Endoscopic Management of Biliary Strictures after Orthotopic Liver Transplantation: A Single Center Experience Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
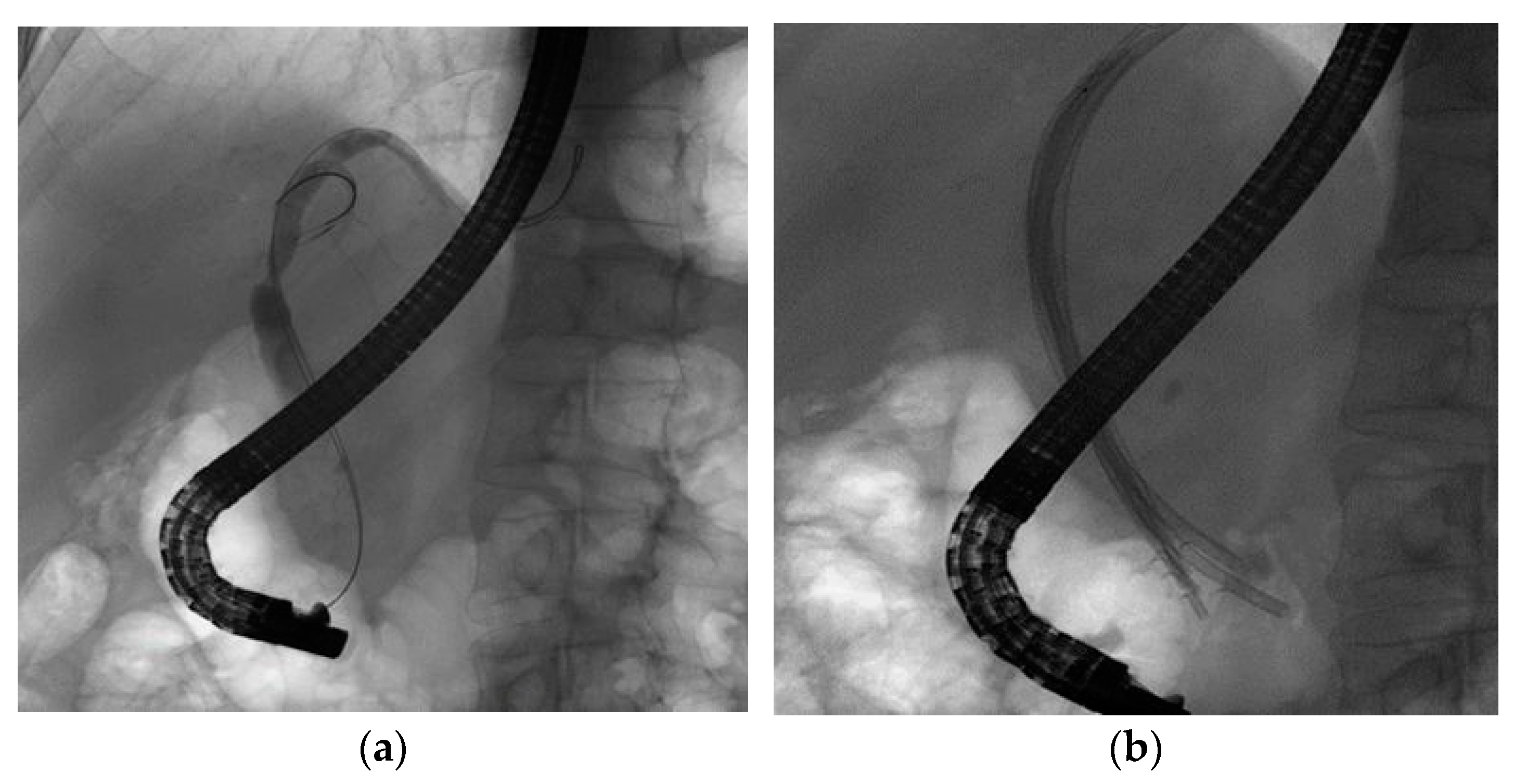
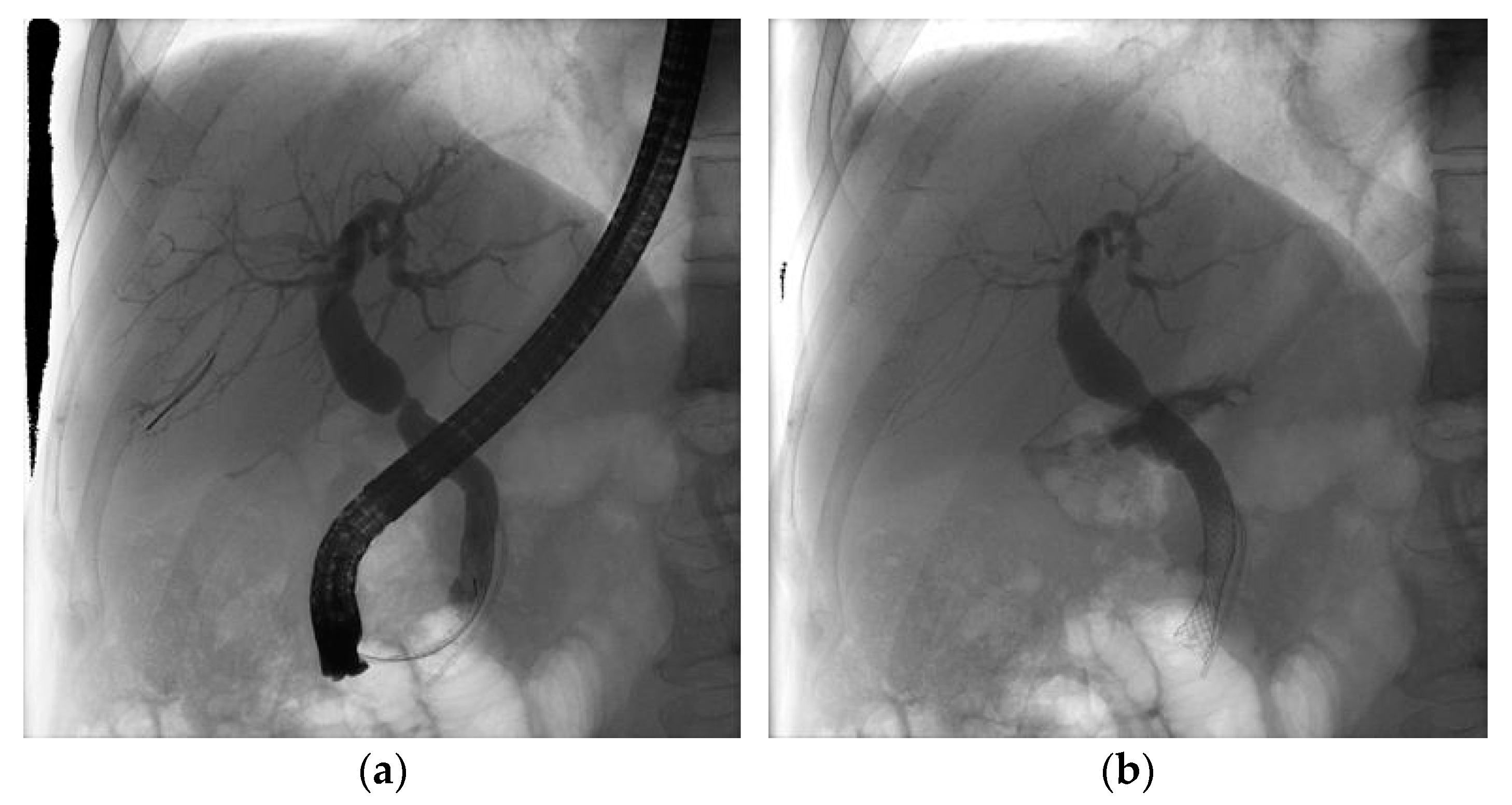
2.2. Procedure
2.3. Outcomes
2.4. Statistical Analysis
3. Results
3.1. Patients’ Characteristics
3.2. Procedures’ Outcomes
3.3. Complications
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lee, H.W.; Shah, N.H.; Lee, S.K. An update on endoscopic management of post-liver transplant biliary complications. Clin. Endosc. 2017, 50, 451–463. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moy, B.T.; Birk, J.W. A Review on the Management of Biliary Complications after Orthotopic Liver Transplantation. J. Clin. Transl. Hepatol. 2019, 7, 61–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koksal, A.S.; Eminler, A.T.; Parlak, E.; Gurakar, A. Management of biliary anastomotic strictures after liver transplantation. Transpl. Rev. (Orlando) 2017, 31, 207–217. [Google Scholar] [CrossRef] [PubMed]
- Yang, Q.; Liu, J.; Ma, W.; Wang, J.; Li, F.; Bramer, W.M.; Peppelenbosch, M.P.; Pan, Q. Efficacy of Different Endoscopic Stents in the Management of Postoperative Biliary Strictures: A Systematic Review and Meta-analysis. J. Clin. Gastroenterol. 2019, 53, 418–426. [Google Scholar] [CrossRef]
- Chang, J.H.; Lee, I.; Choi, M.G.; Han, S.W. Current diagnosis and treatment of benign biliary strictures after living donor liver transplantation. World J. Gastroenterol. 2016, 22, 1593–1606. [Google Scholar] [CrossRef]
- Dai, S.C.; Goldberg, D.; Agarwal, A.; Ma, G.K.; Yam, C.; Ahmad, N.A.; Ginsberg, G.G.; Jaffe, D.L.; Kochman, M.L.; Olthoff, K.M.; et al. Endoscopic Therapy is Effective for Recurrent Anastomotic Biliary Strictures after Orthotopic Liver Transplantation. Ann. Hepatol. 2017, 16, 924–931. [Google Scholar] [CrossRef]
- Landi, F.; de’Angelis, N.; Sepulveda, A.; Martínez-Pérez, A.; Sobhani, I.; Laurent, A.; Soubrane, O. Endoscopic treatment of anastomotic biliary stricture after adult deceased donor liver transplantation with multiple plastic stents versus self-expandable metal stents: A systematic review and meta-analysis. Transpl Int. 2018, 31, 131–151. [Google Scholar] [CrossRef] [Green Version]
- Martins, F.P.; De Paulo, G.A.; Contini, M.L.C.; Ferrari, A.P. Metal versus plastic stents for anastomotic biliary stricturesafter liver transplantation: A randomized controlled trial. Gastrointest. Endosc. 2018, 87, 131.e1–131.e13. [Google Scholar] [CrossRef]
- García-Pajares, F.; Sánchez-Antolín, G.; Pelayo, S.L.; Gómez de la Cuesta, S.; Herranz Bachiller, M.T.; Pérez-Miranda, M.; de La Serna, C.; Vallecillo Sande, M.A.; Alcaide, N.; Llames, R.V.; et al. Covered metal stents for the treatment of biliary complications after orthotopic liver transplantation. Transplant Proc. 2010, 42, 2966–2969. [Google Scholar] [CrossRef]
- Tee, H.P.; James, M.W.; Kaffes, A.J. Placement of removable metal biliary stent in post-orthotopic liver transplantation anastomotic stricture. World J. Gastroenterol. 2010, 16, 3597–3600. [Google Scholar] [CrossRef]
- Tal, A.O.; Finkelmeier, F.; Filmann, N.; Kylänpää, L.; Udd, M.; Parzanese, I.; Cantù, P.; Dechêne, A.; Penndorf, V.; Schnitzbauer, A.; et al. Multiple plastic stents versus covered metal stent for treatment of anastomotic biliary strictures after liver transplantation: A prospective, randomized, multicenter trial. Gastrointest. Endosc. 2017, 86, 1038–1045. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Macías Gómez, C.M.; Dumonceau, J.-M.; Marcolongo, M.; de Santibañes, E.; Ciardullo, M.; Pekolj, J.; Palavecino, M.; Gadano, A.; Dávolos, J. Endoscopic management of biliary complications after adult living-donor versus deceased-donor liver transplantation. Transplantation 2009, 88, 1280–1285. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kahaleh, M.; Behm, B.; Clarke, B.W.; Brock, A.; Shami, V.M.; De La Rue, S.A.; Sundaram, V.; Tokar, J.; Adams, R.B.; Yeaton, P. Temporary placement of covered self-expandable metal stents in benign biliary strictures: A new paradigm? (with video). Gastrointest. Endosc. 2008, 67, 446–454. [Google Scholar] [CrossRef] [PubMed]
- Martins, F.P.; De Paulo, G.A.; Conceição, R.D.; Zurstrassen, M.P.; Thomé, T.; Ferraz-Neto, B.H.; Ferrari, A.P. Incidence, risk factors and ERCP outcome for biliary complications after cadaveric OLT. Hepatogastroenterology 2011, 58, 732–737. [Google Scholar] [PubMed]
- Costamagna, G.; Tringali, A.; Mutignani, M.; Perri, V.; Spada, C.; Pandolfi, M.; Galasso, D. Endotherapy of postoperative biliary strictures with multiple stents: Results after more than 10 years of follow-up. Gastrointest. Endosc. 2010, 72, 551–557. [Google Scholar] [CrossRef] [PubMed]
- Facciorusso, A.; Rosca, E.C.; Ashimi, A.; Ugoeze, K.C.; Pathak, U.; Infante, V.; Muscatiello, N. Management of anastomotic biliary stricture after liver transplantation: Metal versus plastic stent. Ann. Gastroenterol. 2018, 31, 728–734. [Google Scholar] [CrossRef] [PubMed]
- Larghi, A.; Tringali, A.; Rimbaş, M.; Barbaro, F.; Perri, V.; Rizzatti, G.; Gasbarrini, A.; Costamagna, G. Endoscopic Management of Benign Biliary Strictures After Liver Transplantation. Liver Transpl. 2019, 25, 323–335. [Google Scholar] [CrossRef]
- Coté, G.A.; Slivka, A.; Tarnasky, P.; Mullady, D.K.; Elmunzer, B.J.; Elta, G.; Fogel, E.; Lehman, G.; McHenry, L.; Romagnuolo, J.; et al. Effect of Covered Metallic Stents Compared with Plastic Stents on Benign Biliary Stricture Resolution: A Randomized Clinical Trial. JAMA 2016, 315, 1250–1257. [Google Scholar] [CrossRef] [Green Version]
- Kao, D.; Zepeda-Gomez, S.; Tandon, P.; Bain, V.G. Managing the post-liver transplantation anastomotic biliary stricture: Multiple plastic versus metal stents: A systematic review. Gastrointest. Endosc. 2013, 77, 679–691. [Google Scholar] [CrossRef]
- Aparicio, D.; Otoch, J.P.; Montero, E.F.S.; Khan, M.A.; Artifon, E.L.A. Endoscopic approach for management of biliary strictures in liver transplant recipients: A systematic review and meta-analysis. United Eur. Gastroenterol. J. 2017, 5, 827–845. [Google Scholar] [CrossRef] [Green Version]
- Tringali, A.; Tarantino, I.; Barresi, L.; Traina, M.; Bonato, G.; Cintolo, M.; Hassan, C.; Mutignani, M.; Adler, D.G. Multiple plastic versus fully covered metal stents for managing post-liver transplantation anastomotic biliary strictures: A meta-analysis of randomized controlled trials. Ann. Gastroenterol. 2019, 32, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Bartel, M.J.; Higa, J.T.; Tokar, J.L. The Status of SEMS Versus Plastic Stents for Benign Biliary Strictures. Curr. Gastroenterol. Rep. 2019, 21, 29. [Google Scholar] [CrossRef] [PubMed]
- Poley, J.W.; Ponchon, T.; Puespoek, A.; Bruno, M.; Roy, A.; Peetermans, J.; Rousseau, M.; Lépilliez, V.; Dolak, W.; Tringali, A.; et al. Fully covered self-expanding metal stents for benign biliary stricture after orthotopic liver transplant: 5-year outcomes. Gastrointest. Endosc. 2020, 92, 1216–1224. [Google Scholar] [CrossRef] [PubMed]
- Alazmi, W.M.; Fogel, E.L.; Watkins, J.L.; McHenry, L.; Tector, J.A.; Fridell, J.; Mosler, P.; Sherman, S.; Lehman, G.A. Recurrence rate of anastomotic biliary strictures in patients who have had previous successful endoscopic therapy for anastomotic narrowing after orthotopic liver transplantation. Endoscopy 2006, 38, 571–574. [Google Scholar] [CrossRef]
- Sharma, S.; Gurakar, A.; Jabbour, N. Biliary strictures following liver transplantation: Past, present and preventive strategies. Liver Transpl. 2008, 14, 759–769. [Google Scholar] [CrossRef]
- Koizumi, M.; Kumagi, T.; Kuroda, T.; Imamura, Y.; Kanemitsu, K.; Ogawa, K.; Takada, Y.; Hiasa, Y. Endoscopic stent placement above the sphincter of Oddi for biliary strictures after living donor liver transplantation. BMC Gastroenterol. 2020, 20, 92. [Google Scholar] [CrossRef] [Green Version]
- Kaffes, A.; Griffin, S.; Vaughan, R.; James, M.; Chua, T.; Tee, H.; Dinesen, L.; Corte, C.; Gill, R. A randomized trial of a fully covered self-expandable metallic stent versus plastic stents in anastomotic biliary strictures after liver transplantation. Therap. Adv. Gastroenterol. 2014, 7, 64–71. [Google Scholar] [CrossRef] [Green Version]
- Haapamäki, C.; Udd, M.; Halttunen, J.; Lindström, O.; Mäkisalo, H.; Kylänpää, L. Endoscopic treatment of anastomotic biliary complications after liver transplantation using removable, covered, self-expandable metallic stents. Scand. J. Gastroenterol. 2012, 47, 116–121. [Google Scholar] [CrossRef]
- Lee, D.W.; Hara, K. Management of Post-Transplant Anastomotic Stricture Using Self-Expandable Metal Stent. Clin. Endosc. 2020, 53, 261–265. [Google Scholar] [CrossRef]
- Almedia, G.; Donato, P. Biodegradable versus multiple plastic stent implantation in benign biliary strictures: A systematic review and met-analysis. Eur. J. Radiol. 2020, 125, 108899. [Google Scholar] [CrossRef]
| Plastic Stents | Metal Stents | ||
|---|---|---|---|
| Variables | Mean (±Standard Deviation)/ Number (Percentage) | Mean (±Standard Deviation)/ Number (Percentage) | p-Value |
| Age | 53.17 (±12.36) | 60.67 (±10.46) | 0.074 |
| Gender | F (n = 10, 33.3%) | F (n = 1, 16.7%) | 0.643 |
| B (n = 20, 66.7%) | B (n = 5, 83.3%) |
| Indication for Liver Transplant | Number of Patients According to Type of Stent, n (%) | ||
|---|---|---|---|
| Plastic Stents | Metal Stents | Total | |
| Alcoholic liver cirrhosis | 7 (25.9%) | 3 (33%) | 10 (27.7%) |
| Liver cirrhosis HBV + HDV | 4 (15%) | 0 | 4 (11.4%) |
| Liver cirrhosis HCV | 4 (15%) | 2 (22%) | 6 (17%) |
| Cryptogenic hepatic cirrhosis | 2 (7.7%) | 1 (11%) | 3 (8.6%) |
| Liver cirrhosis HBV | 3 (11.5%) | 0 | 3 (8.6%) |
| HCC graft on liver cirrhosis HBV + HDV | 2 (7.7%) | 2 (22%) | 4 (11.5%) |
| Budd Chiari Syndrome | 1 (3.9%) | 0 | 1 (2.8%) |
| Autoimmune liver cirrhosis | 0 | 1 (11%) | 1 (2.8%) |
| Caroli Syndrome | 1 (3.9%) | 0 | 1 (2.8%) |
| Liver cirrhosis HBV + HCV | 1 (3.9%) | 0 | 1 (2.8%) |
| Liver cirrhosis HBV+ Alcoholic liver cirrhosis | 1 (3.9%) | 0 | 1 (2.8%) |
| HCC graft on liver cirrhosis HBV | 1 (3.9%) | 0 | 1 (2.8%) |
| Total | 27 | 9 | 36 |
| MPS | FCSEMS | p-Value | |
|---|---|---|---|
| Total number of ERCP procedures | 87 | 19 | |
| Number of ERCP per patient | |||
| Mean (±SD) | 3.34 (±1.46) | 2.11 (±0.33) | <0.001 |
| Median | 3 | 2 | |
| Range | 2–8 | 2–3 | |
| Total number of stents | 149 | 10 | |
| Number of stents per patient | |||
| Mean (±SD) | 5.73 (±2.64) | 1.16 (±0.40) | <0.001 |
| Median | 6 | 1 | |
| Range | 3–13 | 1–2 | |
| Dilation | 0.317 | ||
| Yes | 20 (77%) | 4 (44%) | |
| No | 6 (23%) | 5 (45%) | |
| Difficult cannulation | 0.391 | ||
| Yes | 18 (70%) | 4 (44%) | |
| No | 8 (30%) | 5 (45%) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sandru, V.; Stan-Ilie, M.; Plotogea, O.-M.; Vladut, C.; Ungureanu, B.S.; Balan, G.G.; Gheonea, D.I.; Constantinescu, G. Endoscopic Management of Biliary Strictures after Orthotopic Liver Transplantation: A Single Center Experience Study. Diagnostics 2022, 12, 1221. https://doi.org/10.3390/diagnostics12051221
Sandru V, Stan-Ilie M, Plotogea O-M, Vladut C, Ungureanu BS, Balan GG, Gheonea DI, Constantinescu G. Endoscopic Management of Biliary Strictures after Orthotopic Liver Transplantation: A Single Center Experience Study. Diagnostics. 2022; 12(5):1221. https://doi.org/10.3390/diagnostics12051221
Chicago/Turabian StyleSandru, Vasile, Madalina Stan-Ilie, Oana-Mihaela Plotogea, Catalina Vladut, Bogdan Silviu Ungureanu, Gheorghe G. Balan, Dan Ionut Gheonea, and Gabriel Constantinescu. 2022. "Endoscopic Management of Biliary Strictures after Orthotopic Liver Transplantation: A Single Center Experience Study" Diagnostics 12, no. 5: 1221. https://doi.org/10.3390/diagnostics12051221
APA StyleSandru, V., Stan-Ilie, M., Plotogea, O.-M., Vladut, C., Ungureanu, B. S., Balan, G. G., Gheonea, D. I., & Constantinescu, G. (2022). Endoscopic Management of Biliary Strictures after Orthotopic Liver Transplantation: A Single Center Experience Study. Diagnostics, 12(5), 1221. https://doi.org/10.3390/diagnostics12051221








