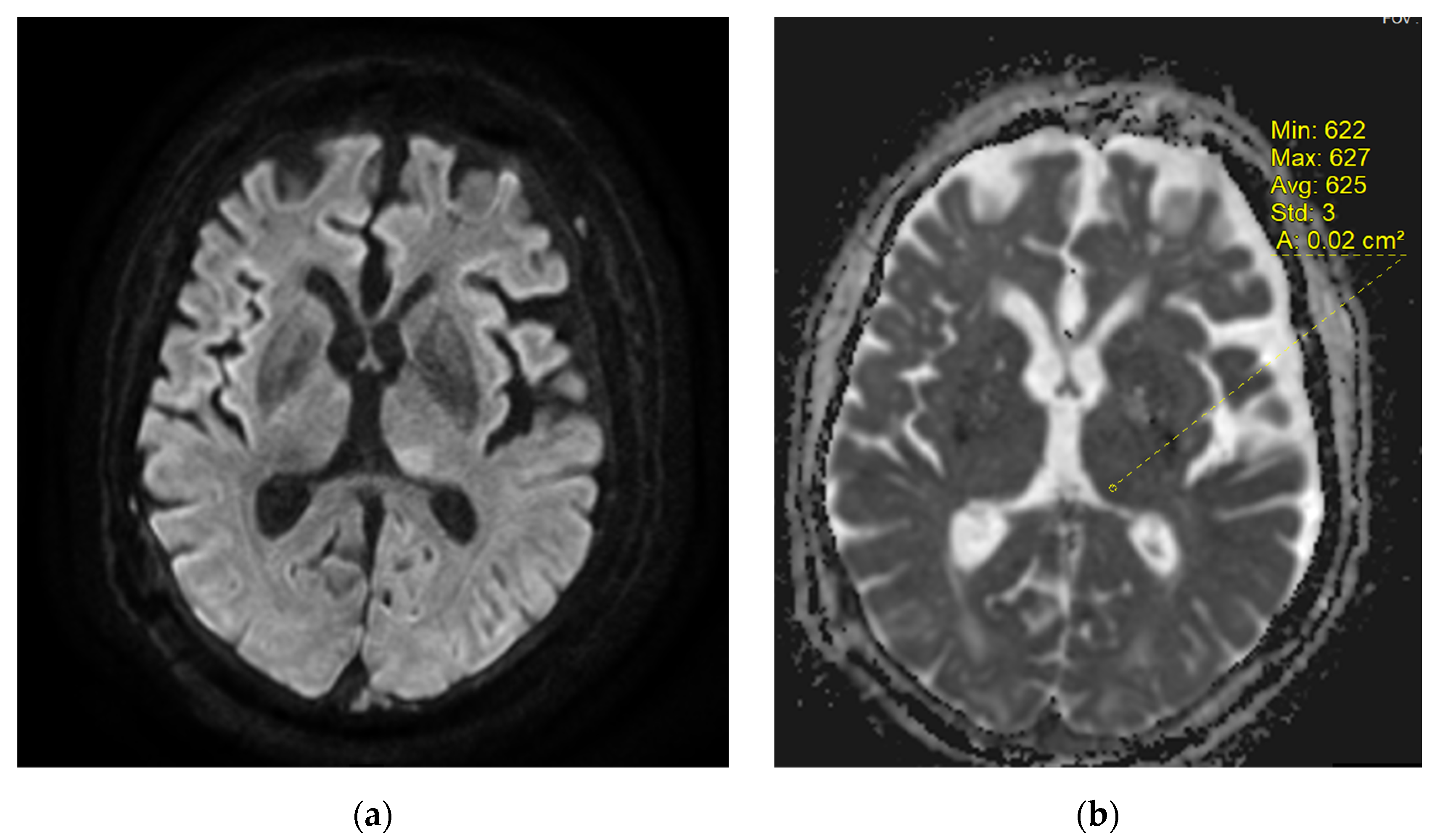
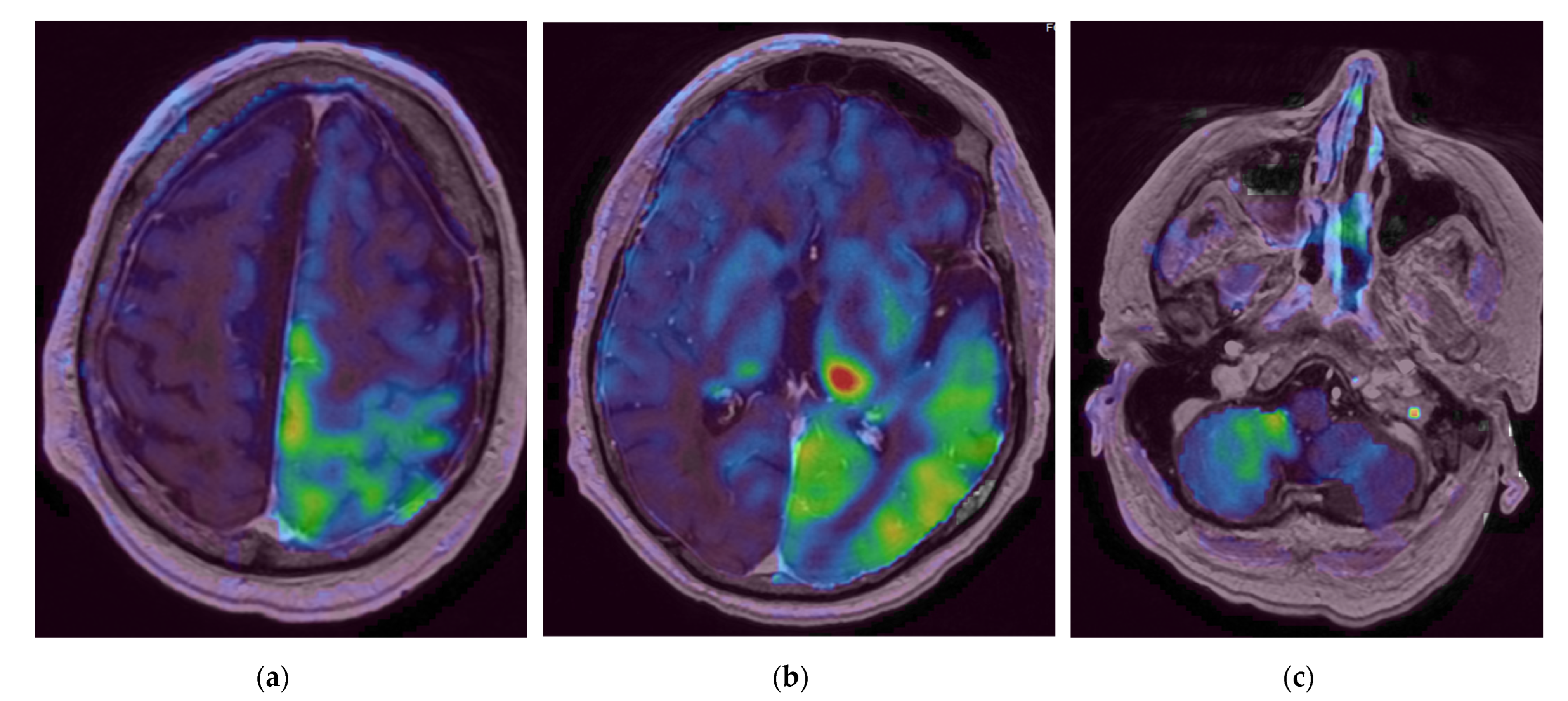
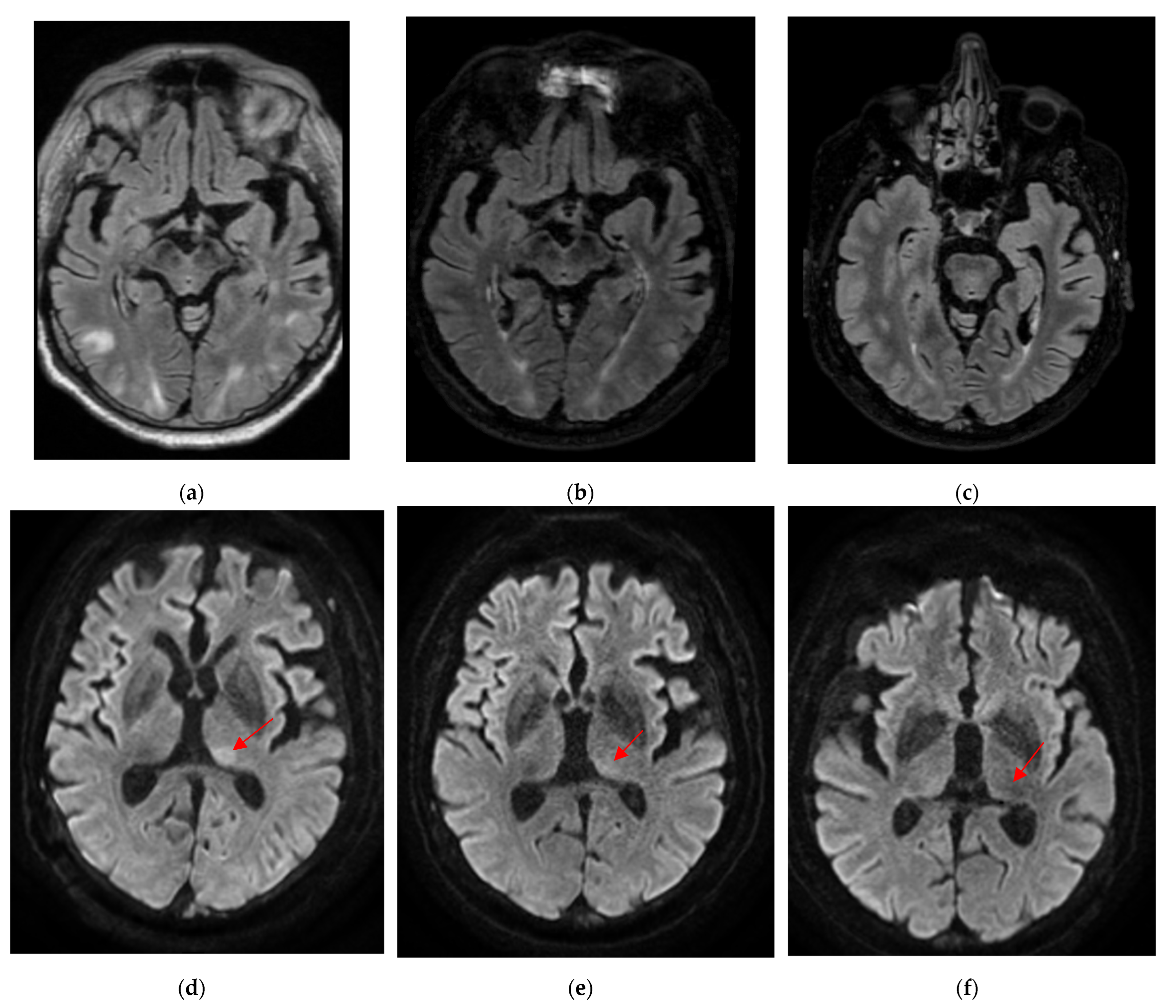
2. Case Report
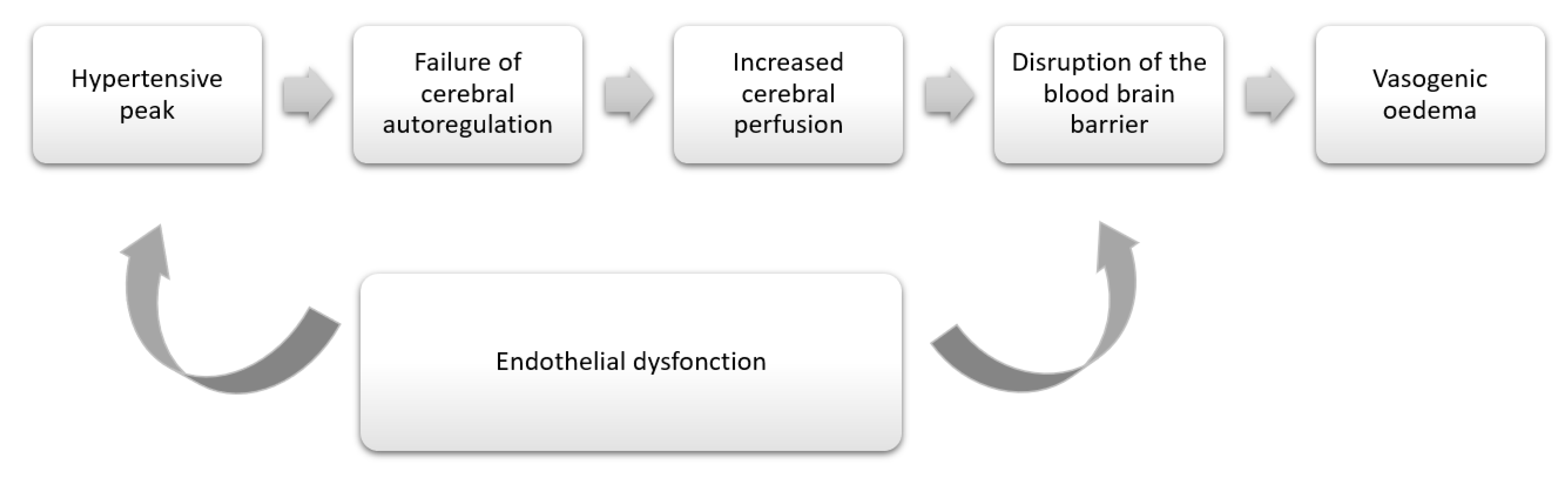
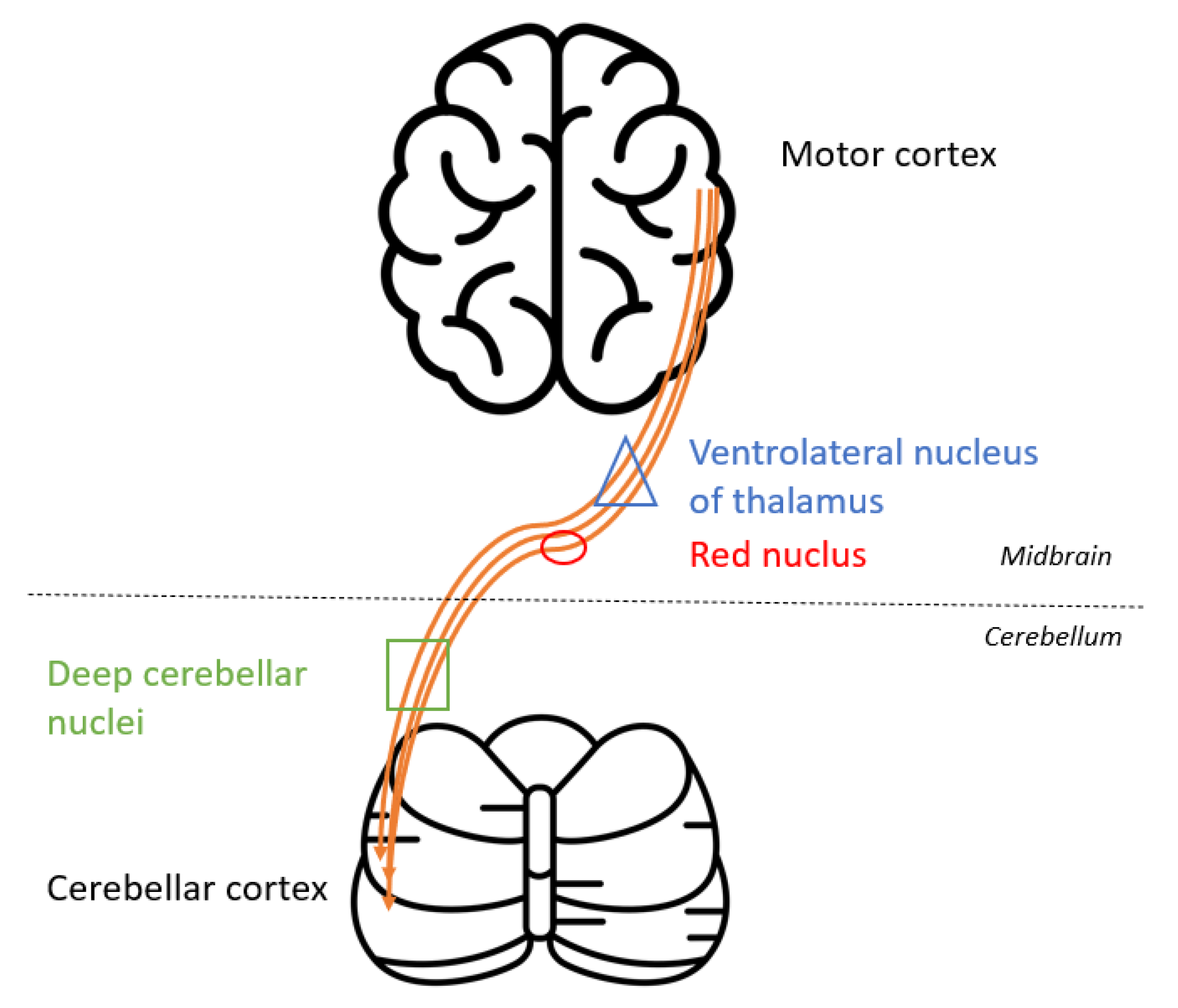
3. Discussion
4. Conclusions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hinchey, J.; Chaves, C.; Appignani, B.; Breen, J.; Pao, L.; Wang, A.; Pessin, M.S.; Lamy, C.; Mas, J.L.; Caplan, L.R. A Reversible Posterior Leukoencephalopathy Syndrome. N. Engl. J. Med. 1996, 334, 494–500. [Google Scholar] [CrossRef] [PubMed]
- Liman, T.G.; Bohner, G.; Heuschmann, P.U.; Endres, M.; Siebert, E. The Clinical and Radiological Spectrum of Posterior Reversible Encephalopathy Syndrome: The Retrospective Berlin PRES Study. J. Neurol. 2012, 259, 155–164. [Google Scholar] [CrossRef] [PubMed]
- Roth, C.; Ferbert, A. The Posterior Reversible Encephalopathy Syndrome: What’s Certain, What’s New? Pract. Neurol. 2011, 11, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Fischer, M.; Schmutzhard, E. Posterior Reversible Encephalopathy Syndrome. J. Neurol. 2017, 264, 1608–1616. [Google Scholar] [CrossRef] [Green Version]
- Imai, H.; Okuno, N.; Ishihara, S.; Nakano, S.; Higuchi, S.; Arai, T.; Tokunaga, M.; Kobayashi, H.; Arai, T.; Mori, M. Reversible Posterior Leukoencephalopathy Syndrome after Carboplatin and Paclitaxel Regimen for Lung Cancer. Intern. Med. 2012, 51, 911–915. [Google Scholar] [CrossRef] [Green Version]
- Jamelot, M.; Fontanilles, M.; Vaschalde, Y. Reversible posterior leukoencephalopathy post-chemotherapy with carboplatin and paclitaxel. Bull. Du Cancer 2019, 106, 1191–1195. [Google Scholar] [CrossRef]
- Vieillot, S.; Pouessel, D.; de Champfleur, N.M.; Becht, C.; Culine, S. Reversible Posterior Leukoencephalopathy Syndrome after Carboplatin Therapy. Ann. Oncol. 2007, 18, 608–609. [Google Scholar] [CrossRef]
- Cacho-Díaz, B.; Lorenzana-Mendoza, N.A.; Salmerón-Moreno, K.; Reyes-Soto, G.; Castillo-Rangel, C.; Corona-Cedillo, R.; Escobar-Ceballos, S.; de la Garza-Salazar, J.G. Chemotherapy-Induced Posterior Reversible Encephalopathy Syndrome: Three Case Reports. Medicine 2019, 98, e15691. [Google Scholar] [CrossRef]
- Ryan, S.A.; Maceneaney, P.; O’Reilly, S.P.; Moylan, E.J.; Power, D.G. Reversible Posterior Leukoencephalopathy Induced by Carboplatin and Etoposide. Med. Oncol. 2012, 29, 1287–1291. [Google Scholar] [CrossRef]
- Barber, F.D. Identification and Management of Posterior Reversible Encephalopathy Syndrome in a Patient Enrolled in an Immunotherapy Combination Phase I Clinical Trial: A Case Study. Asia-Pac. J. Oncol. Nurs. 2021, 8, 103–105. [Google Scholar] [CrossRef]
- Kim, D. Posterior Reversible Encephalopathy Syndrome Induced by Nivolumab Immunotherapy for Non-Small-Cell Lung Cancer. Clin. Case Rep. 2019, 7, 935–938. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fugate, J.E.; Rabinstein, A.A. Posterior Reversible Encephalopathy Syndrome: Clinical and Radiological Manifestations, Pathophysiology, and Outstanding Questions. Lancet Neurol. 2015, 14, 914–925. [Google Scholar] [CrossRef]
- Lamy, C.; Oppenheim, C.; Méder, J.F.; Mas, J.L. Neuroimaging in Posterior Reversible Encephalopathy Syndrome. J. Neuroimaging 2004, 14, 89–96. [Google Scholar] [CrossRef] [PubMed]
- Doelken, M.; Lanz, S.; Rennert, J.; Alibek, S.; Richter, G.; Doerfler, A. Differentiation of Cytotoxic and Vasogenic Edema in a Patient with Reversible Posterior Leukoencephalopathy Syndrome Using Diffusion-Weighted MRI. Diagn. Interv. Radiol. 2007, 13, 125–128. [Google Scholar] [PubMed]
- Casey, S.O.; Sampaio, R.C.; Michel, E.; Truwit, C.L. Posterior Reversible Encephalopathy Syndrome: Utility of Fluid-Attenuated Inversion Recovery MR Imaging in the Detection of Cortical and Subcortical Lesions. AJNR Am. J. Neuroradiol. 2000, 21, 1199–1206. [Google Scholar] [PubMed]
- Sheikh-Bahaei, N.; Acharya, J.; Rajamohan, A.; Kim, P.E. Advanced Imaging Techniques in Diagnosis of Posterior Reversible Encephalopathy Syndrome (PRES). Front. Neurol. 2020, 11, 165. [Google Scholar] [CrossRef] [Green Version]
- Vilela, P. Acute Stroke Differential Diagnosis: Stroke Mimics. Eur. J. Radiol. 2017, 96, 133–144. [Google Scholar] [CrossRef]
- Tetsuka, S.; Ogawa, T. Posterior Reversible Encephalopathy Syndrome: A Review with Emphasis on Neuroimaging Characteristics. J. Neurol. Sci. 2019, 404, 72–79. [Google Scholar] [CrossRef]
- Caplan, L.R. “Top of the Basilar” Syndrome. Neurology 1980, 30, 72–79. [Google Scholar] [CrossRef]
- Ou, S.; Xia, L.; Wang, L.; Xia, L.; Zhou, Q.; Pan, S. Posterior Reversible Encephalopathy Syndrome with Isolated Involving Infratentorial Structures. Front. Neurol. 2018, 9, 843. [Google Scholar] [CrossRef]
- Pilato, F.; Distefano, M.; Calandrelli, R. Posterior Reversible Encephalopathy Syndrome and Reversible Cerebral Vasoconstriction Syndrome: Clinical and Radiological Considerations. Front. Neurol. 2020, 11, 34. [Google Scholar] [CrossRef] [PubMed]
- Katramados, A.M.; Burdette, D.; Patel, S.C.; Schultz, L.R.; Gaddam, S.; Mitsias, P.D. Periictal Diffusion Abnormalities of the Thalamus in Partial Status Epilepticus. Epilepsia 2009, 50, 265–275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yokota, H.; Ida, Y. Crossed Cerebellar Diaschisis in Status Epilepticus. Neurochirurgie 2019, 65, 425–426. [Google Scholar] [CrossRef] [PubMed]
- Poretti, A.; Boltshauser, E. Crossed Cerebro-Cerebellar Diaschisis. Neuropediatrics 2012, 43, 53–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bartynski, W.S. Posterior Reversible Encephalopathy Syndrome, Part 1: Fundamental Imaging and Clinical Features. AJNR Am. J. Neuroradiol. 2008, 29, 1036–1042. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floeter, A.E.; Patel, A.; Tran, M.; Chamberlain, M.C.; Hendrie, P.C.; Gopal, A.K.; Cassaday, R.D. Posterior Reversible Encephalopathy Syndrome Associated With Dose-Adjusted EPOCH (Etoposide, Prednisone, Vincristine, Cyclophosphamide, Doxorubicin) Chemotherapy. Clin. Lymphoma Myeloma Leuk. 2017, 17, 225–230. [Google Scholar] [CrossRef] [PubMed]
- Khanal, P.; Awan, F.; Nguyen, V. Etoposide-Induced Posterior Reversible Encephalopathy Syndrome. Ann. Hematol. 2013, 92, 561–562. [Google Scholar] [CrossRef]
- Ruste, V.; Goldschmidt, V.; Laparra, A.; Messayke, S.; Danlos, F.-X.; Romano-Martin, P.; Champiat, S.; Voisin, A.-L.; Baldini, C.; Massard, C.; et al. The Determinants of Very Severe Immune-Related Adverse Events Associated with Immune Checkpoint Inhibitors: A Prospective Study of the French REISAMIC Registry. Eur. J. Cancer 2021, 158, 217–224. [Google Scholar] [CrossRef]

| Machine | Weighting | Sequence | TR | TE | Slice Thickness |
|---|---|---|---|---|---|
| Discovery MR 750w 3T Installed in 2012, 70 cm tunnel, 32 channels, 50 cm z-axisFOV, gradient 44 mT/m SR 200 T/m/s | T1 pre-contrast | 3D rapid gradient echo | 9 ms | 2.1 ms | 1 mm |
| T2-FLAIR | Turbo spin echo | 7002 ms | 118 ms | 1 mm | |
| DWI | EPI, two-b-values (0 and 1000 mm/s) | 3349 ms | 62.6 ms | 3 mm | |
| T1 post-contrast | 3D rapid gradient echo | 6.1 ms | 2.1 ms | 1 mm |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Evin, C.; Lassau, N.; Balleyguier, C.; Assi, T.; Ammari, S. Posterior Reversible Encephalopathy Syndrome Following Chemotherapy and Immune Checkpoint Inhibitor Combination in a Patient with Small-Cell Lung Cancer. Diagnostics 2022, 12, 1369. https://doi.org/10.3390/diagnostics12061369
Evin C, Lassau N, Balleyguier C, Assi T, Ammari S. Posterior Reversible Encephalopathy Syndrome Following Chemotherapy and Immune Checkpoint Inhibitor Combination in a Patient with Small-Cell Lung Cancer. Diagnostics. 2022; 12(6):1369. https://doi.org/10.3390/diagnostics12061369
Chicago/Turabian StyleEvin, Cécile, Nathalie Lassau, Corinne Balleyguier, Tarek Assi, and Samy Ammari. 2022. "Posterior Reversible Encephalopathy Syndrome Following Chemotherapy and Immune Checkpoint Inhibitor Combination in a Patient with Small-Cell Lung Cancer" Diagnostics 12, no. 6: 1369. https://doi.org/10.3390/diagnostics12061369
APA StyleEvin, C., Lassau, N., Balleyguier, C., Assi, T., & Ammari, S. (2022). Posterior Reversible Encephalopathy Syndrome Following Chemotherapy and Immune Checkpoint Inhibitor Combination in a Patient with Small-Cell Lung Cancer. Diagnostics, 12(6), 1369. https://doi.org/10.3390/diagnostics12061369






