Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Data Sources and Literature Searches
2.2. Study Selection
2.3. Data Extraction and Quality Assessment
2.4. Data Analysis
3. Results
3.1. Study Selection
3.2. Quality Assessment
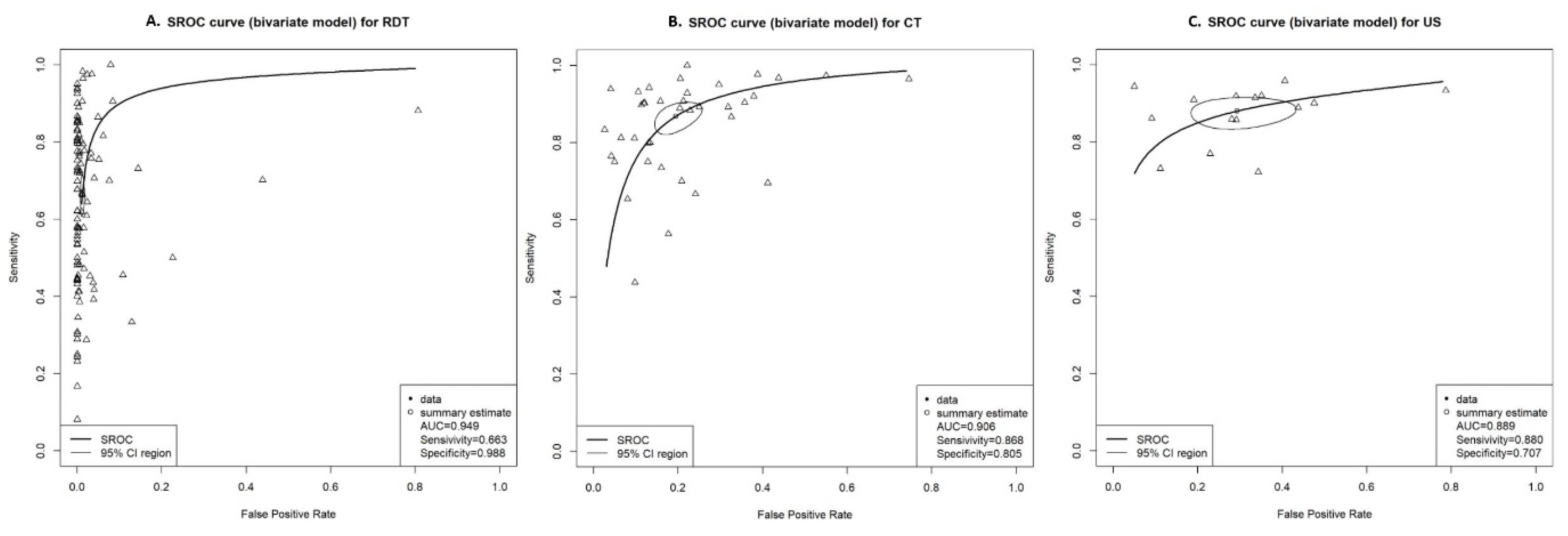
3.3. Pairwise Meta-Analysis for DTA
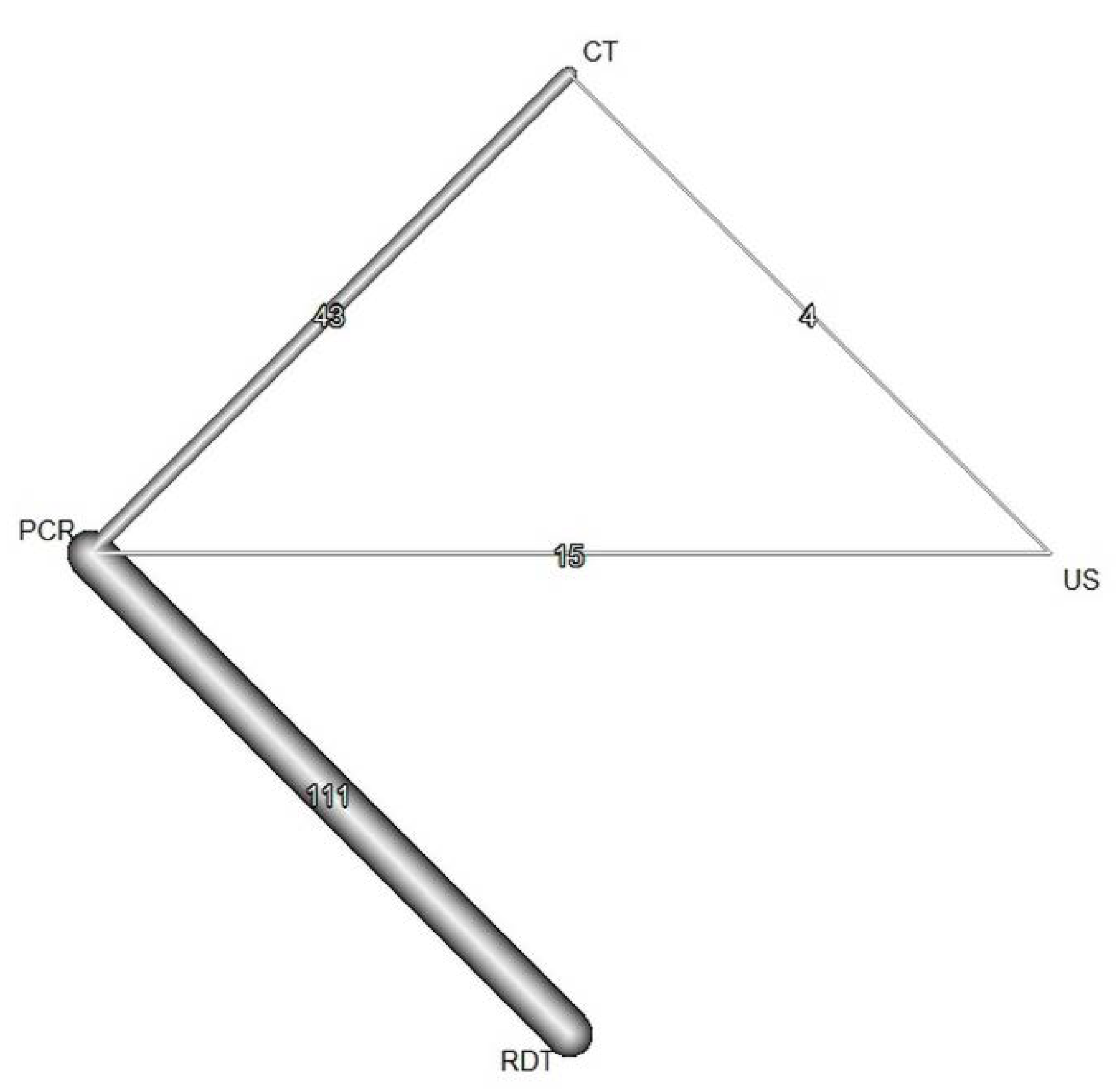
3.4. Network Meta-Analysis
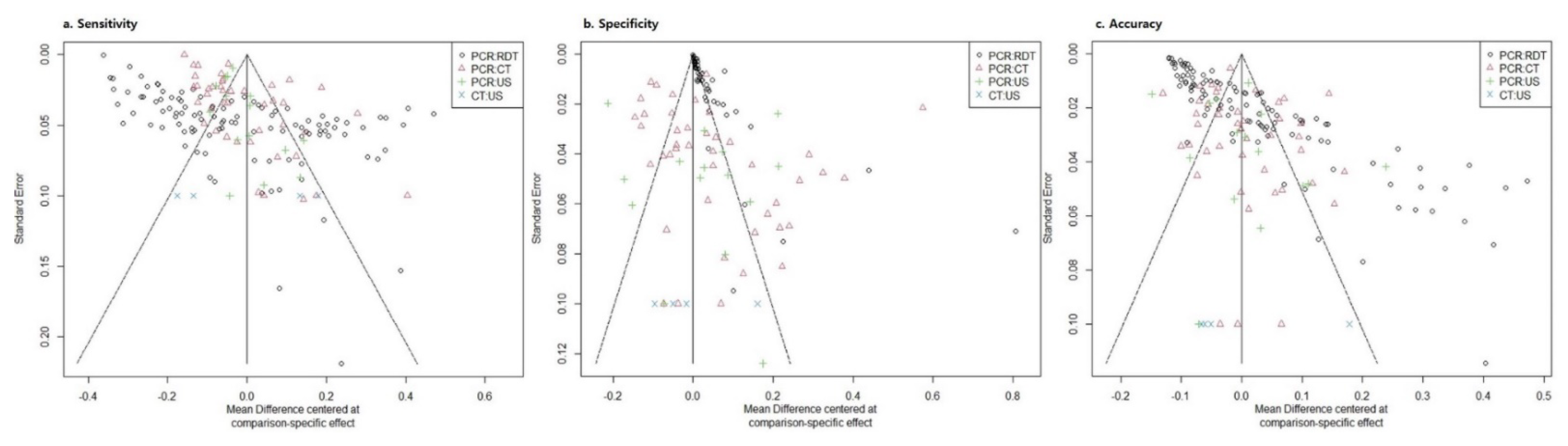
3.5. Publication Bias
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- World Health Organization. Weekly Epidemiological Update on COVID-19. Available online: https://www.who.int/publications/m/item/weeklyoperational-update-on-covid-19 (accessed on 6 January 2022).
- Wu, Y.; Ho, W.; Huang, Y.; Jin, D.Y.; Li, S.; Liu, S.L.; Liu, X.; Qiu, J.; Sang, Y.; Wang, Q.; et al. SARS-CoV-2 is an appropriate name for the new coronavirus. Lancet 2020, 395, 949–950. [Google Scholar] [CrossRef]
- Tahamtan, A.; Ardebili, A. Real-time RT-PCR in COVID-19 detection: Issues affecting the results. Expert Rev. Mol. Diagn. 2020, 20, 453–454. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lieberman, J.A.; Pepper, G.; Naccache, S.N.; Huang, M.L.; Jerome, K.R.; Greninger, A.L. Comparison of Commercially Available and Laboratory-Developed Assays for In Vitro Detection of SARS-CoV-2 in Clinical Laboratories. J. Clin. Microbiol. 2020, 58, e00821-20. [Google Scholar] [CrossRef] [PubMed]
- Watson, J.; Whiting, P.F.; Brush, J.E. Interpreting a covid-19 test result. BMJ 2020, 369, m1808. [Google Scholar] [CrossRef] [PubMed]
- Carter, L.J.; Garner, L.V.; Smoot, J.W.; Li, Y.; Zhou, Q.; Saveson, C.J.; Sasso, J.M.; Gregg, A.C.; Soares, D.J.; Beskid, T.R.; et al. Assay Techniques and Test Development for COVID-19 Diagnosis. ACS Cent. Sci. 2020, 6, 591–605. [Google Scholar] [CrossRef]
- Chen, Z.; Li, Y.; Wu, B.; Hou, Y.; Bao, J.; Deng, X. A Patient with COVID-19 Presenting a False-Negative Reverse Transcriptase Polymerase Chain Reaction Result. Korean J. Radiol. 2020, 21, 623–624. [Google Scholar] [CrossRef] [Green Version]
- Sethuraman, N.; Jeremiah, S.S.; Ryo, A. Interpreting Diagnostic Tests for SARS-CoV-2. JAMA 2020, 323, 2249–2251. [Google Scholar] [CrossRef]
- Winichakoon, P.; Chaiwarith, R.; Liwsrisakun, C.; Salee, P.; Goonna, A.; Limsukon, A.; Kaewpoowat, Q. Negative Nasopharyngeal and Oropharyngeal Swabs Do Not Rule Out COVID-19. J. Clin. Microbiol. 2020, 58, e00297-20. [Google Scholar] [CrossRef] [Green Version]
- Mina, M.J.; Parker, R.; Larremore, D.B. Rethinking COVID-19 Test Sensitivity—A Strategy for Containment. N. Engl. J. Med. 2020, 383, e120. [Google Scholar] [CrossRef]
- World Health Organization. Antigen-Detection in the Diagnosis of SARS-CoV-2 Infection Using Rapid Immunoassays: Interim Guidance; World Health Organization: Geneva, Switzerland, 2020. [Google Scholar]
- Lee, J.; Song, J.U.; Shim, S.R. Comparing the diagnostic accuracy of rapid antigen detection tests to real time polymerase chain reaction in the diagnosis of SARS-CoV-2 infection: A systematic review and meta-analysis. J. Clin. Virol. 2021, 144, 104985. [Google Scholar] [CrossRef]
- Ai, T.; Yang, Z.; Hou, H.; Zhan, C.; Chen, C.; Lv, W.; Tao, Q.; Sun, Z.; Xia, L. Correlation of Chest CT and RT-PCR Testing for Coronavirus Disease 2019 (COVID-19) in China: A Report of 1014 Cases. Radiology 2020, 296, e32–e40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chinese National Health Commission. Chinese Clinical Guidance for COVID-19 Pneumonia Diagnosis and Treatment, 7th ed.; Chinese National Health Commission: Beijing, China, 2020.
- Akl, E.A.; Blažić, I.; Yaacoub, S.; Frija, G.; Chou, R.; Appiah, J.A.; Fatehi, M.; Flor, N.; Hitti, E.; Jafri, H.; et al. Use of Chest Imaging in the Diagnosis and Management of COVID-19: A WHO Rapid Advice Guide. Radiology 2021, 298, e63–e69. [Google Scholar] [CrossRef] [PubMed]
- Peng, Q.Y.; Wang, X.T.; Zhang, L.N. Findings of lung ultrasonography of novel corona virus pneumonia during the 2019-2020 epidemic. Intensive Care Med. 2020, 46, 849–850. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poggiali, E.; Dacrema, A.; Bastoni, D.; Tinelli, V.; Demichele, E.; Mateo Ramos, P.; Marcianò, T.; Silva, M.; Vercelli, A.; Magnacavallo, A. Can Lung US Help Critical Care Clinicians in the Early Diagnosis of Novel Coronavirus (COVID-19) Pneumonia? Radiology 2020, 295, e6. [Google Scholar] [CrossRef] [PubMed]
- Shim, S.R.; Kim, S.J.; Lee, J. Diagnostic test accuracy: Application and practice using R software. Epidemiol. Health 2019, 41, e2019007. [Google Scholar] [CrossRef]
- Leeflang, M.M.; Deeks, J.J.; Gatsonis, C.; Bossuyt, P.M. Systematic reviews of diagnostic test accuracy. Ann. Intern. Med. 2008, 149, 889–897. [Google Scholar] [CrossRef]
- Hutton, B.; Salanti, G.; Caldwell, D.M.; Chaimani, A.; Schmid, C.H.; Cameron, C.; Ioannidis, J.P.; Straus, S.; Thorlund, K.; Jansen, J.P.; et al. The PRISMA extension statement for reporting of systematic reviews incorporating network meta-analyses of health care interventions: Checklist and explanations. Ann. Intern. Med. 2015, 162, 777–784. [Google Scholar] [CrossRef] [Green Version]
- Whiting, P.F.; Rutjes, A.W.; Westwood, M.E.; Mallett, S.; Deeks, J.J.; Reitsma, J.B.; Leeflang, M.M.; Sterne, J.A.; Bossuyt, P.M. QUADAS-2: A revised tool for the quality assessment of diagnostic accuracy studies. Ann. Intern. Med. 2011, 155, 529–536. [Google Scholar] [CrossRef]
- Shim, S.R.; Kim, S.J.; Lee, J.; Rücker, G. Network meta-analysis: Application and practice using R software. Epidemiol. Health 2019, 41, e2019013. [Google Scholar] [CrossRef] [Green Version]
- Lu, G.; Ades, A.E. Combination of direct and indirect evidence in mixed treatment comparisons. Stat. Med. 2004, 23, 3105–3124. [Google Scholar] [CrossRef]
- Salanti, G.; Ades, A.E.; Ioannidis, J.P. Graphical methods and numerical summaries for presenting results from multiple-treatment meta-analysis: An overview and tutorial. J. Clin. Epidemiol. 2011, 64, 163–171. [Google Scholar] [CrossRef] [PubMed]
- Rücker, G.; Schwarzer, G. Ranking treatments in frequentist network meta-analysis works without resampling methods. BMC Med. Res. Methodol. 2015, 15, 58. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, Y.; Yang, H. The transmission and diagnosis of 2019 novel coronavirus infection disease (COVID-19): A Chinese perspective. J. Med. Virol. 2020, 92, 639–644. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, X.; Liu, B.; Yu, Y.; Wang, X.; Du, Y.; Gu, J.; Wu, X. The characteristics and clinical value of chest CT images of novel coronavirus pneumonia. Clin. Radiol. 2020, 75, 335–340. [Google Scholar] [CrossRef]
- Toptan, T.; Eckermann, L.; Pfeiffer, A.E.; Hoehl, S.; Ciesek, S.; Drosten, C.; Corman, V.M. Evaluation of a SARS-CoV-2 rapid antigen test: Potential to help reduce community spread? J. Clin. Virol. 2021, 135, 104713. [Google Scholar] [CrossRef] [PubMed]
- Brümmer, L.E.; Katzenschlager, S.; Gaeddert, M.; Erdmann, C.; Schmitz, S.; Bota, M.; Grilli, M.; Larmann, J.; Weigand, M.A.; Pollock, N.R.; et al. Accuracy of novel antigen rapid diagnostics for SARS-CoV-2: A living systematic review and meta-analysis. PLoS Med. 2021, 18, e1003735. [Google Scholar] [CrossRef]
- Chen, C.C.; Lu, S.C.; Bai, C.H.; Wang, P.Y.; Lee, K.Y.; Wang, Y.H. Diagnostic Accuracy of SARS-CoV-2 Antigen Tests for Community Transmission Screening: A Systematic Review and Meta-Analysis. Int. J. Environ. Res. Public Health 2021, 18, 11451. [Google Scholar] [CrossRef]
- Dinnes, J.; Deeks, J.J.; Berhane, S.; Taylor, M.; Adriano, A.; Davenport, C.; Dittrich, S.; Emperador, D.; Takwoingi, Y.; Cunningham, J.; et al. Rapid, point-of-care antigen and molecular-based tests for diagnosis of SARS-CoV-2 infection. Cochrane Database Syst. Rev. 2021, 3, Cd013705. [Google Scholar] [CrossRef]
- General Office of National Health Committee. Notice on the Issuance of a Program for the Diagnosis and Treatment of Novel Coronavirus (2019-nCoV) Infected Pneumonia (Trial Sixth Edition). Available online: http://www.nhc.gov.cn/yzygj/s7653p/202002/8334a8326dd94d329df351d7da8aefc2.shtml (accessed on 6 January 2022).
- De Smet, K.; De Smet, D.; Ryckaert, T.; Laridon, E.; Heremans, B.; Vandenbulcke, R.; Demedts, I.; Bouckaert, B.; Gryspeerdt, S.; Martens, G.A. Diagnostic Performance of Chest CT for SARS-CoV-2 Infection in Individuals with or without COVID-19 Symptoms. Radiology 2021, 298, e30–e37. [Google Scholar] [CrossRef]
- Mair, M.D.; Hussain, M.; Siddiqui, S.; Das, S.; Baker, A.; Conboy, P.; Valsamakis, T.; Uddin, J.; Rea, P. A systematic review and meta-analysis comparing the diagnostic accuracy of initial RT-PCR and CT scan in suspected COVID-19 patients. Br. J. Radiol. 2021, 94, 20201039. [Google Scholar] [CrossRef]
- Xu, B.; Xing, Y.; Peng, J.; Zheng, Z.; Tang, W.; Sun, Y.; Xu, C.; Peng, F. Chest CT for detecting COVID-19: A systematic review and meta-analysis of diagnostic accuracy. Eur. Radiol. 2020, 30, 5720–5727. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.P.; Tay, E.T.; Elikashvili, I.; Sanders, J.E.; Paul, A.Z.; Nelson, B.P.; Spina, L.A.; Tsung, J.W. Feasibility and Safety of Substituting Lung Ultrasonography for Chest Radiography When Diagnosing Pneumonia in Children: A Randomized Controlled Trial. Chest 2016, 150, 131–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zieleskiewicz, L.; Cornesse, A.; Hammad, E.; Haddam, M.; Brun, C.; Vigne, C.; Meyssignac, B.; Remacle, A.; Chaumoitre, K.; Antonini, F.; et al. Implementation of lung ultrasound in polyvalent intensive care unit: Impact on irradiation and medical cost. Anaesth. Crit. Care Pain Med. 2015, 34, 41–44. [Google Scholar] [CrossRef] [PubMed]
- Zieleskiewicz, L.; Markarian, T.; Lopez, A.; Taguet, C.; Mohammedi, N.; Boucekine, M.; Baumstarck, K.; Besch, G.; Mathon, G.; Duclos, G.; et al. Comparative study of lung ultrasound and chest computed tomography scan in the assessment of severity of confirmed COVID-19 pneumonia. Intensive Care Med. 2020, 46, 1707–1713. [Google Scholar] [CrossRef]
- Brenner, D.S.; Liu, G.Y.; Omron, R.; Tang, O.; Garibaldi, B.T.; Fong, T.C. Diagnostic accuracy of lung ultrasound for SARS-CoV-2: A retrospective cohort study. Ultrasound J. 2021, 13, 12. [Google Scholar] [CrossRef]
- Islam, N.; Ebrahimzadeh, S.; Salameh, J.P.; Kazi, S.; Fabiano, N.; Treanor, L.; Absi, M.; Hallgrimson, Z.; Leeflang, M.M.; Hooft, L.; et al. Thoracic imaging tests for the diagnosis of COVID-19. Cochrane Database Syst. Rev. 2021, 3, Cd013639. [Google Scholar] [CrossRef]
- Butler-Laporte, G.; Lawandi, A.; Schiller, I.; Yao, M.; Dendukuri, N.; McDonald, E.G.; Lee, T.C. Comparison of Saliva and Nasopharyngeal Swab Nucleic Acid Amplification Testing for Detection of SARS-CoV-2: A Systematic Review and Meta-analysis. JAMA Intern. Med. 2021, 181, 353–360. [Google Scholar] [CrossRef]
- Tsang, N.N.Y.; So, H.C.; Ng, K.Y.; Cowling, B.J.; Leung, G.M.; Ip, D.K.M. Diagnostic performance of different sampling approaches for SARS-CoV-2 RT-PCR testing: A systematic review and meta-analysis. Lancet Infect. Dis. 2021, 21, 1233–1245. [Google Scholar] [CrossRef]
- Agulló, V.; Fernández-González, M.; Ortiz de la Tabla, V.; Gonzalo-Jiménez, N.; García, J.A.; Masiá, M.; Gutiérrez, F. Evaluation of the rapid antigen test Panbio COVID-19 in saliva and nasal swabs in a population-based point-of-care study. J. Infect. 2021, 82, 186–230. [Google Scholar] [CrossRef]
- Albert, E.; Torres, I.; Bueno, F.; Huntley, D.; Molla, E.; Fernández-Fuentes, M.; Martínez, M.; Poujois, S.; Forqué, L.; Valdivia, A.; et al. Field evaluation of a rapid antigen test (Panbio™ COVID-19 Ag Rapid Test Device) for COVID-19 diagnosis in primary healthcare centres. Clin. Microbiol. Infect. 2021, 27, 472.e410–472.e477. [Google Scholar] [CrossRef]
- Alemany, A.; Baró, B.; Ouchi, D.; Rodó, P.; Ubals, M.; Corbacho-Monné, M.; Vergara-Alert, J.; Rodon, J.; Segalés, J.; Esteban, C.; et al. Analytical and clinical performance of the panbio COVID-19 antigen-detecting rapid diagnostic test. J. Infect. 2021, 82, 186–230. [Google Scholar] [CrossRef] [PubMed]
- Asai, N.; Sakanashi, D.; Ohashi, W.; Nakamura, A.; Kawamoto, Y.; Miyazaki, N.; Ohno, T.; Yamada, A.; Chida, S.; Shibata, Y.; et al. Efficacy and validity of automated quantitative chemiluminescent enzyme immunoassay for SARS-CoV-2 antigen test from saliva specimen in the diagnosis of COVID-19. J. Infect. Chemother. 2021, 27, 1039–1042. [Google Scholar] [CrossRef] [PubMed]
- Aslan, S.; Bekçi, T.; Çakır İ, M.; Ekiz, M.; Yavuz, İ.; Şahin, A.M. Diagnostic performance of low-dose chest CT to detect COVID-19: A Turkish population study. Diagn. Interv. Radiol. 2021, 27, 181–187. [Google Scholar] [CrossRef] [PubMed]
- Barbosa, P.; Bitencourt, A.G.V.; de Miranda, G.D.; Almeida, M.F.A.; Chojniak, R. Chest CT accuracy in the diagnosis of SARS-CoV-2 infection: Initial experience in a cancer center. Radiol. Bras. 2020, 53, 211–215. [Google Scholar] [CrossRef]
- Baro, B.; Rodo, P.; Ouchi, D.; Bordoy, A.E.; Saya Amaro, E.N.; Salsench, S.V.; Molinos, S.; Alemany, A.; Ubals, M.; Corbacho-Monné, M.; et al. Performance characteristics of five antigen-detecting rapid diagnostic test (Ag-RDT) for SARS-CoV-2 asymptomatic infection: A head-to-head benchmark comparison. J. Infect. 2021, 82, 269–275. [Google Scholar] [CrossRef]
- Basso, D.; Aita, A.; Padoan, A.; Cosma, C.; Navaglia, F.; Moz, S.; Contran, N.; Zambon, C.F.; Maria Cattelan, A.; Plebani, M. Salivary SARS-CoV-2 antigen rapid detection: A prospective cohort study. Clin. Chim. Acta 2021, 517, 54–59. [Google Scholar] [CrossRef]
- Beck, E.T.; Paar, W.; Fojut, L.; Serwe, J.; Jahnke, R.R. Comparison of the Quidel Sofia SARS FIA Test to the Hologic Aptima SARS-CoV-2 TMA Test for Diagnosis of COVID-19 in Symptomatic Outpatients. J. Clin. Microbiol. 2021, 59, e02727-20. [Google Scholar] [CrossRef]
- Bellini, D.; Panvini, N.; Rengo, M.; Vicini, S.; Lichtner, M.; Tieghi, T.; Ippoliti, D.; Giulio, F.; Orlando, E.; Iozzino, M.; et al. Diagnostic accuracy and interobserver variability of CO-RADS in patients with suspected coronavirus disease-2019: A multireader validation study. Eur. Radiol. 2021, 31, 1932–1940. [Google Scholar] [CrossRef]
- Berger, A.; Nsoga, M.T.N.; Perez-Rodriguez, F.J.; Aad, Y.A.; Sattonnet-Roche, P.; Gayet-Ageron, A.; Jaksic, C.; Torriani, G.; Boehm, E.; Kronig, I.; et al. Diagnostic accuracy of two commercial SARS-CoV-2 antigen-detecting rapid tests at the point of care in community-based testing centers. PLoS ONE 2021, 16, e0248921. [Google Scholar] [CrossRef]
- Besutti, G.; Giorgi Rossi, P.; Iotti, V.; Spaggiari, L.; Bonacini, R.; Nitrosi, A.; Ottone, M.; Bonelli, E.; Fasano, T.; Canovi, S.; et al. Accuracy of CT in a cohort of symptomatic patients with suspected COVID-19 pneumonia during the outbreak peak in Italy. Eur. Radiol. 2020, 30, 6818–6827. [Google Scholar] [CrossRef]
- Bianchi, S.; Savinelli, C.; Paolucci, E.; Pelagatti, L.; Sibona, E.; Fersini, N.; Buggea, M.; Tozzi, C.; Allescia, G.; Paolini, D.; et al. Point-of-care ultrasound (PoCUS) in the early diagnosis of novel coronavirus 2019 disease (COVID-19) in a first-level emergency department during a SARS-CoV-2 outbreak in Italy: A real-life analysis. Intern. Emerg. Med. 2022, 17, 193–204. [Google Scholar] [CrossRef] [PubMed]
- Bosso, G.; Allegorico, E.; Pagano, A.; Porta, G.; Serra, C.; Minerva, V.; Mercurio, V.; Russo, T.; Altruda, C.; Arbo, P.; et al. Lung ultrasound as diagnostic tool for SARS-CoV-2 infection. Intern. Emerg. Med. 2021, 16, 471–476. [Google Scholar] [CrossRef] [PubMed]
- Mboumba Bouassa, R.S.; Veyer, D.; Péré, H.; Bélec, L. Analytical performances of the point-of-care SIENNA™ COVID-19 Antigen Rapid Test for the detection of SARS-CoV-2 nucleocapsid protein in nasopharyngeal swabs: A prospective evaluation during the COVID-19 second wave in France. Int. J. Infect. Dis. 2021, 106, 8–12. [Google Scholar] [CrossRef] [PubMed]
- Gaia, C.; Maria Chiara, C.; Silvia, L.; Chiara, A.; Maria Luisa, C.; Giulia, B.; Silvia, P.; Lucia, C.; Alessandra, T.; Annarita, S.; et al. Chest CT for early detection and management of coronavirus disease (COVID-19): A report of 314 patients admitted to Emergency Department with suspected pneumonia. Radiol. Med. 2020, 125, 931–942. [Google Scholar] [CrossRef] [PubMed]
- Caruana, G.; Croxatto, A.; Kampouri, E.; Kritikos, A.; Opota, O.; Foerster, M.; Brouillet, R.; Senn, L.; Lienhard, R.; Egli, A.; et al. Implementing SARS-CoV-2 Rapid Antigen Testing in the Emergency Ward of a Swiss University Hospital: The INCREASE Study. Microorganisms 2021, 9, 798. [Google Scholar] [CrossRef]
- Caruso, D.; Zerunian, M.; Polici, M.; Pucciarelli, F.; Polidori, T.; Rucci, C.; Guido, G.; Bracci, B.; De Dominicis, C.; Laghi, A. Chest CT Features of COVID-19 in Rome, Italy. Radiology 2020, 296, E79–E85. [Google Scholar] [CrossRef]
- Cerutti, F.; Burdino, E.; Milia, M.G.; Allice, T.; Gregori, G.; Bruzzone, B.; Ghisetti, V. Urgent need of rapid tests for SARS CoV-2 antigen detection: Evaluation of the SD-Biosensor antigen test for SARS-CoV-2. J. Clin. Virol. 2020, 132, 104654. [Google Scholar] [CrossRef]
- Chaimayo, C.; Kaewnaphan, B.; Tanlieng, N.; Athipanyasilp, N.; Sirijatuphat, R.; Chayakulkeeree, M.; Angkasekwinai, N.; Sutthent, R.; Puangpunngam, N.; Tharmviboonsri, T.; et al. Rapid SARS-CoV-2 antigen detection assay in comparison with real-time RT-PCR assay for laboratory diagnosis of COVID-19 in Thailand. Virol. J. 2020, 17, 177. [Google Scholar] [CrossRef]
- Ciotti, M.; Maurici, M.; Pieri, M.; Andreoni, M.; Bernardini, S. Performance of a rapid antigen test in the diagnosis of SARS-CoV-2 infection. J. Med. Virol. 2021, 93, 2988–2991. [Google Scholar] [CrossRef]
- Colombi, D.; Petrini, M.; Maffi, G.; Villani, G.D.; Bodini, F.C.; Morelli, N.; Milanese, G.; Silva, M.; Sverzellati, N.; Michieletti, E. Comparison of admission chest computed tomography and lung ultrasound performance for diagnosis of COVID-19 pneumonia in populations with different disease prevalence. Eur. J. Radiol. 2020, 133, 109344. [Google Scholar] [CrossRef]
- Debray, M.P.; Tarabay, H.; Males, L.; Chalhoub, N.; Mahdjoub, E.; Pavlovsky, T.; Visseaux, B.; Bouzid, D.; Borie, R.; Wackenheim, C.; et al. Observer agreement and clinical significance of chest CT reporting in patients suspected of COVID-19. Eur. Radiol. 2021, 31, 1081–1089. [Google Scholar] [CrossRef] [PubMed]
- Dofferhoff, A.S.M.; Swinkels, A.; Sprong, T.; Berk, Y.; Spanbroek, M.; Nabuurs-Franssen, M.H.; Vermaat, M.; van de Kerkhof, B.; Willekens, M.H.C.; Voss, A. [Diagnostic algorithm for COVID-19 at the ER]. Ned. Tijdschr. Geneeskd. 2020, 164, D5042. [Google Scholar] [PubMed]
- Domínguez Fernández, M.; Peña Rodríguez, M.F.; Lamelo Alfonsín, F.; Bou Arévalo, G. Experience with Panbio™ rapid antigens test device for the detection of SARS-CoV-2 in nursing homes. Enferm. Infect. Microbiol. Clin. (Engl. Ed.) 2021, 40, 42–43. [Google Scholar] [CrossRef] [PubMed]
- Drain, P.K.; Ampajwala, M.; Chappel, C.; Gvozden, A.B.; Hoppers, M.; Wang, M.; Rosen, R.; Young, S.; Zissman, E.; Montano, M. A Rapid, High-Sensitivity SARS-CoV-2 Nucleocapsid Immunoassay to Aid Diagnosis of Acute COVID-19 at the Point of Care: A Clinical Performance Study. Infect. Dis. Ther. 2021, 10, 753–761. [Google Scholar] [CrossRef]
- Ducray, V.; Vlachomitrou, A.S.; Bouscambert-Duchamp, M.; Si-Mohamed, S.; Gouttard, S.; Mansuy, A.; Wickert, F.; Sigal, A.; Gaymard, A.; Talbot, F.; et al. Chest CT for rapid triage of patients in multiple emergency departments during COVID-19 epidemic: Experience report from a large French university hospital. Eur. Radiol. 2021, 31, 795–803. [Google Scholar] [CrossRef]
- Falaschi, Z.; Danna, P.S.C.; Arioli, R.; Pasché, A.; Zagaria, D.; Percivale, I.; Tricca, S.; Barini, M.; Aquilini, F.; Andreoni, S.; et al. Chest CT accuracy in diagnosing COVID-19 during the peak of the Italian epidemic: A retrospective correlation with RT-PCR testing and analysis of discordant cases. Eur. J. Radiol. 2020, 130, 109192. [Google Scholar] [CrossRef]
- Favresse, J.; Gillot, C.; Oliveira, M.; Cadrobbi, J.; Elsen, M.; Eucher, C.; Laffineur, K.; Rosseels, C.; Van Eeckhoudt, S.; Nicolas, J.B.; et al. Head-to-Head Comparison of Rapid and Automated Antigen Detection Tests for the Diagnosis of SARS-CoV-2 Infection. J. Clin. Med. 2021, 10, 265. [Google Scholar] [CrossRef]
- Fenollar, F.; Bouam, A.; Ballouche, M.; Fuster, L.; Prudent, E.; Colson, P.; Tissot-Dupont, H.; Million, M.; Drancourt, M.; Raoult, D.; et al. Evaluation of the Panbio COVID-19 Rapid Antigen Detection Test Device for the Screening of Patients with COVID-19. J. Clin. Microbiol. 2021, 59, e02589-20. [Google Scholar] [CrossRef]
- Ferguson, J.; Dunn, S.; Best, A.; Mirza, J.; Percival, B.; Mayhew, M.; Megram, O.; Ashford, F.; White, T.; Moles-Garcia, E.; et al. Validation testing to determine the sensitivity of lateral flow testing for asymptomatic SARS-CoV-2 detection in low prevalence settings: Testing frequency and public health messaging is key. PLoS Biol. 2021, 19, e3001216. [Google Scholar] [CrossRef]
- Fonsi, G.B.; Sapienza, P.; Brachini, G.; Andreoli, C.; De Cicco, M.L.; Cirillo, B.; Meneghini, S.; Pugliese, F.; Crocetti, D.; Fiori, E.; et al. Is Lung Ultrasound Imaging a Worthwhile Procedure for Severe Acute Respiratory Syndrome Coronavirus 2 Pneumonia Detection? J. Ultrasound. Med. 2021, 40, 1113–1123. [Google Scholar] [CrossRef]
- Fujioka, T.; Takahashi, M.; Mori, M.; Tsuchiya, J.; Yamaga, E.; Horii, T.; Yamada, H.; Kimura, M.; Kimura, K.; Kitazume, Y.; et al. Evaluation of the Usefulness of CO-RADS for Chest CT in Patients Suspected of Having COVID-19. Diagnostics (Basel) 2020, 10, 608. [Google Scholar] [CrossRef] [PubMed]
- Gezer, N.S.; Ergan, B.; Barış, M.M.; Appak, Ö.; Sayıner, A.A.; Balcı, P.; Kuruüzüm, Z.; Çavuş, S.A.; Kılınç, O. COVID-19 S: A new proposal for diagnosis and structured reporting of COVID-19 on computed tomography imaging. Diagn. Interv. Radiol. 2020, 26, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Giannitto, C.; Sposta, F.M.; Repici, A.; Vatteroni, G.; Casiraghi, E.; Casari, E.; Ferraroli, G.M.; Fugazza, A.; Sandri, M.T.; Chiti, A.; et al. Chest CT in patients with a moderate or high pretest probability of COVID-19 and negative swab. Radiol. Med. 2020, 125, 1260–1270. [Google Scholar] [CrossRef] [PubMed]
- Gietema, H.A.; Zelis, N.; Nobel, J.M.; Lambriks, L.J.G.; van Alphen, L.B.; Oude Lashof, A.M.L.; Wildberger, J.E.; Nelissen, I.C.; Stassen, P.M. CT in relation to RT-PCR in diagnosing COVID-19 in The Netherlands: A prospective study. PLoS ONE 2020, 15, e0235844. [Google Scholar] [CrossRef]
- Gili, A.; Paggi, R.; Russo, C.; Cenci, E.; Pietrella, D.; Graziani, A.; Stracci, F.; Mencacci, A. Evaluation of Lumipulse® G SARS-CoV-2 antigen assay automated test for detecting SARS-CoV-2 nucleocapsid protein (NP) in nasopharyngeal swabs for community and population screening. Int. J. Infect. Dis. 2021, 105, 391–396. [Google Scholar] [CrossRef]
- Gremmels, H.; Winkel, B.M.F.; Schuurman, R.; Rosingh, A.; Rigter, N.A.M.; Rodriguez, O.; Ubijaan, J.; Wensing, A.M.J.; Bonten, M.J.M.; Hofstra, L.M. Real-life validation of the Panbio™ COVID-19 antigen rapid test (Abbott) in community-dwelling subjects with symptoms of potential SARS-CoV-2 infection. EClinicalMedicine 2021, 31, 100677. [Google Scholar] [CrossRef]
- Guillo, E.; Bedmar Gomez, I.; Dangeard, S.; Bennani, S.; Saab, I.; Tordjman, M.; Jilet, L.; Chassagnon, G.; Revel, M.P. COVID-19 pneumonia: Diagnostic and prognostic role of CT based on a retrospective analysis of 214 consecutive patients from Paris, France. Eur. J. Radiol. 2020, 131, 109209. [Google Scholar] [CrossRef]
- Gupta, A.; Khurana, S.; Das, R.; Srigyan, D.; Singh, A.; Mittal, A.; Singh, P.; Soneja, M.; Kumar, A.; Singh, A.K.; et al. Rapid chromatographic immunoassay-based evaluation of COVID-19: A cross-sectional, diagnostic test accuracy study & its implications for COVID-19 management in India. Indian J. Med. Res. 2021, 153, 126. [Google Scholar] [CrossRef]
- Haak, S.L.; Renken, I.J.; Jager, L.C.; Lameijer, H.; van der Kolk, B.B.Y. Diagnostic accuracy of point-of-care lung ultrasound in COVID-19. Emerg. Med. J. 2021, 38, 94–99. [Google Scholar] [CrossRef]
- He, J.L.; Luo, L.; Luo, Z.D.; Lyu, J.X.; Ng, M.Y.; Shen, X.P.; Wen, Z. Diagnostic performance between CT and initial real-time RT-PCR for clinically suspected 2019 coronavirus disease (COVID-19) patients outside Wuhan, China. Respir. Med. 2020, 168, 105980. [Google Scholar] [CrossRef]
- Hermans, J.J.R.; Groen, J.; Zwets, E.; Boxma-De Klerk, B.M.; Van Werkhoven, J.M.; Ong, D.S.Y.; Hanselaar, W.; Waals-Prinzen, L.; Brown, V. Chest CT for triage during COVID-19 on the emergency department: Myth or truth? Emerg. Radiol. 2020, 27, 641–651. [Google Scholar] [CrossRef] [PubMed]
- Hernigou, J.; Cornil, F.; Poignard, A.; El Bouchaibi, S.; Mani, J.; Naouri, J.F.; Younes, P.; Hernigou, P. Thoracic computerised tomography scans in one hundred eighteen orthopaedic patients during the COVID-19 pandemic: Identification of chest lesions; added values; help in managing patients; burden on the computerised tomography scan department. Int. Orthop. 2020, 44, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Herpe, G.; Lederlin, M.; Naudin, M.; Ohana, M.; Chaumoitre, K.; Gregory, J.; Vilgrain, V.; Freitag, C.A.; De Margerie-Mellon, C.; Flory, V.; et al. Efficacy of Chest CT for COVID-19 Pneumonia Diagnosis in France. Radiology 2021, 298, E81–E87. [Google Scholar] [CrossRef] [PubMed]
- Hirotsu, Y.; Maejima, M.; Shibusawa, M.; Amemiya, K.; Nagakubo, Y.; Hosaka, K.; Sueki, H.; Hayakawa, M.; Mochizuki, H.; Tsutsui, T.; et al. Prospective study of 1308 nasopharyngeal swabs from 1033 patients using the LUMIPULSE SARS-CoV-2 antigen test: Comparison with RT-qPCR. Int. J. Infect. Dis. 2021, 105, 7–14. [Google Scholar] [CrossRef]
- Homza, M.; Zelena, H.; Janosek, J.; Tomaskova, H.; Jezo, E.; Kloudova, A.; Mrazek, J.; Svagera, Z.; Prymula, R. Five Antigen Tests for SARS-CoV-2: Virus Viability Matters. Viruses 2021, 13, 684. [Google Scholar] [CrossRef] [PubMed]
- Houston, H.; Gupta-Wright, A.; Toke-Bjolgerud, E.; Biggin-Lamming, J.; John, L. Diagnostic accuracy and utility of SARS-CoV-2 antigen lateral flow assays in medical admissions with possible COVID-19. J. Hosp. Infect. 2021, 110, 203–205. [Google Scholar] [CrossRef]
- Igloi, Z.; Velzing, J.; van Beek, J.; van de Vijver, D.; Aron, G.; Ensing, R.; Benschop, K.; Han, W.; Boelsums, T.; Koopmans, M.; et al. Clinical Evaluation of Roche SD Biosensor Rapid Antigen Test for SARS-CoV-2 in Municipal Health Service Testing Site, the Netherlands. Emerg. Infect. Dis. 2021, 27, 1323–1329. [Google Scholar] [CrossRef]
- Jääskeläinen, A.E.; Ahava, M.J.; Jokela, P.; Szirovicza, L.; Pohjala, S.; Vapalahti, O.; Lappalainen, M.; Hepojoki, J.; Kurkela, S. Evaluation of three rapid lateral flow antigen detection tests for the diagnosis of SARS-CoV-2 infection. J. Clin. Virol. 2021, 137, 104785. [Google Scholar] [CrossRef]
- Jalil, B.A.; Khan, A.; Kugasia, I.R.; Ijaz, M. Lung ultrasound in early SARS-CoV-2 pneumonia and the LUS-CoV criteria. Proc. (Bayl. Univ. Med. Cent.) 2020, 34, 1–4. [Google Scholar] [CrossRef]
- James, A.E.; Gulley, T.; Kothari, A.; Holder, K.; Garner, K.; Patil, N. Performance of the BinaxNOW coronavirus disease 2019 (COVID-19) Antigen Card test relative to the severe acute respiratory coronavirus virus 2 (SARS-CoV-2) real-time reverse transcriptase polymerase chain reaction (rRT-PCR) assay among symptomatic and asymptomatic healthcare employees. Infect. Control. Hosp. Epidemiol. 2022, 43, 99–101. [Google Scholar] [CrossRef]
- Kannian, P.; Lavanya, C.; Ravichandran, K.; Bagavad Gita, J.; Mahanathi, P.; Ashwini, V.; Kumarasamy, N.; Rajan, G.; Ranganathan, K.; Challacombe, S.J.; et al. SARS-CoV2 antigen in whole mouth fluid may be a reliable rapid detection tool. Oral Dis. 2021. [Google Scholar] [CrossRef] [PubMed]
- Kilic, A.; Hiestand, B.; Palavecino, E. Evaluation of Performance of the BD Veritor SARS-CoV-2 Chromatographic Immunoassay Test in Patients with Symptoms of COVID-19. J. Clin. Microbiol. 2021, 59, e00260-210. [Google Scholar] [CrossRef] [PubMed]
- Kohmer, N.; Toptan, T.; Pallas, C.; Karaca, O.; Pfeiffer, A.; Westhaus, S.; Widera, M.; Berger, A.; Hoehl, S.; Kammel, M.; et al. The Comparative Clinical Performance of Four SARS-CoV-2 Rapid Antigen Tests and Their Correlation to Infectivity In Vitro. J. Clin. Med. 2021, 10, 328. [Google Scholar] [CrossRef]
- Korevaar, D.A.; Kootte, R.S.; Smits, L.P.; van den Aardweg, J.G.; Bonta, P.I.; Schinkel, J.; Vigeveno, R.M.; van den Berk, I.A.H.; Scheerder, M.J.; Lemkes, B.A.; et al. Added value of chest computed tomography in suspected COVID-19: An analysis of 239 patients. Eur. Respir. J. 2020, 56, 2001377. [Google Scholar] [CrossRef] [PubMed]
- Krdzalic, J.; de Jaegere, T.M.H.; Kwee, R.M. Diagnostic performance of chest CT in screening patients with suspected COVID-19 infection in a Western population. Br. J. Radiol. 2020, 93, 20200643. [Google Scholar] [CrossRef] [PubMed]
- Krüttgen, A.; Cornelissen, C.G.; Dreher, M.; Hornef, M.W.; Imöhl, M.; Kleines, M. Comparison of the SARS-CoV-2 Rapid antigen test to the real star Sars-CoV-2 RT PCR kit. J. Virol. Methods 2021, 288, 114024. [Google Scholar] [CrossRef]
- Kuzan, T.Y.; Murzoğlu Altıntoprak, K.; Çiftçi, H.; Ergül, U.; Ünal Özdemir, N.B.; Bulut, M.; Yiyit, N. A comparison of clinical, laboratory and chest CT findings of laboratory-confirmed and clinically diagnosed COVID-19 patients at first admission. Diagn. Interv. Radiol. 2021, 27, 336–343. [Google Scholar] [CrossRef]
- Lambert-Niclot, S.; Cuffel, A.; Le Pape, S.; Vauloup-Fellous, C.; Morand-Joubert, L.; Roque-Afonso, A.M.; Le Goff, J.; Delaugerre, C. Evaluation of a Rapid Diagnostic Assay for Detection of SARS-CoV-2 Antigen in Nasopharyngeal Swabs. J. Clin. Microbiol. 2020, 58, e00977-20. [Google Scholar] [CrossRef]
- Lefever, S.; Indevuyst, C.; Cuypers, L.; Dewaele, K.; Yin, N.; Cotton, F.; Padalko, E.; Oyaert, M.; Descy, J.; Cavalier, E.; et al. Comparison of the Quantitative DiaSorin Liaison Antigen Test to Reverse Transcription-PCR for the Diagnosis of COVID-19 in Symptomatic and Asymptomatic Outpatients. J. Clin. Microbiol. 2021, 59, e0037421. [Google Scholar] [CrossRef]
- Li, Z.; Zeng, B.; Lei, P.; Liu, J.; Fan, B.; Shen, Q.; Pang, P.; Xu, R. Differentiating pneumonia with and without COVID-19 using chest CT images: From qualitative to quantitative. J. Xray Sci. Technol. 2020, 28, 583–589. [Google Scholar] [CrossRef]
- Lieveld, A.W.E.; Kok, B.; Schuit, F.H.; Azijli, K.; Heijmans, J.; van Laarhoven, A.; Assman, N.L.; Kootte, R.S.; Olgers, T.J.; Nanayakkara, P.W.B.; et al. Diagnosing COVID-19 pneumonia in a pandemic setting: Lung Ultrasound versus CT (LUVCT)—A multicentre, prospective, observational study. ERJ Open Res. 2020, 6, 00539–2020. [Google Scholar] [CrossRef] [PubMed]
- Linares, M.; Pérez-Tanoira, R.; Carrero, A.; Romanyk, J.; Pérez-García, F.; Gómez-Herruz, P.; Arroyo, T.; Cuadros, J. Panbio antigen rapid test is reliable to diagnose SARS-CoV-2 infection in the first 7 days after the onset of symptoms. J. Clin. Virol. 2020, 133, 104659. [Google Scholar] [CrossRef] [PubMed]
- Lindner, A.K.; Nikolai, O.; Kausch, F.; Wintel, M.; Hommes, F.; Gertler, M.; Krüger, L.J.; Gaeddert, M.; Tobian, F.; Lainati, F.; et al. Head-to-head comparison of SARS-CoV-2 antigen-detecting rapid test with self-collected nasal swab versus professional-collected nasopharyngeal swab. Eur. Respir. J. 2021, 57, 2003961. [Google Scholar] [CrossRef] [PubMed]
- Liotti, F.M.; Menchinelli, G.; Lalle, E.; Palucci, I.; Marchetti, S.; Colavita, F.; La Sorda, M.; Sberna, G.; Bordi, L.; Sanguinetti, M.; et al. Performance of a novel diagnostic assay for rapid SARS-CoV-2 antigen detection in nasopharynx samples. Clin. Microbiol. Infect. 2021, 27, 487–488. [Google Scholar] [CrossRef] [PubMed]
- Luo, L.; Luo, Z.; Jia, Y.; Zhou, C.; He, J.; Lyu, J.; Shen, X. CT differential diagnosis of COVID-19 and non-COVID-19 in symptomatic suspects: A practical scoring method. BMC Pulm. Med. 2020, 20, 129. [Google Scholar] [CrossRef] [PubMed]
- Luo, N.; Zhang, H.; Zhou, Y.; Kong, Z.; Sun, W.; Huang, N.; Zhang, A. Utility of chest CT in diagnosis of COVID-19 pneumonia. Diagn. Interv. Radiol. 2020, 26, 437–442. [Google Scholar] [CrossRef]
- Mei, X.; Lee, H.C.; Diao, K.; Huang, M.; Lin, B.; Liu, C.; Xie, Z.; Ma, Y.; Robson, P.M.; Chung, M.; et al. Artificial intelligence-enabled rapid diagnosis of COVID-19 patients. medRxiv, 2020; in press. [Google Scholar] [CrossRef]
- Merino, P.; Guinea, J.; Muñoz-Gallego, I.; González-Donapetry, P.; Galán, J.C.; Antona, N.; Cilla, G.; Hernáez-Crespo, S.; Díaz-de Tuesta, J.L.; Gual-de Torrella, A.; et al. Multicenter evaluation of the Panbio™ COVID-19 rapid antigen-detection test for the diagnosis of SARS-CoV-2 infection. Clin. Microbiol. Infect. 2021, 27, 758–761. [Google Scholar] [CrossRef]
- Mertens, P.; De Vos, N.; Martiny, D.; Jassoy, C.; Mirazimi, A.; Cuypers, L.; Van den Wijngaert, S.; Monteil, V.; Melin, P.; Stoffels, K.; et al. Development and Potential Usefulness of the COVID-19 Ag Respi-Strip Diagnostic Assay in a Pandemic Context. Front. Med. (Lausanne) 2020, 7, 225. [Google Scholar] [CrossRef]
- Miranda Magalhães Santos, J.M.; Paula Alves Fonseca, A.; Pinheiro Zarattini Anastacio, E.; Formagio Minenelli, F.; Furtado de Albuquerque Cavalcanti, C.; Borges da Silva Teles, G. Initial Results of the Use of a Standardized Diagnostic Criteria for Chest Computed Tomography Findings in Coronavirus Disease 2019. J. Comput. Assist. Tomogr. 2020, 44, 647–651. [Google Scholar] [CrossRef]
- Möckel, M.; Corman, V.M.; Stegemann, M.S.; Hofmann, J.; Stein, A.; Jones, T.C.; Gastmeier, P.; Seybold, J.; Offermann, R.; Bachmann, U.; et al. SARS-CoV-2 antigen rapid immunoassay for diagnosis of COVID-19 in the emergency department. Biomarkers 2021, 26, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Nalumansi, A.; Lutalo, T.; Kayiwa, J.; Watera, C.; Balinandi, S.; Kiconco, J.; Nakaseegu, J.; Olara, D.; Odwilo, E.; Serwanga, J.; et al. Field evaluation of the performance of a SARS-CoV-2 antigen rapid diagnostic test in Uganda using nasopharyngeal samples. Int. J. Infect. Dis 2021, 104, 282–286. [Google Scholar] [CrossRef] [PubMed]
- Narinx, N.; Smismans, A.; Symons, R.; Frans, J.; Demeyere, A.; Gillis, M. Feasibility of using point-of-care lung ultrasound for early triage of COVID-19 patients in the emergency room. Emerg. Radiol. 2020, 27, 663–670. [Google Scholar] [CrossRef] [PubMed]
- Okoye, N.C.; Barker, A.P.; Curtis, K.; Orlandi, R.R.; Snavely, E.A.; Wright, C.; Hanson, K.E.; Pearson, L.N. Performance Characteristics of BinaxNOW COVID-19 Antigen Card for Screening Asymptomatic Individuals in a University Setting. J. Clin. Microbiol. 2021, 59. [Google Scholar] [CrossRef]
- Olearo, F.; Nörz, D.; Heinrich, F.; Sutter, J.P.; Roedl, K.; Schultze, A.; Wiesch, J.S.Z.; Braun, P.; Oestereich, L.; Kreuels, B.; et al. Handling and accuracy of four rapid antigen tests for the diagnosis of SARS-CoV-2 compared to RT-qPCR. J. Clin. Virol. 2021, 137, 104782. [Google Scholar] [CrossRef]
- Osterman, A.; Baldauf, H.M.; Eletreby, M.; Wettengel, J.M.; Afridi, S.Q.; Fuchs, T.; Holzmann, E.; Maier, A.; Döring, J.; Grzimek-Koschewa, N.; et al. Evaluation of two rapid antigen tests to detect SARS-CoV-2 in a hospital setting. Med. Microbiol. Immunol. 2021, 210, 65–72. [Google Scholar] [CrossRef]
- Parada-Ricart, E.; Gomez-Bertomeu, F.; Picó-Plana, E.; Olona-Cabases, M. Usefulness of the antigen test for diagnosing SARS-CoV-2 infection in patients with and without symptoms. Enferm. Infect. Microbiol. Clin. (Engl. Ed.) 2021, 39, 357–358. [Google Scholar] [CrossRef]
- Pare, J.R.; Camelo, I.; Mayo, K.C.; Leo, M.M.; Dugas, J.N.; Nelson, K.P.; Baker, W.E.; Shareef, F.; Mitchell, P.M.; Schechter-Perkins, E.M. Point-of-care Lung Ultrasound Is More Sensitive than Chest Radiograph for Evaluation of COVID-19. West J. Emerg. Med. 2020, 21, 771–778. [Google Scholar] [CrossRef]
- Pekosz, A.; Parvu, V.; Li, M.; Andrews, J.C.; Manabe, Y.C.; Kodsi, S.; Gary, D.S.; Roger-Dalbert, C.; Leitch, J.; Cooper, C.K. Antigen-Based Testing but Not Real-Time Polymerase Chain Reaction Correlates With Severe Acute Respiratory Syndrome Coronavirus 2 Viral Culture. Clin. Infect. Dis. 2021, 73, e2861–e2866. [Google Scholar] [CrossRef]
- Pérez-García, F.; Romanyk, J.; Gómez-Herruz, P.; Arroyo, T.; Pérez-Tanoira, R.; Linares, M.; Pérez Ranz, I.; Labrador Ballestero, A.; Moya Gutiérrez, H.; Ruiz-Álvarez, M.J.; et al. Diagnostic performance of CerTest and Panbio antigen rapid diagnostic tests to diagnose SARS-CoV-2 infection. J. Clin. Virol. 2021, 137, 104781. [Google Scholar] [CrossRef]
- Pilarowski, G.; Lebel, P.; Sunshine, S.; Liu, J.; Crawford, E.; Marquez, C.; Rubio, L.; Chamie, G.; Martinez, J.; Peng, J.; et al. Performance characteristics of a rapid SARS-CoV-2 antigen detection assay at a public plaza testing site in San Francisco. medRxiv, 2020; in press. [Google Scholar] [CrossRef]
- Pivetta, E.; Goffi, A.; Tizzani, M.; Locatelli, S.M.; Porrino, G.; Losano, I.; Leone, D.; Calzolari, G.; Vesan, M.; Steri, F.; et al. Lung Ultrasonography for the Diagnosis of SARS-CoV-2 Pneumonia in the Emergency Department. Ann. Emerg. Med. 2021, 77, 385–394. [Google Scholar] [CrossRef] [PubMed]
- Pollock, N.R.; Jacobs, J.R.; Tran, K.; Cranston, A.E.; Smith, S.; O’Kane, C.Y.; Roady, T.J.; Moran, A.; Scarry, A.; Carroll, M.; et al. Performance and Implementation Evaluation of the Abbott BinaxNOW Rapid Antigen Test in a High-Throughput Drive-Through Community Testing Site in Massachusetts. J. Clin. Microbiol. 2021, 59, e00083-21. [Google Scholar] [CrossRef] [PubMed]
- Porte, L.; Legarraga, P.; Vollrath, V.; Aguilera, X.; Munita, J.M.; Araos, R.; Pizarro, G.; Vial, P.; Iruretagoyena, M.; Dittrich, S.; et al. Evaluation of a novel antigen-based rapid detection test for the diagnosis of SARS-CoV-2 in respiratory samples. Int. J. Infect. Dis. 2020, 99, 328–333. [Google Scholar] [CrossRef] [PubMed]
- Prokop, M.; van Everdingen, W.; van Rees Vellinga, T.; Quarles van Ufford, H.; Stöger, L.; Beenen, L.; Geurts, B.; Gietema, H.; Krdzalic, J.; Schaefer-Prokop, C.; et al. CO-RADS: A Categorical CT Assessment Scheme for Patients Suspected of Having COVID-19-Definition and Evaluation. Radiology 2020, 296, E97–E104. [Google Scholar] [CrossRef]
- Ristić, M.; Nikolić, N.; Čabarkapa, V.; Turkulov, V.; Petrović, V. Validation of the STANDARD Q COVID-19 antigen test in Vojvodina, Serbia. PLoS ONE 2021, 16, e0247606. [Google Scholar] [CrossRef]
- Rottenstreich, A.; Zarbiv, G.; Kabiri, D.; Porat, S.; Sompolinsky, Y.; Reubinoff, B.; Benenson, S.; Oster, Y. Rapid antigen detection testing for universal screening for severe acute respiratory syndrome coronavirus 2 in women admitted for delivery. Am. J. Obstet. Gynecol. 2021, 224, 539–540. [Google Scholar] [CrossRef]
- Salvagno, G.L.; Gianfilippi, G.; Bragantini, D.; Henry, B.M.; Lippi, G. Clinical assessment of the Roche SARS-CoV-2 rapid antigen test. Diagnosis (Berl) 2021, 8, 322–326. [Google Scholar] [CrossRef]
- Sberna, G.; Lalle, E.; Capobianchi, M.R.; Bordi, L.; Amendola, A. Letter of concern re: “Immunochromatographic test for the detection of SARS-CoV-2 in saliva. J. Infect. Chemother. 2021, 27, 384–386. [Google Scholar] [CrossRef]
- Schildgen, V.; Demuth, S.; Lüsebrink, J.; Schildgen, O. Limits and Opportunities of SARS-CoV-2 Antigen Rapid Tests: An Experienced-Based Perspective. Pathogens 2021, 10, 38. [Google Scholar] [CrossRef]
- Schmid, B.; Feuerstein, D.; Lang, C.N.; Fink, K.; Steger, R.; Rieder, M.; Duerschmied, D.; Busch, H.J.; Damjanovic, D. Lung ultrasound in the emergency department—A valuable tool in the management of patients presenting with respiratory symptoms during the SARS-CoV-2 pandemic. BMC Emerg. Med. 2020, 20, 96. [Google Scholar] [CrossRef]
- Schulze-Hagen, M.; Hübel, C.; Meier-Schroers, M.; Yüksel, C.; Sander, A.; Sähn, M.; Kleines, M.; Isfort, P.; Cornelissen, C.; Lemmen, S.; et al. Low-Dose Chest CT for the Diagnosis of COVID-19—A Systematic, Prospective Comparison With PCR. Dtsch. Arztebl. Int. 2020, 117, 389–395. [Google Scholar] [CrossRef]
- Scohy, A.; Anantharajah, A.; Bodéus, M.; Kabamba-Mukadi, B.; Verroken, A.; Rodriguez-Villalobos, H. Low performance of rapid antigen detection test as frontline testing for COVID-19 diagnosis. J. Clin. Virol. 2020, 129, 104455. [Google Scholar] [CrossRef]
- Seitz, T.; Schindler, S.; Winkelmeyer, P.; Zach, B.; Wenisch, C.; Zoufaly, A.; Allerberger, F. Evaluation of rapid antigen tests based on saliva for the detection of SARS-CoV-2. J. Med. Virol. 2021, 93, 4161–4162. [Google Scholar] [CrossRef]
- Shrestha, B.; Neupane, A.K.; Pant, S.; Shrestha, A.; Bastola, A.; Rajbhandari, B.; Thapa, A.; Singh, A. Sensitivity and Specificity of Lateral Flow Antigen Test Kits for COVID-19 in Asymptomatic Population of Quarantine Centre of Province 3. Kathmandu Univ. Med. J. (KUMJ) 2020, 18, 36–39. [Google Scholar] [CrossRef]
- Song, S.; Wu, F.; Liu, Y.; Jiang, H.; Xiong, F.; Guo, X.; Zhang, H.; Zheng, C.; Yang, F. Correlation Between Chest CT Findings and Clinical Features of 211 COVID-19 Suspected Patients in Wuhan, China. Open Forum Infect. Dis. 2020, 7, ofaa171. [Google Scholar] [CrossRef]
- Sorlini, C.; Femia, M.; Nattino, G.; Bellone, P.; Gesu, E.; Francione, P.; Paternò, M.; Grillo, P.; Ruffino, A.; Bertolini, G.; et al. The role of lung ultrasound as a frontline diagnostic tool in the era of COVID-19 outbreak. Intern. Emerg. Med. 2021, 16, 749–756. [Google Scholar] [CrossRef]
- Steuwe, A.; Rademacher, C.; Valentin, B.; Köhler, M.H.; Appel, E.; Keitel, V.; Timm, J.; Antoch, G.; Aissa, J. Dose-optimised chest computed tomography for diagnosis of Coronavirus Disease 2019 (COVID-19)—Evaluation of image quality and diagnostic impact. J. Radiol. Prot. 2020, 40, 877–891. [Google Scholar] [CrossRef]
- Stokes, W.; Berenger, B.M.; Portnoy, D.; Scott, B.; Szelewicki, J.; Singh, T.; Venner, A.A.; Turnbull, L.; Pabbaraju, K.; Shokoples, S.; et al. Clinical performance of the Abbott Panbio with nasopharyngeal, throat, and saliva swabs among symptomatic individuals with COVID-19. Eur. J. Clin. Microbiol. Infect. Dis. 2021, 40, 1721–1726. [Google Scholar] [CrossRef]
- Takeuchi, Y.; Akashi, Y.; Kato, D.; Kuwahara, M.; Muramatsu, S.; Ueda, A.; Notake, S.; Nakamura, K.; Ishikawa, H.; Suzuki, H. The evaluation of a newly developed antigen test (QuickNavi™-COVID19 Ag) for SARS-CoV-2: A prospective observational study in Japan. J. Infect. Chemother. 2021, 27, 890–894. [Google Scholar] [CrossRef]
- Thakur, P.; Saxena, S.; Manchanda, V.; Rana, N.; Goel, R.; Arora, R. Utility of Antigen-Based Rapid Diagnostic Test for Detection of SARS-CoV-2 Virus in Routine Hospital Settings. Lab. Med. 2021, 52, e154–e158. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; Poujois, S.; Albert, E.; Álvarez, G.; Colomina, J.; Navarro, D. Point-of-care evaluation of a rapid antigen test (CLINITEST(®) Rapid COVID-19 Antigen Test) for diagnosis of SARS-CoV-2 infection in symptomatic and asymptomatic individuals. J. Infect. 2021, 82, e11–e12. [Google Scholar] [CrossRef] [PubMed]
- Torres, I.; Poujois, S.; Albert, E.; Colomina, J.; Navarro, D. Evaluation of a rapid antigen test (Panbio™ COVID-19 Ag rapid test device) for SARS-CoV-2 detection in asymptomatic close contacts of COVID-19 patients. Clin. Microbiol. Infect. 2021, 27, 636.e631–636.e634. [Google Scholar] [CrossRef] [PubMed]
- Tung-Chen, Y.; Algora-Martín, A.; Llamas-Fuentes, R.; Rodríguez-Fuertes, P.; Martínez Virto, A.M.; Sanz-Rodríguez, E.; Alonso-Martínez, B.; Rivera Núñez, M.A. Point-of-care ultrasonography in the initial characterization of patients with COVID-19. Med. Clin. (Engl. Ed.) 2021, 156, 477–484. [Google Scholar] [CrossRef] [PubMed]
- Turcato, G.; Zaboli, A.; Pfeifer, N.; Ciccariello, L.; Sibilio, S.; Tezza, G.; Ausserhofer, D. Clinical application of a rapid antigen test for the detection of SARS-CoV-2 infection in symptomatic and asymptomatic patients evaluated in the emergency department: A preliminary report. J. Infect. 2021, 82, e14–e16. [Google Scholar] [CrossRef] [PubMed]
- Veyrenche, N.; Bolloré, K.; Pisoni, A.; Bedin, A.S.; Mondain, A.M.; Ducos, J.; Segondy, M.; Montes, B.; Pastor, P.; Morquin, D.; et al. Diagnosis value of SARS-CoV-2 antigen/antibody combined testing using rapid diagnostic tests at hospital admission. J. Med. Virol. 2021, 93, 3069–3076. [Google Scholar] [CrossRef]
- Villaverde, S.; Domínguez-Rodríguez, S.; Sabrido, G.; Pérez-Jorge, C.; Plata, M.; Romero, M.P.; Grasa, C.D.; Jiménez, A.B.; Heras, E.; Broncano, A.; et al. Diagnostic Accuracy of the Panbio Severe Acute Respiratory Syndrome Coronavirus 2 Antigen Rapid Test Compared with Reverse-Transcriptase Polymerase Chain Reaction Testing of Nasopharyngeal Samples in the Pediatric Population. J. Pediatr. 2021, 232, 287–289.e284. [Google Scholar] [CrossRef]
- Volpicelli, G.; Gargani, L.; Perlini, S.; Spinelli, S.; Barbieri, G.; Lanotte, A.; Casasola, G.G.; Nogué-Bou, R.; Lamorte, A.; Agricola, E.; et al. Lung ultrasound for the early diagnosis of COVID-19 pneumonia: An international multicenter study. Intensive Care Med. 2021, 47, 444–454. [Google Scholar] [CrossRef]
- Wang, T.; Xiong, Z.; Zhou, H.; Luo, W.; Tang, H.; Liu, J. Design, validation, and clinical practice of standardized imaging diagnostic report for COVID-19. Zhong Nan Da Xue Xue Bao Yi Xue Ban 2020, 45, 229–235. [Google Scholar] [CrossRef]
- Weitzel, T.; Legarraga, P.; Iruretagoyena, M.; Pizarro, G.; Vollrath, V.; Araos, R.; Munita, J.M.; Porte, L. Comparative evaluation of four rapid SARS-CoV-2 antigen detection tests using universal transport medium. Travel. Med. Infect. Dis. 2021, 39, 101942. [Google Scholar] [CrossRef]
- Xiong, Z.; Fu, L.; Zhou, H.; Liu, J.K.; Wang, A.M.; Huang, Y.; Huang, X.; Yi, B.; Wu, J.; Li, C.H.; et al. [Construction and evaluation of a novel diagnosis pathway for 2019-Corona Virus Disease]. Zhonghua Yi Xue Za Zhi 2020, 100, 1223–1229. [Google Scholar] [CrossRef] [PubMed]
- Young, B.C.; Eyre, D.W.; Jeffery, K. Use of lateral flow devices allows rapid triage of patients with SARS-CoV-2 on admission to hospital. J. Infect. 2021, 82, 276–316. [Google Scholar] [CrossRef] [PubMed]
- Young, S.; Taylor, S.N.; Cammarata, C.L.; Varnado, K.G.; Roger-Dalbert, C.; Montano, A.; Griego-Fullbright, C.; Burgard, C.; Fernandez, C.; Eckert, K.; et al. Clinical Evaluation of BD Veritor SARS-CoV-2 Point-of-Care Test Performance Compared to PCR-Based Testing and versus the Sofia 2 SARS Antigen Point-of-Care Test. J. Clin. Microbiol. 2020, 59, e02338-20. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shim, S.R.; Kim, S.-J.; Hong, M.; Lee, J.; Kang, M.-G.; Han, H.W. Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis. Diagnostics 2022, 12, 1302. https://doi.org/10.3390/diagnostics12061302
Shim SR, Kim S-J, Hong M, Lee J, Kang M-G, Han HW. Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis. Diagnostics. 2022; 12(6):1302. https://doi.org/10.3390/diagnostics12061302
Chicago/Turabian StyleShim, Sung Ryul, Seong-Jang Kim, Myunghee Hong, Jonghoo Lee, Min-Gyu Kang, and Hyun Wook Han. 2022. "Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis" Diagnostics 12, no. 6: 1302. https://doi.org/10.3390/diagnostics12061302
APA StyleShim, S. R., Kim, S.-J., Hong, M., Lee, J., Kang, M.-G., & Han, H. W. (2022). Diagnostic Performance of Antigen Rapid Diagnostic Tests, Chest Computed Tomography, and Lung Point-of-Care-Ultrasonography for SARS-CoV-2 Compared with RT-PCR Testing: A Systematic Review and Network Meta-Analysis. Diagnostics, 12(6), 1302. https://doi.org/10.3390/diagnostics12061302





