Regional Cerebral Blood Flow Correlates of Neuropsychiatric Symptom Domains in Early Alzheimer’s Disease
Abstract
:1. Introduction
2. Materials and Methods
2.1. Participants
2.2. Clinical Assessment
2.3. SPECT Acquisition and Analysis
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Gauthier, S.; Cummings, J.; Ballard, C.; Brodaty, H.; Grossberg, G.; Robert, P.; Lyketsos, C. Management of behavioral problems in Alzheimer’s disease. Int. Psychogeriatr. 2010, 22, 346–372. [Google Scholar] [CrossRef] [PubMed]
- Fernandez-Martinez, M.; Molano, A.; Castro, J.; Zarranz, J.J. Prevalence of neuropsychiatric symptoms in mild cognitive impairment and Alzheimer’s disease, and its relationship with cognitive impairment. Curr. Alzheimer Res. 2010, 7, 517–526. [Google Scholar] [CrossRef] [PubMed]
- Peters, M.E.; Schwartz, S.; Han, D.; Rabins, P.V.; Steinberg, M.; Tschanz, J.T.; Lyketsos, C.G. Neuropsychiatric symptoms as predictors of progression to severe Alzheimer’s dementia and death: The Cache County Dementia Progression Study. Am. J. Psychiatry 2015, 172, 460–465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shin, I.S.; Carter, M.; Masterman, D.; Fairbanks, L.; Cummings, J.L. Neuropsychiatric symptoms and quality of life in Alzheimer disease. Am. J. Geriatr. Psychiatry 2005, 13, 469–474. [Google Scholar] [CrossRef]
- Clement, A.; Wiborg, O.; Asuni, A.A. Steps Towards Developing Effective Treatments for Neuropsychiatric Disturbances in Alzheimer’s Disease: Insights From Preclinical Models, Clinical Data, and Future Directions. Front. Aging Neurosci. 2020, 12, 56. [Google Scholar] [CrossRef]
- Rosenberg, P.B.; Nowrangi, M.A.; Lyketsos, C.G. Neuropsychiatric symptoms in Alzheimer’s disease: What might be associated brain circuits? Mol. Asp. Med. 2015, 43–44, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Boublay, N.; Schott, A.M.; Krolak-Salmon, P. Neuroimaging correlates of neuropsychiatric symptoms in Alzheimer’s disease: A review of 20 years of research. Eur. J. Neurol. 2016, 23, 1500–1509. [Google Scholar] [CrossRef]
- Ng, K.P.; Chiew, H.J.; Rosa-Neto, P.; Kandiah, N.; Ismail, Z.; Gauthier, S. Brain Metabolic Dysfunction in Early Neuropsychiatric Symptoms of Dementia. Front. Pharmacol. 2019, 10, 1398. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Dang, M.; Zhang, Z. Brain mechanisms underlying neuropsychiatric symptoms in Alzheimer’s disease: A systematic review of symptom-general and -specific lesion patterns. Mol. Neurodegener. 2021, 16, 38. [Google Scholar] [CrossRef]
- Nowrangi, M.A.; Lyketsos, C.G.; Rosenberg, P.B. Principles and management of neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimers Res. Ther. 2015, 7, 12. [Google Scholar] [CrossRef] [Green Version]
- Balthazar, M.L.; Pereira, F.R.; Lopes, T.M.; da Silva, E.L.; Coan, A.C.; Campos, B.M.; Duncan, N.W.; Stella, F.; Northoff, G.; Damasceno, B.P.; et al. Neuropsychiatric symptoms in Alzheimer’s disease are related to functional connectivity alterations in the salience network. Hum. Brain Mapp. 2014, 35, 1237–1246. [Google Scholar] [CrossRef] [PubMed]
- Ballarini, T.; Iaccarino, L.; Magnani, G.; Ayakta, N.; Miller, B.L.; Jagust, W.J.; Gorno-Tempini, M.L.; Rabinovici, G.D.; Perani, D. Neuropsychiatric subsyndromes and brain metabolic network dysfunctions in early onset Alzheimer’s disease. Hum. Brain Mapp. 2016, 37, 4234–4247. [Google Scholar] [CrossRef] [Green Version]
- Chang, Y.T.; Hsu, J.L.; Huang, S.H.; Hsu, S.W.; Lee, C.C.; Chang, C.C. Functional connectome and neuropsychiatric symptom clusters of Alzheimer’s disease. J. Affect. Disord. 2020, 273, 48–54. [Google Scholar] [CrossRef] [PubMed]
- Aalten, P.; Verhey, F.R.; Boziki, M.; Brugnolo, A.; Bullock, R.; Byrne, E.J.; Camus, V.; Caputo, M.; Collins, D.; De Deyn, P.P.; et al. Consistency of neuropsychiatric syndromes across dementias: Results from the European Alzheimer Disease Consortium. Part II. Dement. Geriatr. Cogn. Disord. 2008, 25, 1–8. [Google Scholar] [CrossRef]
- Pickut, B.A.; Dierckx, R.A.; Dobbeleir, A.; Audenaert, K.; Van Laere, K.; Vervaet, A.; De Deyn, P.P. Validation of the cerebellum as a reference region for SPECT quantification in patients suffering from dementia of the Alzheimer type. Psychiatry Res. 1999, 90, 103–112. [Google Scholar] [CrossRef]
- Bora, E.; Harrison, B.J.; Davey, C.G.; Yucel, M.; Pantelis, C. Meta-analysis of volumetric abnormalities in cortico-striatal-pallidal-thalamic circuits in major depressive disorder. Psychol. Med. 2012, 42, 671–681. [Google Scholar] [CrossRef]
- Frank, D.W.; Dewitt, M.; Hudgens-Haney, M.; Schaeffer, D.J.; Ball, B.H.; Schwarz, N.F.; Hussein, A.A.; Smart, L.M.; Sabatinelli, D. Emotion regulation: Quantitative meta-analysis of functional activation and deactivation. Neurosci. Biobehav. Rev. 2014, 45, 202–211. [Google Scholar] [CrossRef]
- Buyukdura, J.S.; McClintock, S.M.; Croarkin, P.E. Psychomotor retardation in depression: Biological underpinnings, measurement, and treatment. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Guo, Z.; Liu, X.; Jia, X.; Hou, H.; Cao, Y.; Wei, F.; Li, J.; Chen, X.; Zhang, Y.; Shen, Y.; et al. Regional Coherence Changes in Alzheimer’s Disease Patients with Depressive Symptoms: A Resting-State Functional MRI Study. J. Alzheimers Dis. 2015, 48, 603–611. [Google Scholar] [CrossRef]
- Pizzagalli, D.A.; Holmes, A.J.; Dillon, D.G.; Goetz, E.L.; Birk, J.L.; Bogdan, R.; Dougherty, D.D.; Iosifescu, D.V.; Rauch, S.L.; Fava, M. Reduced caudate and nucleus accumbens response to rewards in unmedicated individuals with major depressive disorder. Am. J. Psychiatry 2009, 166, 702–710. [Google Scholar] [CrossRef] [Green Version]
- Brommelhoff, J.A.; Spann, B.M.; Go, J.L.; Mack, W.J.; Gatz, M. Striatal Hypodensities, Not White Matter Hypodensities on CT, Are Associated with Late-Onset Depression in Alzheimer’s Disease. J. Aging Res. 2011, 2011, 187219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, Z.; Herrmann, N.; Rothenburg, L.S.; Cotter, A.; Leibovitch, F.S.; Rafi-Tari, S.; Black, S.E.; Lanctot, K.L. A functional neuroimaging study of appetite loss in Alzheimer’s disease. J. Neurol. Sci. 2008, 271, 97–103. [Google Scholar] [CrossRef]
- Kouneiher, F.; Charron, S.; Koechlin, E. Motivation and cognitive control in the human prefrontal cortex. Nat. Neurosci. 2009, 12, 939–945. [Google Scholar] [CrossRef] [PubMed]
- Kang, J.Y.; Lee, J.S.; Kang, H.; Lee, H.W.; Kim, Y.K.; Jeon, H.J.; Chung, J.K.; Lee, M.C.; Cho, M.J.; Lee, D.S. Regional cerebral blood flow abnormalities associated with apathy and depression in Alzheimer disease. Alzheimer Dis. Assoc. Disord. 2012, 26, 217–224. [Google Scholar] [CrossRef]
- Alfano, V.; Longarzo, M.; Mele, G.; Esposito, M.; Aiello, M.; Salvatore, M.; Grossi, D.; Cavaliere, C. Identifying a Common Functional Framework for Apathy Large-Scale Brain Network. J. Pers. Med. 2021, 11, 679. [Google Scholar] [CrossRef]
- D’Amelio, M.; Puglisi-Allegra, S.; Mercuri, N. The role of dopaminergic midbrain in Alzheimer’s disease: Translating basic science into clinical practice. Pharmacol. Res. 2018, 130, 414–419. [Google Scholar] [CrossRef]
- Lawrence, A.D.; Goerendt, I.K.; Brooks, D.J. Apathy blunts neural response to money in Parkinson’s disease. Soc. Neurosci. 2011, 6, 653–662. [Google Scholar] [CrossRef]
- Cho, S.S.; Ko, J.H.; Pellecchia, G.; Van Eimeren, T.; Cilia, R.; Strafella, A.P. Continuous theta burst stimulation of right dorsolateral prefrontal cortex induces changes in impulsivity level. Brain Stimul. 2010, 3, 170–176. [Google Scholar] [CrossRef] [Green Version]
- Rosen, H.J.; Wilson, M.R.; Schauer, G.F.; Allison, S.; Gorno-Tempini, M.L.; Pace-Savitsky, C.; Kramer, J.H.; Levenson, R.W.; Weiner, M.; Miller, B.L. Neuroanatomical correlates of impaired recognition of emotion in dementia. Neuropsychologia 2006, 44, 365–373. [Google Scholar] [CrossRef]
- Keszycki, R.M.; Fisher, D.W.; Dong, H. The Hyperactivity-Impulsivity-Irritiability-Disinhibition-Aggression-Agitation Domain in Alzheimer’s Disease: Current Management and Future Directions. Front. Pharmacol. 2019, 10, 1109. [Google Scholar] [CrossRef] [Green Version]
- Jung, M.S.; Lee, Y.M.; Park, J.M.; Lee, B.D.; Moon, E.S.; Jeong, H.J. Neuroanatomical Correlation of Agitation/Aggression in Alzheimer’s Disease. J. Korean Geriatr. Psychiatry 2013, 17, 69–73. [Google Scholar]
- Joseph, S.; Zomorrodi, R.; Ghazala, Z.; Knezevic, D.; Blumberger, D.M.; Daskalakis, Z.J.; Mulsant, B.H.; Pollock, B.G.; Rajji, T.K.; Kumar, S. Dorsolateral prefrontal cortex excitability assessed using TMS-EEG and its relationship with neuropsychiatric symptoms in Alzheimer’s dementia. Alzheimer’s Dement. 2020, 16, e042956. [Google Scholar] [CrossRef]
- Perez-Costas, E.; Melendez-Ferro, M.; Roberts, R.C. Basal ganglia pathology in schizophrenia: Dopamine connections and anomalies. J. Neurochem. 2010, 113, 287–302. [Google Scholar] [CrossRef]
- Sheffield, J.M.; Kandala, S.; Burgess, G.C.; Harms, M.P.; Barch, D.M. Cingulo-opercular network efficiency mediates the association between psychotic-like experiences and cognitive ability in the general population. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2016, 1, 498–506. [Google Scholar] [CrossRef] [Green Version]
- Harrison, B.J.; Yucel, M.; Shaw, M.; Brewer, W.J.; Nathan, P.J.; Strother, S.C.; Olver, J.S.; Egan, G.F.; Velakoulis, D.; McGorry, P.D.; et al. Dysfunction of dorsolateral prefrontal cortex in antipsychotic-naive schizophreniform psychosis. Psychiatry Res. 2006, 148, 23–31. [Google Scholar] [CrossRef]
- Kerns, J.G.; Cohen, J.D.; MacDonald, A.W., 3rd; Johnson, M.K.; Stenger, V.A.; Aizenstein, H.; Carter, C.S. Decreased conflict- and error-related activity in the anterior cingulate cortex in subjects with schizophrenia. Am. J. Psychiatry 2005, 162, 1833–1839. [Google Scholar] [CrossRef] [Green Version]
- Caligiuri, M.P.; Peavy, G. An instrumental study of the relationship between extrapyramidal signs and psychosis in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 34–39. [Google Scholar] [CrossRef]
- Holroyd, S.; Shepherd, M.L.; Downs, J.H., 3rd. Occipital atrophy is associated with visual hallucinations in Alzheimer’s disease. J. Neuropsychiatry Clin. Neurosci. 2000, 12, 25–28. [Google Scholar] [CrossRef]
- Jeste, D.V.; Finkel, S.I. Psychosis of Alzheimer’s disease and related dementias. Diagnostic criteria for a distinct syndrome. Am. J. Geriatr. Psychiatry 2000, 8, 29–34. [Google Scholar] [CrossRef]
- Collerton, D.; Perry, E.; McKeith, I. Why people see things that are not there: A novel Perception and Attention Deficit model for recurrent complex visual hallucinations. Behav. Brain Sci. 2005, 28, 737–757; discussion 757–794. [Google Scholar] [CrossRef] [Green Version]
| Domain | Frequency (%) † | Mean ± SD | Symptom | Frequency (%) † | Mean ± SD |
|---|---|---|---|---|---|
| Affective | 61.0 | 1.53 ± 1.86 | Anxiety | 30.5 | 0.61 ± 1.20 |
| Depression | 54.2 | 0.92 ± 1.22 | |||
| Apathy | 57.6 | 2.54 ± 3.54 | Apathy | 44.1 | 1.07 ± 1.81 |
| Eating abnormalities | 30.5 | 1.47 ± 2.81 | |||
| Hyperactivity | 67.8 | 2.71 ± 3.78 | Aberrant motor behavior | 17.0 | 0.76 ± 2.25 |
| Agitation | 18.6 | 0.29 ± 0.70 | |||
| Disinhibition | 23.7 | 0.46 ± 1.12 | |||
| Euphoria | 5.1 | 0.07 ± 0.31 | |||
| Irritability | 54.2 | 1.14 ± 1.57 | |||
| Psychosis | 32.2 | 1.02 ± 1.88 | Delusions | 11.9 | 0.25 ± 0.90 |
| Hallucinations | 5.1 | 0.05 ± 0.22 | |||
| Sleep disturbances | 25.4 | 0.71 ± 1.57 |
| Brain Regions | t | p | Cluster Size (Voxels) | MNI Coordinates |
|---|---|---|---|---|
| Positive correlations with the affective domain | ||||
| None | ||||
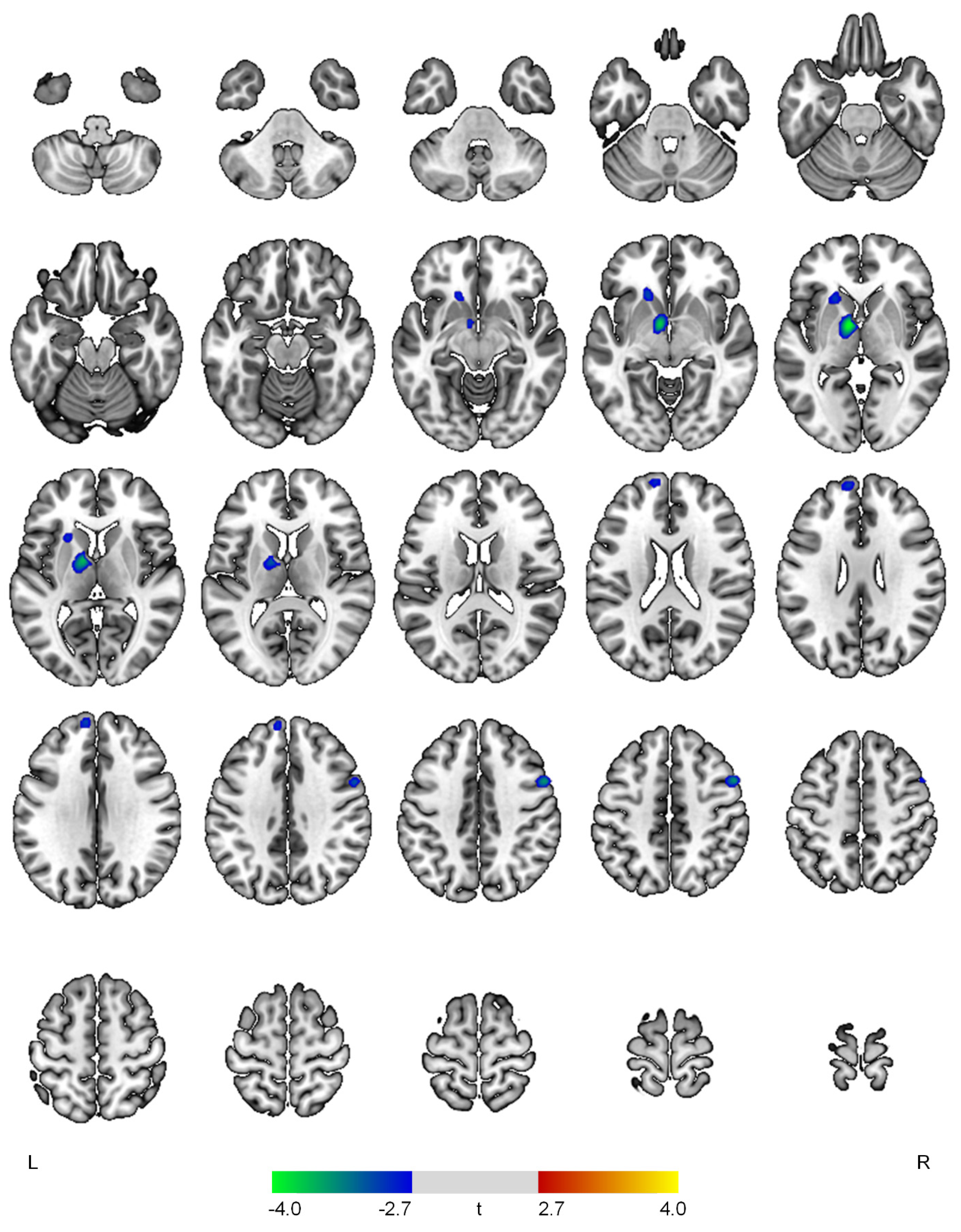
| Negative correlations with the affective domain | ||||
| L thalamus | 4.08 | <0.001 | 427 | −8, −2, −2 |
| R precentral gyrus | 3.59 | <0.001 | 172 | 54, 8, 42 |
| L superior frontal gyrus | 3.06 | 0.002 | 160 | −8, 56, 30 |
| L caudate | 3.05 | 0.002 | 165 | −18, 22, −4 |
| Positive correlations with the apathy domain | ||||
| None | ||||
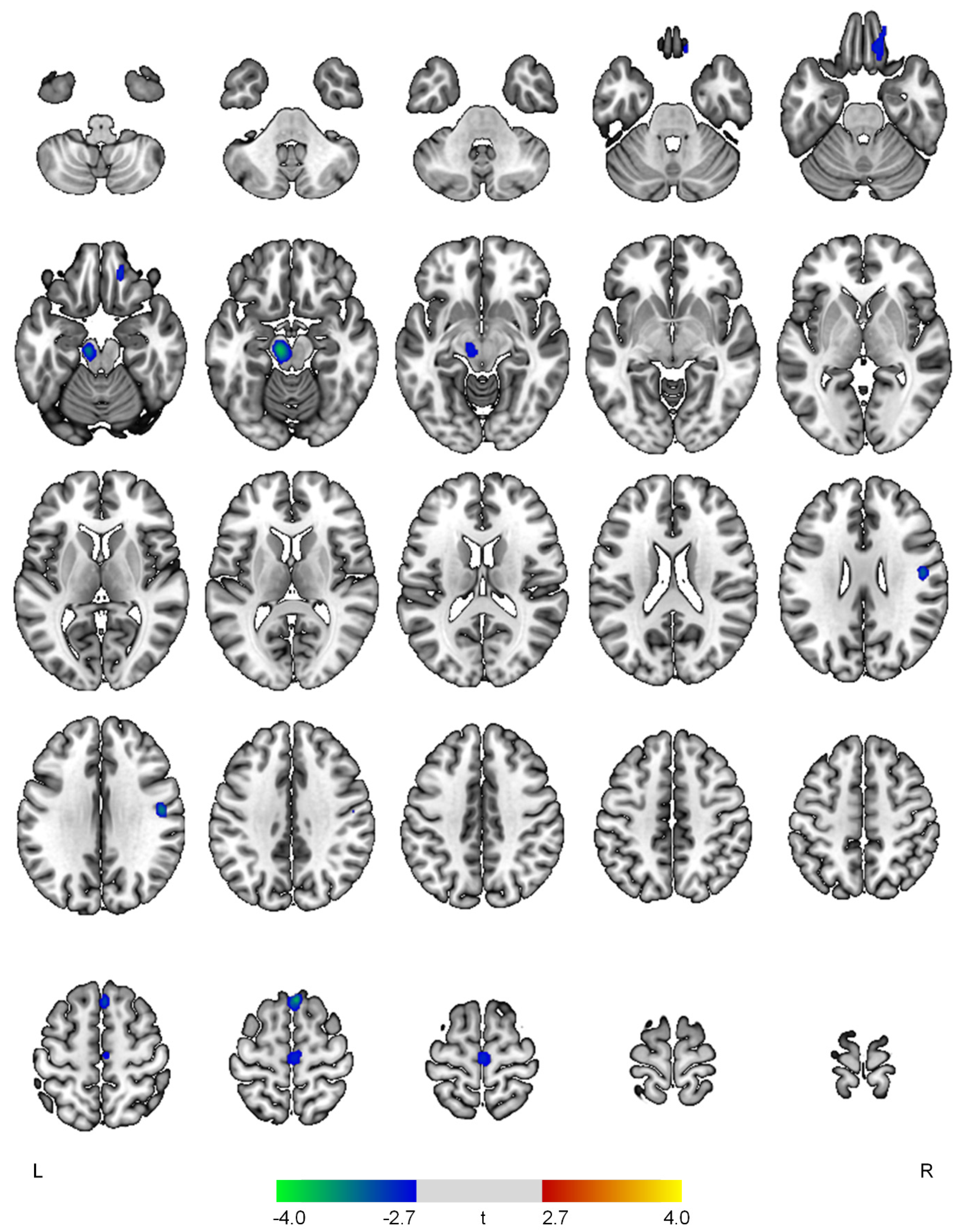
| Negative correlations with the apathy domain | ||||
| R superior frontal gyrus | 3.74 | <0.001 | 138 | 4, 26, 64 |
| L midbrain | 3.69 | <0.001 | 265 | −10, −20, −16 |
| R postcentral gyrus | 3.33 | 0.001 | 111 | 48, −10, 30 |
| R medial orbital gyrus | 3.01 | 0.002 | 177 | 16, 40, −22 |
| R precentral gyrus | 2.89 | 0.003 | 156 | 2, −20, 64 |
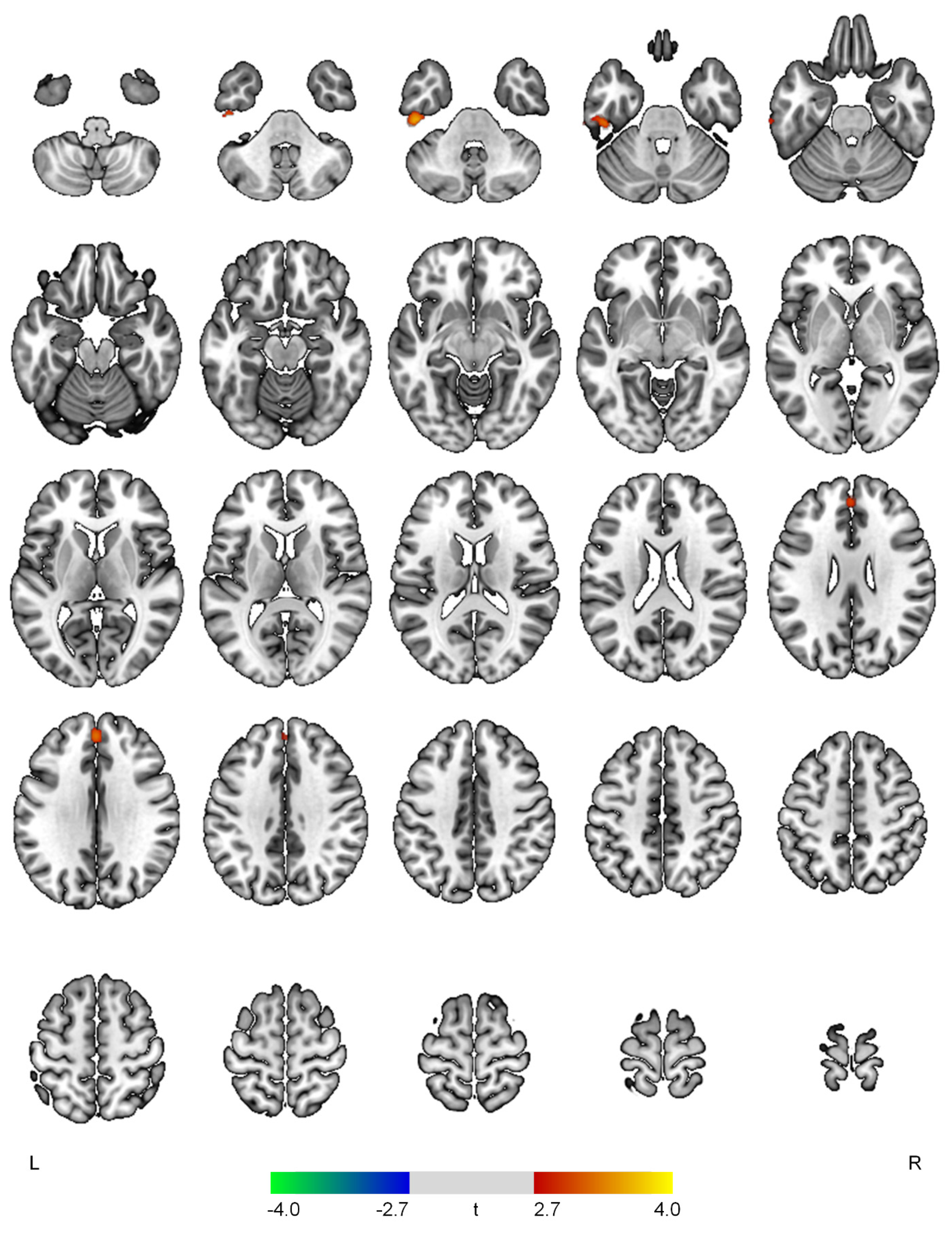
| Positive correlations with the hyperactivity domain | ||||
| L inferior temporal gyrus | 3.66 | <0.001 | 315 | −46, −26, −36 |
| Superior frontal gyrus | 3.30 | 0.001 | 171 | 0, 46, 32 |
| Negative correlations with the hyperactivity domain | ||||
| None | ||||
| Positive correlations with the psychosis domain | ||||
| R cuneus | 3.53 | <0.001 | 305 | 4, −76, 34 |
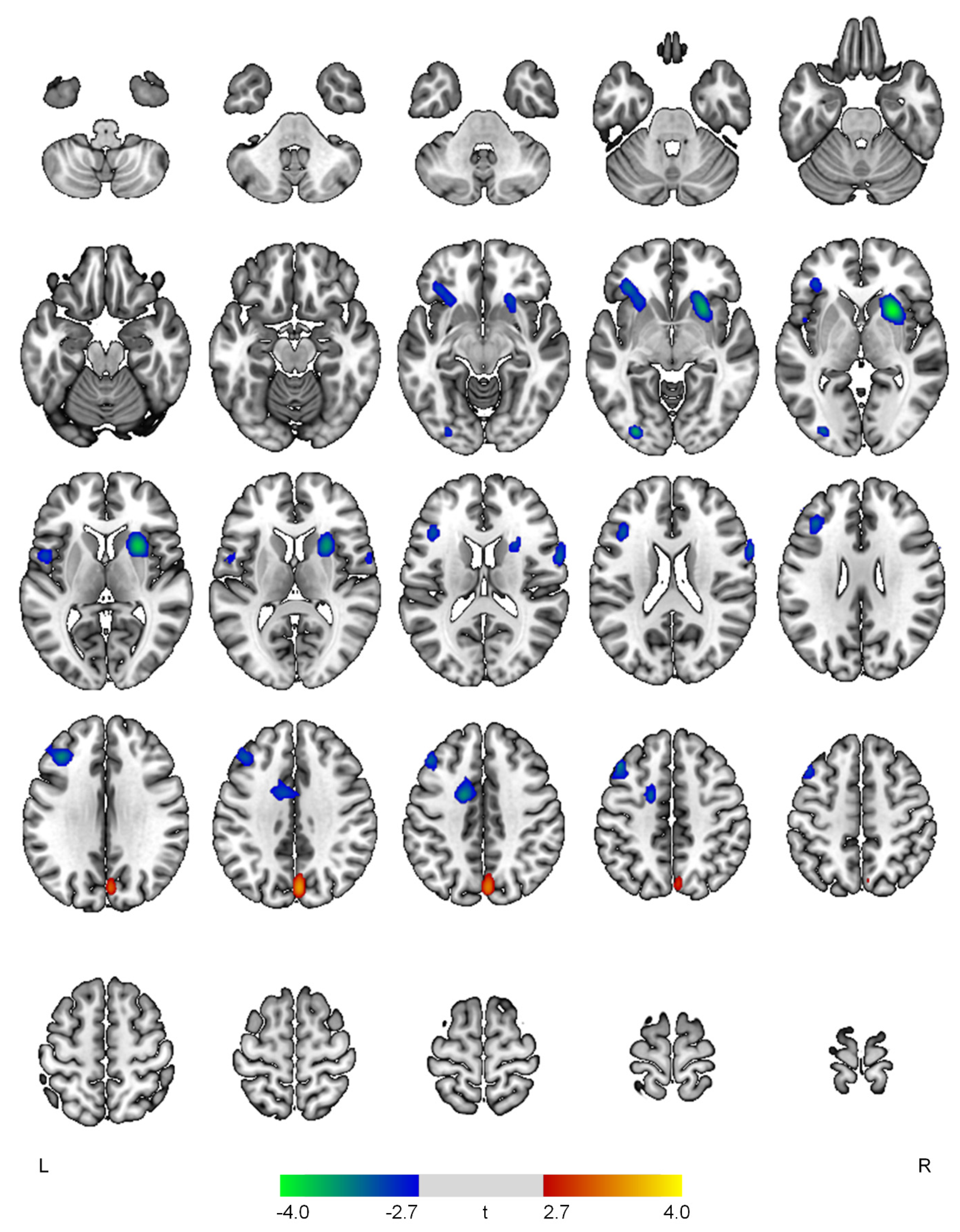
| Negative correlations with the psychosis domain | ||||
| R putamen | 4.06 | <0.001 | 921 | 26, 12, 0 |
| L inferior occipital gyrus | 3.64 | <0.001 | 131 | −30, −84, −4 |
| L middle frontal gyrus | 3.49 | 0.001 | 702 | −44, 24, 44 |
| L anterior cingulate gyrus | 3.46 | 0.001 | 350 | −14, 0, 42 |
| L inferior frontal gyrus | 3.30 | 0.001 | 368 | −34, 30, −6 |
| R precentral gyrus | 3.20 | 0.001 | 191 | 64, 10, 20 |
| L central operculum | 3.04 | 0.002 | 118 | −50, 4, 6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, H.; Kang, I.; Park, J.-S.; Na, S.-H.; Kim, S.; Yoon, S.; Song, I.-U.; Chung, Y.-A. Regional Cerebral Blood Flow Correlates of Neuropsychiatric Symptom Domains in Early Alzheimer’s Disease. Diagnostics 2022, 12, 1246. https://doi.org/10.3390/diagnostics12051246
Jeong H, Kang I, Park J-S, Na S-H, Kim S, Yoon S, Song I-U, Chung Y-A. Regional Cerebral Blood Flow Correlates of Neuropsychiatric Symptom Domains in Early Alzheimer’s Disease. Diagnostics. 2022; 12(5):1246. https://doi.org/10.3390/diagnostics12051246
Chicago/Turabian StyleJeong, Hyeonseok, Ilhyang Kang, Jong-Sik Park, Seung-Hee Na, Seunghee Kim, Sujung Yoon, In-Uk Song, and Yong-An Chung. 2022. "Regional Cerebral Blood Flow Correlates of Neuropsychiatric Symptom Domains in Early Alzheimer’s Disease" Diagnostics 12, no. 5: 1246. https://doi.org/10.3390/diagnostics12051246
APA StyleJeong, H., Kang, I., Park, J.-S., Na, S.-H., Kim, S., Yoon, S., Song, I.-U., & Chung, Y.-A. (2022). Regional Cerebral Blood Flow Correlates of Neuropsychiatric Symptom Domains in Early Alzheimer’s Disease. Diagnostics, 12(5), 1246. https://doi.org/10.3390/diagnostics12051246







