Transient Polyhydramnios during Pregnancy Complicated with Gestational Diabetes Mellitus: Case Report and Systematic Review
Abstract
:1. Introduction
2. Case Report
- -
- Blood type and Rh were determined at the first prenatal visit—patient with negative Rh. During pregnancy, the anti-Rh antibodies were undetectable.
- -
- TORCH profile (performed during the 1st trimester)—normal.
- -
- The patient underwent screening evaluation for fetal abnormalities at the gestational age of 12 weeks and 5 days, with free β-HCG = 1.177 MoM, PAPP-A = 2.050 MoM, and the following risks for trisomy: trisomy 21 (Down syndrome), 1:3987; trisomy 18, 1:9641; and trisomy 13, 1:1514. These values include patients in the low-risk group for fetal genetic abnormalities.
- -
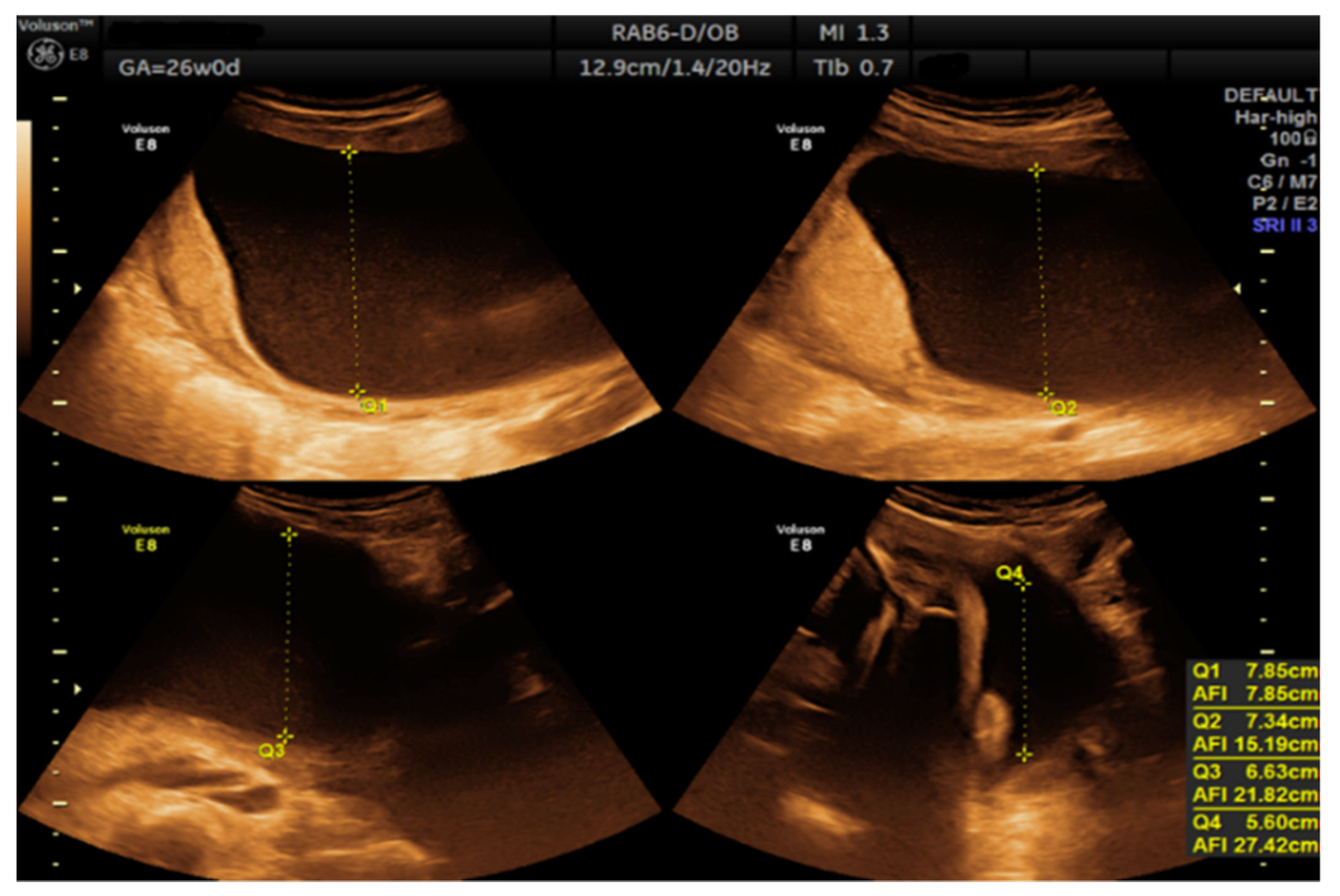
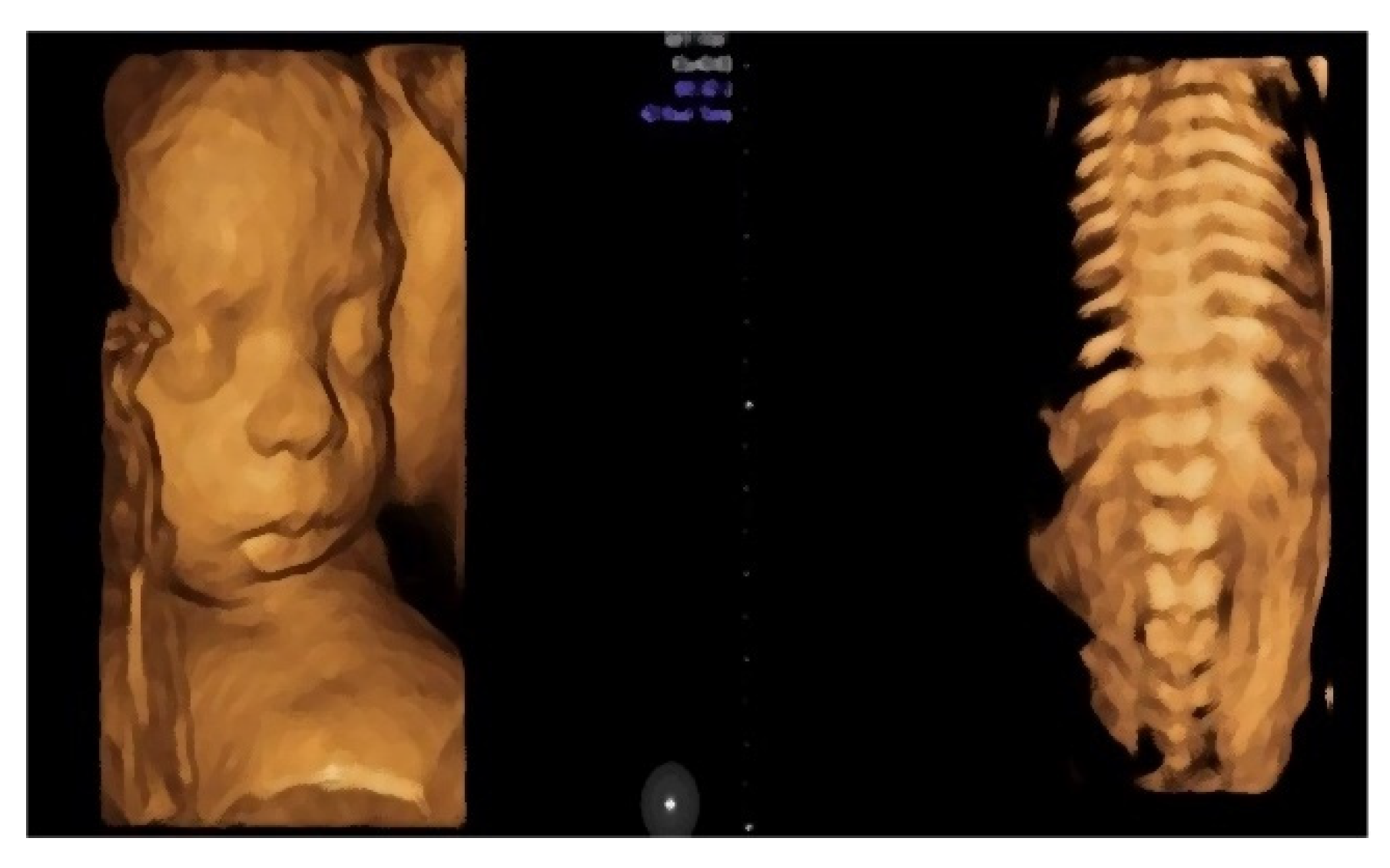
- Morphological examination during the 2nd trimester highlights the normal anatomy of the fetus (Figure 3).
- -
- Muscular and skeletal diseases were excluded based on the presence of long bones with a normal shape and echogenicity without any visible asymmetry.
- -
- A digestive system with a normal aspect: complete anterior abdominal wall, stomach in a left position under the diaphragm, normal fetal intestinal development with normal echogenicity.
- -
- Central nervous system with no symmetric changes and a normal aspect. Complete fetal spine. No neural-tube defects were observed.
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Magann, E.F.; Chauhan, S.P.; Doherty, D.A.; Lutgendorf, M.A.; Magann, M.I.; Morrison, J.C. A review of idiopathic hydramnios and pregnancy outcomes. Obstet. Gynecol. Surv. 2007, 62, 795–802. [Google Scholar] [CrossRef] [PubMed]
- Alexander, E.; Spitz, H.; Clark, R. Sonography of polyhydramnios. Am. J. Roentgenol. 1982, 138, 343–346. [Google Scholar] [CrossRef] [PubMed]
- Hill, L.M.; Breckle, R.; Thomas, M.L.; Fries, J.K. Polyhydramnios: Ultrasonically detected prevalence and neonatal outcome. Obs. Gynecol. 1987, 69, 21–25. [Google Scholar]
- Matěcha, J.; Nováčková, M. Idiopathic polyhydramnios. Ceska Gynekol. 2020, 85, 417–421. [Google Scholar]
- Zeino, S.; Carbillon, L.; Pharisien, I.; Tigaizin, A.; Benchimol, M.; Murtada, R.; Boujenah, J. Delivery outcomes of term pregnancy complicated by idiopathic polyhydramnios. J. Gynecol. Obstet. Hum. Reprod. 2017, 46, 349–354. [Google Scholar] [CrossRef]
- Coombe-Patterson, J. Amniotic Fluid Assessment: Amniotic Fluid Index versus Maximum Vertical Pocket. J. Diagn. Med. Sonogr. 2017, 33, 280–283. [Google Scholar] [CrossRef] [Green Version]
- Phelan, J.P.; Ahn, M.O.; Smith, C.V.; Rutherford, S.E.; Anderson, E. Amniotic fluid index measurements during pregnancy. J. Reprod. Med. 1987, 32, 601–604. [Google Scholar]
- Fawad, A.; Danish, N. Frequency, causes and outcome of polyhydramnios. Gomal. J. Med. Sci. 2008, 6, 106–109. [Google Scholar]
- Hamza, A.; Herr, D.; Solomayer, E.F.; Meyberg-Solomayer, G. Polyhydramnios: Causes, Diagnosis and Therapy. Geburtshilfe und Frauenheilkunde 2013, 73, 1241–1246. [Google Scholar] [CrossRef] [Green Version]
- Frank Wolf, M.; Peleg, D.; Stahl-Rosenzweig, T.; Kurzweil, Y.; Yogev, Y. Isolated polyhydramnios in the third trimester: Is a gestational diabetes evaluation of value? Gynecol. Endocrinol. 2017, 33, 849–852. [Google Scholar] [CrossRef]
- Berezowsky, A.; Ashwal, E.; Hiersch, L.; Yogev, Y.; Aviram, A. Transient Isolated Polyhydramnios and Perinatal Outcomes. Ultraschall Med. 2019, 40, 749–756. [Google Scholar] [CrossRef] [PubMed]
- Aviram, A.; Salzer, L.; Hiersch, L.; Ashwal, E.; Golan, G.; Pardo, J.; Wiznitzer, A.; Yogev, Y. Association of Isolated Polyhydramnios at or Beyond 34 Weeks of Gestation and Pregnancy Outcome. Obstet. Gynecol. 2015, 125, 825–832. [Google Scholar] [CrossRef] [PubMed]
- Magann, E.F.; Doherty, D.A.; Lutgendorf, M.A.; Magann, M.I.; Chauhan, S.P.; Morrison, J.C. Peripartum outcomes of high-risk pregnancies complicated by oligo- and polyhydramnios: A prospective longitudinal study. J. Obs. Gynaecol. Res. 2010, 36, 268–277. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.-S.; Zhang, Y.-X.; Meng, H.; Wu, X.-N.; Qi, Q.-W. Adducted thumb as an isolated morphologic finding: An early sonographic sign of impaired neurodevelopment A STROBE compliant study. Medicine 2018, 97, e12437. [Google Scholar] [CrossRef]
- Desai, T.; Vishwanath, U. Placental Chorioangioma: A Planned Successful Outcome. J. Obs. Gynecol. India 2021, 71, 181–183. [Google Scholar] [CrossRef]
- Iwahata, H.; Iwahata, Y.; Homma, C.; Kurasaki, A.; Hasegawa, J.; Suzuki, N. Degenerative type of placental chorioangioma requiring fetal blood transfusion. J. Obs. Gynaecol. Res. 2021, 47, 1191–1194. [Google Scholar] [CrossRef]
- Singh, A.; Sivaranjani, S.; Bagga, R.; Saha, P.; Dey, P. Successful outcome of giant chorioangioma. J. Fam. Med. Prim. Care 2021, 10, 2038. [Google Scholar] [CrossRef]
- Sakaria, R.P.; Mostafavi, R.; Miller, S.; Ward, J.C.; Pivnick, E.K.; Talati, A.J. Kagami-Ogata Syndrome: Case Series and Review of Literature. AJP Rep. 2021, 11, e65–e75. [Google Scholar] [CrossRef]
- Thakur, S.; Kumar, M.; Malhotra, S.; Paliwal, P.; Thareja, V.; Sahi, G. Severe Polyhydramnios with Consistent Fetal Full Bladder: A Novel Sign of Antenatal Bartter’s Disease. J. Pediatr. Genet. 2020, 9, 296–300. [Google Scholar] [CrossRef]
- Altmann, J.; Horn, D.; Korinth, D.; Eggermann, T.; Henrich, W.; Verlohren, S. Kagami-Ogata syndrome: An important differential diagnosis to Beckwith-Wiedemann syndrome. J. Clin. Ultrasound 2020, 48, 240–243. [Google Scholar] [CrossRef] [Green Version]
- Huang, H.; Mikami, Y.; Shigematsu, K.; Uemura, N.; Shinsaka, M.; Iwatani, A.; Miyake, F.; Kabe, K.; Takai, Y.; Saitoh, M.; et al. Kagami–Ogata syndrome in a fetus presenting with polyhydramnios, malformations, and preterm delivery: A case report. J. Med. Case Rep. 2019, 13, 340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, N.; Ko, J.M.; Shin, S.H.; Kim, E.K.; Kim, H.S.; Song, M.K.; Choi, C.W. Phenotypic and Genetic Characteristics of Five Korean Patients with Costello Syndrome. Cytogenet. Genome Res. 2019, 158, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Nam, G.; Cho, A.; Park, M.-H. A Rare Cause of Refractory Severe Polyhydramnios: Antenatal Bartter Syndrome. Medicina 2021, 57, 272. [Google Scholar] [CrossRef] [PubMed]
- Meyer, M.; Berrios, M.; Lo, C. Transient Antenatal Bartter’s Syndrome: A Case Report. Front. Pediatr. 2018, 6, 51. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.T.; Kim, H.; Kim, H.H.; Lee, N.H.; Han, Y.; Sung, S.I.; Chang, Y.S.; Park, W.S. A Rare Case of Lethal Prenatal-Onset Infantile Cortical Hyperostosis. Yonsei Med. J. 2019, 60, 484. [Google Scholar] [CrossRef] [PubMed]
- Arthuis, C.J.; Nizon, M.; Kömhoff, M.; Beck, B.B.; Riehmer, V.; Bihouée, T.; Bruel, A.; Benbrik, N.; Winer, N.; Isidor, B. A step towards precision medicine in management of severe transient polyhydramnios: MAGED2 variant. J. Obstet. Gynaecol. 2019, 39, 395–397. [Google Scholar] [CrossRef]
- Carvalho, A.; Estevinho, C.; Coelho, M.; Rocha, J.; Marinho, C.; Rodrigues, G. Abnormal fetal profile at first-trimester ultrasound scan complicated by severe polyhydramnios at the second half of pregnancy. J. Med. Ultrasound 2021, 29, 65. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Tanaka, H.; Furuhashi, F.H.; Tanaka, K.; Kondo, E.; Ikeda, T. Antenatal Indomethacin Treatment for Congenital Myotonic Dystrophy. Case Rep. Obs. Gynecol. 2019, 2019, 4290145. [Google Scholar] [CrossRef]
- Sakamoto, N.; Mitsuzuka, K.; Kanno, Y.; Hayashi, M.; Goto, Y.; Ueno, S.; Ishimoto, H. Atypical Presentation of Duodenal Atresia Concomitant with Type-A Esophageal Atresia in Fetal Life: A Case Report. Tokai J. Exp. Clin. Med. 2019, 44, 31–33. [Google Scholar]
- Wang, Y.; Zhu, C.; Du, L.; Li, Q.; Lin, M.-F.; Férec, C.; Cooper, D.N.; Chen, J.-M.; Zhou, Y. Compound Heterozygosity for Novel Truncating Variants in the LMOD3 Gene as the Cause of Polyhydramnios in Two Successive Fetuses. Front. Genet. 2019, 10, 835. [Google Scholar] [CrossRef]
- Gica, N.; Iliescu, D.G.; Mat, C.; Panaitescu, A.M.; Peltecu, G.; Veduta, A. Differential Diagnosis of Polyhydramnios in a Patient with Gestational Diabetes and Structurally Abnormal Fetus. Maedica 2019, 14, 301–304. [Google Scholar] [CrossRef] [PubMed]
- Ardabili, S.; Uerlings, V.; Kaelin Agten, A.; Hodel, M. Fetal congenital midaortic syndrome with unilateral renal artery stenosis prenatally presenting with polyhydramnios and postpartum as hyponatremic hypertensive syndrome. BMJ Case Rep. 2020, 13, e234459. [Google Scholar] [CrossRef] [PubMed]
- Willis, C.; Ferguson, S.; Soydemir, F. Placental chorioangioma associated with polyhydramnios and hydrops fetalis. BMJ Case Rep. 2019, 12, e227828. [Google Scholar] [CrossRef] [PubMed]
- Murata, T.; Fukuda, T.; Kanno, A.; Kyozuka, H.; Yamaguchi, A.; Shimizu, H.; Watanabe, T.; Fujimori, K. Polyhydramnios and abnormal foetal heart rate patterns in a foetus with Prader-Willi syndrome: A case report. Case Rep. Women’s Health 2020, 27, e00227. [Google Scholar] [CrossRef]
- Nabeshima, T.; Fujii, T.; Nagamatsu, T.; Hashimoto, A.; Seyama, T.; Kubota, K.; Sayama, S.; Nakayama, T.; Kumasawa, K.; Iriyama, T.; et al. Polyhydramnios is associated with postnatal dysphagia determining short-term prognosis of the newborn with 22q11.2 deletion syndrome-A case series analysis. Taiwan. J. Obstet. Gynecol. 2020, 59, 744–747. [Google Scholar] [CrossRef]
- Coll, C.M.; Bach, J.S.; Renau, M.I.; Allen, A.A.; Monk, D.; del Rincón, O.G.; Recasens, M.M.; Crespo, J.M.M. Prenatal diagnosis of Kagami-Ogata syndrome. J. Clin. Ultrasound 2021, 49, 498–501. [Google Scholar] [CrossRef]
- Mata, R.P.; Alves, T.; Figueiredo, A.; Santos, A. Prenatal diagnosis of congenital mesoblastic nephroma: A case with poor prognosis. BMJ Case Rep. 2019, 12, e230297. [Google Scholar] [CrossRef]
- Che, M.; Yang, F.; Huang, H.; Zhang, H.; Han, C.; Sun, N. Prenatal diagnosis of fetal congenital mesoblastic nephroma by ultrasonography combined with MR imaging: A case report and literature review. Medicine 2021, 100, e24034. [Google Scholar] [CrossRef]
- Takemori, S.; Tanigaki, S.; Nozu, K.; Yoshihashi, H.; Uchiumi, Y.; Sakaguchi, K.; Tsushima, K.; Kitamura, A.; Kobayashi, C.; Matsuhima, M.; et al. Prenatal diagnosis of MAGED2 gene mutation causing transient antenatal Bartter syndrome. Eur. J. Med. Genet. 2021, 64, 104308. [Google Scholar] [CrossRef]
- Lai, S.-T.; Chen, C.-P.; Lin, C.-J.; Chen, S.-W.; Town, D.-D.; Wang, W. Prenatal diagnosis of persistent left superior vena cava, polyhydramnios and a small gastric bubble in a fetus with VACTERL association. Taiwan. J. Obstet. Gynecol. 2021, 60, 355–358. [Google Scholar] [CrossRef]
- Katsura, D.; Moritani, S.; Tsuji, S.; Hayashi, K.; Yamada, K.; Tokoro, S.; Suzuki, K.; Kimura, F.; Murakami, T. Prenatal Diagnosis of Umbilical Cord Ulcer: A Report of Two Cases. Case Rep. Obstet. Gynecol. 2019, 2019, 3768761. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, H.; Sun, Y.; Fan, L.; Zhang, X. Prenatal drainage of fetal urinoma with polyhydramnios: A case report and literature review. J. Matern.-Fetal Neonatal Med. 2018, 31, 264–266. [Google Scholar] [CrossRef] [PubMed]
- Kuan Arguello, M.E.; Martín-Crespo, R.M.; Ramírez Velandia, H.J.; Carrera Guermeur, N.; López Menau, M.C.; Maruszeswki, P.; Pantoja Bajo, A.; Moreno de Prado, J.C.; Mialdea, R.L. Pyloric atresia and Down’s syndrome: Prenatal double bubble false sign. Cir. Pediatr. 2021, 34, 211–214. [Google Scholar] [PubMed]
- Baró, A.M.; Perez, S.P.; Costa, M.M.; Heredia, C.L.; Azuara, L.S.; Juanos, J.L.; Lapiedra, M.Z. Sacrococcygeal teratoma with preterm delivery: A case report. J. Med. Case Rep. 2020, 14, 72. [Google Scholar] [CrossRef] [PubMed]
- Perri, A.; Patti, M.L.; Sbordone, A.; Vento, G.; Luciano, R. Unexpected tracheal agenesis with prenatal diagnosis of aortic coarctation, lung hyperecogenicity and polyhydramnios: A case report. Ital. J. Pediatr. 2020, 46, 96. [Google Scholar] [CrossRef]
- Nunes, S.; Xavier, M.; Lourenço, C.; Melo, M.; Godinho, C. Schaaf-Yang Syndrome: A Real Challenge for Prenatal Diagnosis. Cureus 2021, 13, e20414. [Google Scholar] [CrossRef]
- Traisrisilp, K.; Sirikunalai, P.; Sirilert, S.; Chareonsirisuthigul, T.; Tongsong, T. Cardiac rhabdomyoma as a possible new prenatal sonographic feature of Prader–Willi syndrome. J. Obstet. Gynaecol. Res. 2022, 48, 239–243. [Google Scholar] [CrossRef]
- Wu, X.; Huang, L.; Luo, C.; Liu, Y.; Niu, J. A Case Report and Literature Review of a Novel Mutation in the MAGED2 Gene of a Patient with Severe Transient Polyhydramnios. Front. Pediatr. 2021, 9, 778814. [Google Scholar] [CrossRef]
- Biggio, J. Hydramnios prediction of adverse perinatal outcome. Obstet. Gynecol. 1999, 94, 773–777. [Google Scholar] [CrossRef]
- Peng, W.K.; Chen, L.; Boehm, B.O.; Han, J.; Loh, T.P. Molecular phenotyping of oxidative stress in diabetes mellitus with point-of-care NMR system. Npj Aging Mech. Dis. 2020, 6, 11. [Google Scholar] [CrossRef]
- Peng, W.K.; Ng, T.-T.; Loh, T.P. Machine learning assistive rapid, label-free molecular phenotyping of blood with two-dimensional NMR correlational spectroscopy. Commun. Biol. 2020, 3, 535. [Google Scholar] [CrossRef] [PubMed]
- Howlader, K.C.; Satu, S.; Awal, A.; Islam, R.; Islam, S.M.S.; Quinn, J.M.W.; Moni, M.A. Machine learning models for classification and identification of significant attributes to detect type 2 diabetes. Health Inf. Sci. Syst. 2022, 10, 2. [Google Scholar] [CrossRef] [PubMed]

| First Author | Number of Patients | Parity | Age (Years) | Polyhydramnios (Max Value) | GA AT Discovery (W) | Cause | GA AT Delivery (W) | Type of Delivery | Birth Weight (G) | APGAR Score (1 min, 5 min) | Maternal Complications | Neonatal Outcome |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Ouyang et al. [14], 2018 | 4 | N/A | N/A | DVP = 8.1 cm | 28 | central nervous system abnormalities | N/A | N/A | N/A | N/A | none | Intermittent convulsion. |
| N/A | N/A | DVP = 13.2 cm | 25 | central nervous system abnormalities | N/A | N/A | N/A | N/A | none | Swallowing difficulty; intermittent convulsion, death at 2 months. | ||
| N/A | N/A | DVP = 12.7 cm | 32 | central nervous system abnormalities | N/A | N/A | N/A | N/A | none | Swallowing difficulty; death at 22 days. | ||
| N/A | N/A | DVP = 13.7 cm | 32 | central nervous system abnormalities | N/A | N/A | N/A | N/A | none | Breathing difficulty; death at birth. | ||
| Desai et al. [15], 2020 | 1 | 1 | 26 | DVP = 11.5 cm | 21 | Chorioangioma | 32 | Emergency C-section | 1560 | 6, 8 | none | Respiratory distress with NICU admission for 14 days. |
| Iwahata et al. [16], 2021 | 1 | 1 | 39 | AFI = 26 cm | 26 | old placental chorioangioma | 38 | Vaginal delivery | 2886 | 9, 10 | none | Favorable. |
| Singh et al. [17], 2021 | 1 | 1 | 25 | present | 24 | Chorioangioma | 30 | Emergency C-section | 1200 | 7, 9 | none | Favorable. |
| Sakaria et al. [18], 2021 | 2 | 1 | 21 | AFI = 35 cm | 22 | Kagami-Ogata Syndrome | 28 | Emergency C-Section | N/A | 2, 4 | none | Anasarca; micrognathia; large omphalocele containing part of the liver and the bladder; severe respiratory acidosis; life-sustaining measures were discontinued at 2.5 months of life. |
| N/A | N/A | N/A | N/A | Kagami-Ogata Syndrome | 37 | Emergency C-Section | 3000 | 3, 6 | none | Flattened nasal bridge; short limbs; cryptorchidism; hypotonia; respiratory distress; coat-hanger appearance of the ribs. | ||
| Thakur et al. [19], 2020 | 3 | 1 | 23 | AFI = 34 cm | 28 | Bartter syndrome | 36 | Vaginal delivery | 2060 | stillborn | none | Stillborn neonate. |
| Altmann et al. [20], 2020 | 1 | 1 | 30 | AFI = 31 cm | 20 | Kagami-Ogata Syndrome | 35 | Elective C-Section | 3660 | 6, 7 | none | Abnormal shape of the thorax; facial dysmorphism; need for ventilation; generalized muscular hypotonia. |
| Huang et al. [21], 2019 | 1 | 1 | 38 | present | 19 | Kagami-Ogata Syndrome | 35 | Emergency C-Section | 3188 | 3, 6 | none | Generalized hypotonia; a flat nasal bridge; respiratory distress; omphalocele; abnormal swallowing function; morphological abnormality of the ribs; facial dysmorphism. |
| Choi et al. [22], 2019 | 5 | N/A | N/A | present | N/A | Costello Syndrome | 34 | Emergency C-Section | 2560 | 4, 6 | none | Atrial tachycardia; feeding problems; growth retardation; cardiac structural anomalies; respiratory distress. |
| 3 | N/A | present | N/A | Costello Syndrome | 37 | Emergency C-Section | 4420 | 1, 4 | none | Atrial tachycardia; feeding problems; cardiac structural anomalies; respiratory distress; growth retardation. | ||
| 1 | N/A | present | N/A | Costello Syndrome | 36 | Emergency C-Section | 3700 | 7, 10 | none | Atrial tachycardia; feeding problems; cardiac structural anomalies; respiratory distress; growth retardation. | ||
| 2 | N/A | N/A | N/A | Costello Syndrome | 37 | Emergency C-Section | 4700 | N/A | none | Atrial tachycardia; feeding problems; growth retardation; cardiac structural anomalies; respiratory distress; growth retardation. | ||
| N/A | N/A | present | N/A | Costello Syndrome | 31 | Emergency C-Section | 2290 | N/A | none | Atrial tachycardia; feeding problems; growth retardation; cardiac structural anomalies; respiratory distress; growth retardation. | ||
| Nam et al. [23], 2021 | 1 | 1 | 33 | AFI = 45 cm | 27 | Antenatal Bartter syndrome | 36 | Elective C-Section | 2210 | 9, 10 | Aggravating dyspnea; abdominal distension | Hyponatremia; hypokalemia; elevation of plasma renin and aldosterone; 3 months follow-up–good clinical condition. |
| Meyer et al. [24], 2018 | 1 | 2 | 36 | AFI = 49 cm | 21 | Bartter syndrome | 29 | Vaginal delivery | N/A | N/A | none | Severe polyuria; elevated serum aldosterone and renin activity levels; hydronephrosis. |
| Kim et al. [25], 2019 | 1 | 2 | 37 | N/A | 27 | Infantile cortical hyperostosis | 27 | Emergency C-section | 1970 | N/A | none | Severe prematurity; persistent pulmonary hypertension; liver failure; cortical thickening. uncontrolled sepsis on day 38 with death. |
| Arthuis et al. [26], 2019 | 1 | 1 | 23 | AFI = 64 cm | 19 | Antenatal Bartter syndrome | 36 | Vaginal delivery | 3575 | N/A | none | Right aortic arch; retro-esophageal left; subclavian artery; moderate pulmonary stenosis; 12 months follow-up–good clinical condition. |
| Carvalho et al. [27], 2020 | 1 | 3 | 37 | AFI = 35 cm | 3 | Isolated Pierre Robin sequence | 37 | Emergency C-section | 2760 | 5, 7 | none | Micrognathia; posterior cleft palate; glossoptosis. |
| Yamaguchi et al. [28], 2019 | 1 | 1 | 31 | AFI = 47 cm | 24 | Myotonic dystrophy | 38 | Elective C-Section | 2838 | 5, 6 | myotonic dystrophy | Distal dominant hypotonia; weakness of breathing; swallowing dysfunction. |
| Sakamoto et al. [29], 2019 | 1 | 1 | 33 | AFI = 28 cm | 28 | duodenal and esophageal atresia without tracheo-esophageal fistula | 36 | Emergency C-section | 2860 | 8, 10 | none | Duodenal and esophageal atresia without tracheo-esophageal fistula; good clinical condition after surgical treatment. |
| Wang et al. [30], 2019 | 1 | 3 | 35 | AFI = 30.7 cm | 29 | 24 LMOD3 mutation-positive case of nemaline myopathy | 37 | Vaginal delivery | N/A | 5, 6 | none | Stiffness of limbs, little movement; died of respiratory failure 2 days after birth. |
| Gica et al. [31], 2019 | 1 | N/A | 29 | AFI = 36 cm | 25 | esophageal atresia | 35 | N/A | 2400 | N/A | GDM | Cardiomegaly; atypical esophageal atresia. |
| Ardabil et al. [32], 2020 | 1 | 1 | 34 | AFI = 75 cm | 33 | the midaortic syndrome | 33 | Emergency C-section | 2140 | N/A | none | Respiratory distress; biventricular myocardial hypertrophy; renal artery stenosis on the left. |
| Willis et al. [33], 2019 | 1 | 3 | 27 | present | 30 | Chorioangioma | 33 | Emergency C-section | 2850 | 6, 9 | GDM | Fetal anemia; non-immune hydrops fetalis. |
| Murata et al. [34], 2020 | 1 | 1 | 29 | AFI = 31 cm | 27 | Prader-Willi Syndrome | 38 | Elective C-Section | 2492 | 6, 6 | none | Severe hypotonia; cryptorchidism. |
| Nabeshima et al. [35], 2020 | 1 | 2 | 30 | AFI = 41 cm | 35 | 22qDS syndrome | 38 | Emergency C-section | 2377 | 9, 10 | none | Small ventricular septal defect; right aortic arch; severe dysphagia. |
| Molinet COLL et al. [36], 2020 | 1 | 3 | 35 | AFI = 45 cm | 30 | Kagami-Ogata Syndrome | 36 | Emergency C-Section | 2050 | N/A, 8 | none | Respiratory distress; died at 41 days of life. |
| Mata et al. [37], 2019 | 1 | 2 | 36 | AFI = 35 cm | 33 | congenital mesoblastic nephroma | 35 | Emergency C-Section | 2150 | 8, 9 | none | Congenital mesoblastic nephroma. |
| Che et al. [38], 2021 | 1 | 1 | 29 | AFI = 25.3 cm | 31 | congenital mesoblastic nephroma | 38 | Vaginal delivery | 3250 | 5, N/A | none | Congenital mesoblastic nephroma. |
| Takemori et al. [39], 2021 | 1 | 2 | 35 | AFI = 30 cm | 29 | Bartter syndrome | 32 | Emergency C-Section | 2125 | 6, 7 | none | Serum electrolyte imbalance; polyuria; retinopathy of prematurity. |
| Lai et al. [40], 2021 | 1 | 1 | 34 | present | 30 | VACTERL syndrome | 36 | Emergency C-section | 1832 | N/A | none | Esophageal atresia with distal tracheoesophageal fistula; death on day 4 postpartum. |
| Katsura et al. [41], 2019 | 2 | N/A | 31 | AFI = 30 cm | 31 | fetal duodenal atresia | 36 | Emergency C-section | 2282 | 7, 9 | none | Fetal duodenal atresia. |
| N/A | 38 | AFI = 31.5 cm | 30 | fetal duodenal atresia | 34 | Emergency C-section | 2086 | 1, 3 | none | Fetal duodenal atresia. | ||
| Zhang et al. [42], 2018 | 2 | 1 | 32 | AFI = 30.2 cm | 28 | fetal urinoma | 41 | Vaginal delivery | 3820 | 7, 9 | none | Right renal dysplasia; hydronephrosis; pyelo-ureteric junction obstruction. |
| 2 | 33 | present | 31 | fetal urinoma | 37 | Vaginal delivery | 3870 | N/A | none | Persistent fetal urinoma. | ||
| Arguello et al. [43], 2021 | 1 | N/A | N/A | AFI = 36 cm | 30 | fetal pyloric atresia | 33 | Emergency C-section | 1925 | N/A | none | Down syndrome; respiratory distress; fetal type C pyloric atresia. |
| 1 | N/A | AFI = 26.2 cm | 25 | Bartter syndrome | 32 | Vaginal delivery | 1760 | N/A | none | Developmental delay. | ||
| 1 | 25 | AFI = 34 cm | 25 | Bartter syndrome | 30 | Vaginal delivery | 1700 | N/A | none | Neonatal death on day 6. | ||
| Baro et al. [44], 2020 | 1 | 2 | 26 | AFI = 37 cm | N/A | GDM | 35 | Emergency C-section | 4030 | 9, 10 | GDM | Sacrococcygeal teratoma. |
| Perri et al. [45], 2020 | 1 | N/A | N/A | present | 35 | Congenital defects | 35 | Emergency C-section | 2280 | 2, 5 | none | Aortic coarctation; tracheal agenesis; death 150 min postpartum. |
| Nunes et al. [46], 2021 | 1 | 1 | 37 | AFI = 25 cm | 28 | Schaaf-Yang Syndrome | 40 | Emergency C-section | 3000 | 8, 8 | none | Clubfoot; bilateral clindactyly; global hypotonia; distal arthrogryposis. |
| Traisrisilp et al. [47], 2021 | 1 | 2 | 40 | AFI = 30 cm | 28 | Prader-Willi Syndrome | 38 | Emergency C-section | 2420 | 8, 6 | none | Hypotonia; mild chest retraction; difficult feeding. |
| Wu et al. [48], 2021 | 1 | 1 | 27 | AFI = 35 cm | 21 | Bartter syndrome | 35 | Vaginal delivery | 2800 | 10, 10 | none | Favorable. |
| Age (years old) | 31.58 ± 5.04 |
| Gestational age at discovery (weeks) | 27.47 ± 4.23 |
| Gestational age at delivery (weeks) | 34.93 ± 3.06 |
| Birth weight (g) | 2676.9 ± 797.23 |
| Apgar score 1 min | 5.96 ± 2.53 |
| Apgar score 5 min | 7.5 ± 2.09 |
| AFI (cm) | 36.47 ± 11.02 |
| DVP (cm) | 12.03 ± 2.06 |
| Parity > 1 | 40% |
| Emergency C-Section | 64.28% |
| Causes of Polyhydramnios | Number of Studies | Expressed as Percentage |
|---|---|---|
| Placental tumors | 4 | 11.42% |
| Gestational diabetes mellitus | 1 | 2.85% |
| Central nervous system abnormalities | 1 | 2.85% |
| Musculoskeletal Disorders (MSDs) | 3 | 8.57% |
| Congenital abnormalities of the kidneys and the urinary tract | 9 | 25.71% |
| Congenital anomalies | ||
| Gastrointestinal tract | 4 | 11.42% |
| Others | 4 | 11.42% |
| Genetic Disorders | ||
| Kagami-Ogata syndrome | 4 | 11.42% |
| Costello syndrome | 1 | 2.85% |
| Prader-Willi syndrome | 2 | 5.71% |
| Others | 2 | 5.71% |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Preda, A.; Ștefan, A.G.; Preda, S.D.; Comănescu, A.C.; Forțofoiu, M.-C.; Vladu, M.I.; Forțofoiu, M.; Moța, M. Transient Polyhydramnios during Pregnancy Complicated with Gestational Diabetes Mellitus: Case Report and Systematic Review. Diagnostics 2022, 12, 1340. https://doi.org/10.3390/diagnostics12061340
Preda A, Ștefan AG, Preda SD, Comănescu AC, Forțofoiu M-C, Vladu MI, Forțofoiu M, Moța M. Transient Polyhydramnios during Pregnancy Complicated with Gestational Diabetes Mellitus: Case Report and Systematic Review. Diagnostics. 2022; 12(6):1340. https://doi.org/10.3390/diagnostics12061340
Chicago/Turabian StylePreda, Agnesa, Adela Gabriela Ștefan, Silviu Daniel Preda, Alexandru Cristian Comănescu, Mircea-Cătălin Forțofoiu, Mihaela Ionela Vladu, Maria Forțofoiu, and Maria Moța. 2022. "Transient Polyhydramnios during Pregnancy Complicated with Gestational Diabetes Mellitus: Case Report and Systematic Review" Diagnostics 12, no. 6: 1340. https://doi.org/10.3390/diagnostics12061340
APA StylePreda, A., Ștefan, A. G., Preda, S. D., Comănescu, A. C., Forțofoiu, M.-C., Vladu, M. I., Forțofoiu, M., & Moța, M. (2022). Transient Polyhydramnios during Pregnancy Complicated with Gestational Diabetes Mellitus: Case Report and Systematic Review. Diagnostics, 12(6), 1340. https://doi.org/10.3390/diagnostics12061340









