Brain Death and Its Prediction in Out-of-Hospital Cardiac Arrest Patients Treated with Targeted Temperature Management
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. Postcardiac Arrest Care
2.3. Analyses of Outcome Predictors
2.4. Declaration of BD and Organ Donation (OD)
2.5. Statistical Analysis
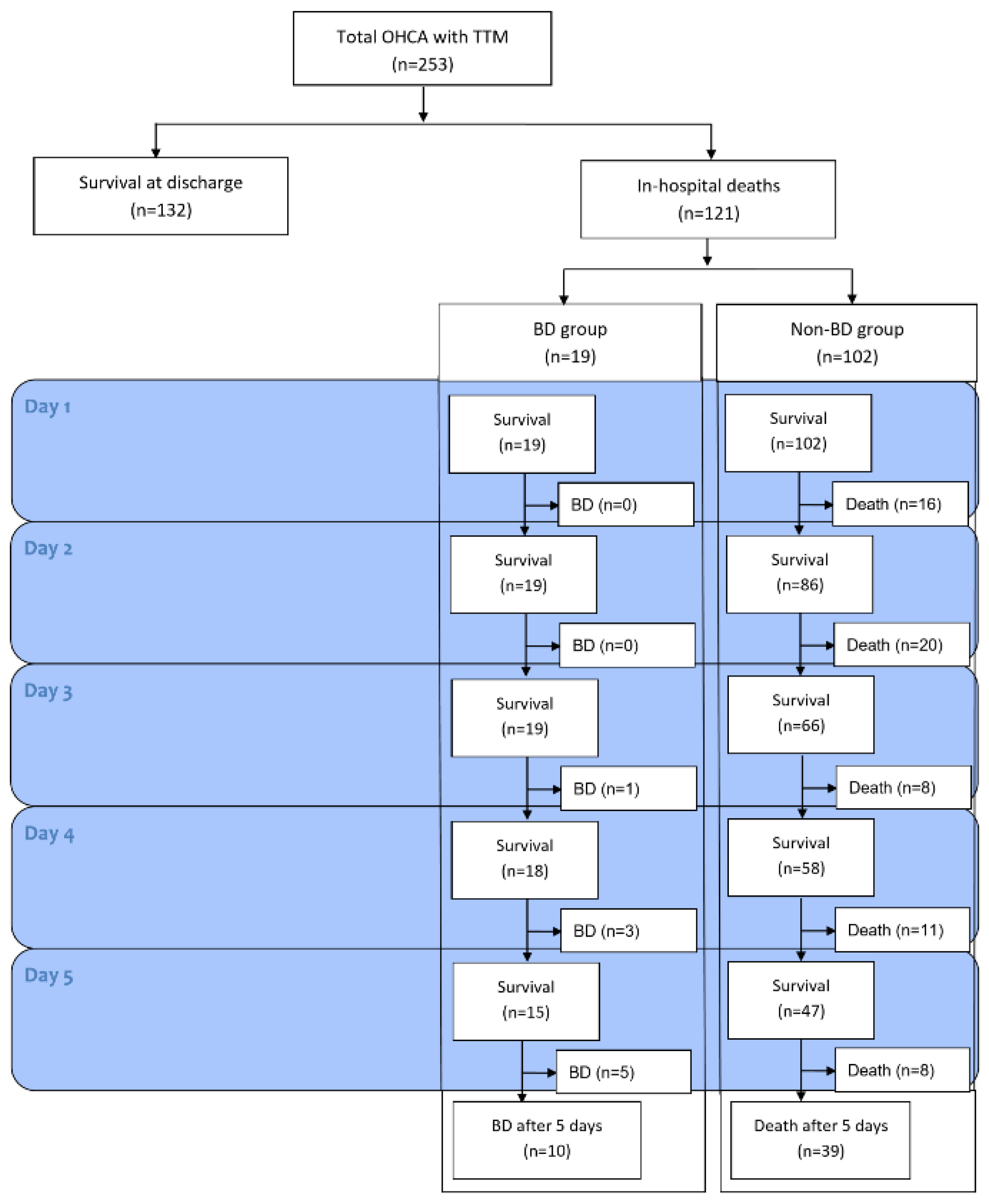
3. Results
3.1. Baseline Characteristics of Participants
3.2. Clinical Course of Patients with BD
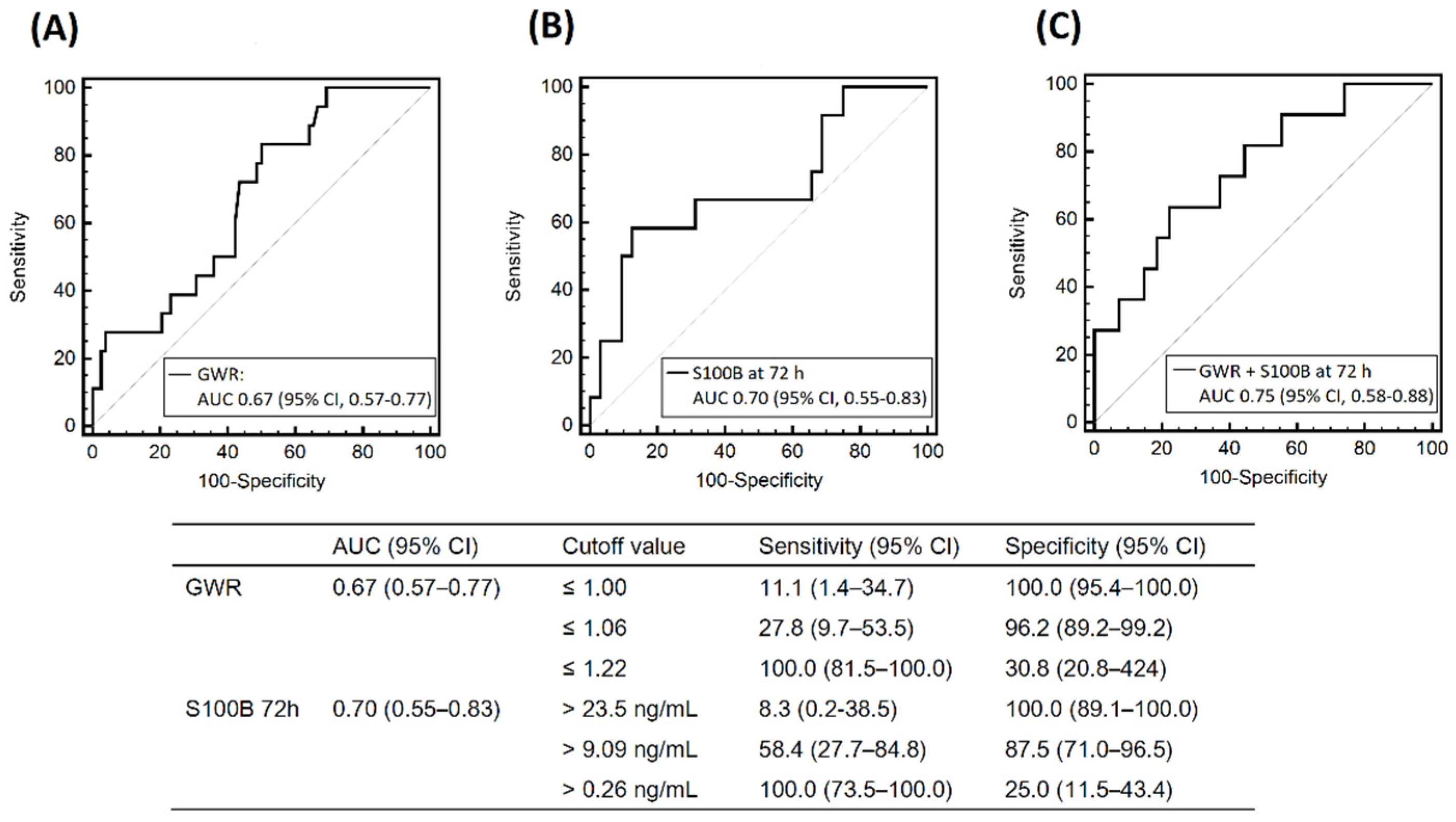
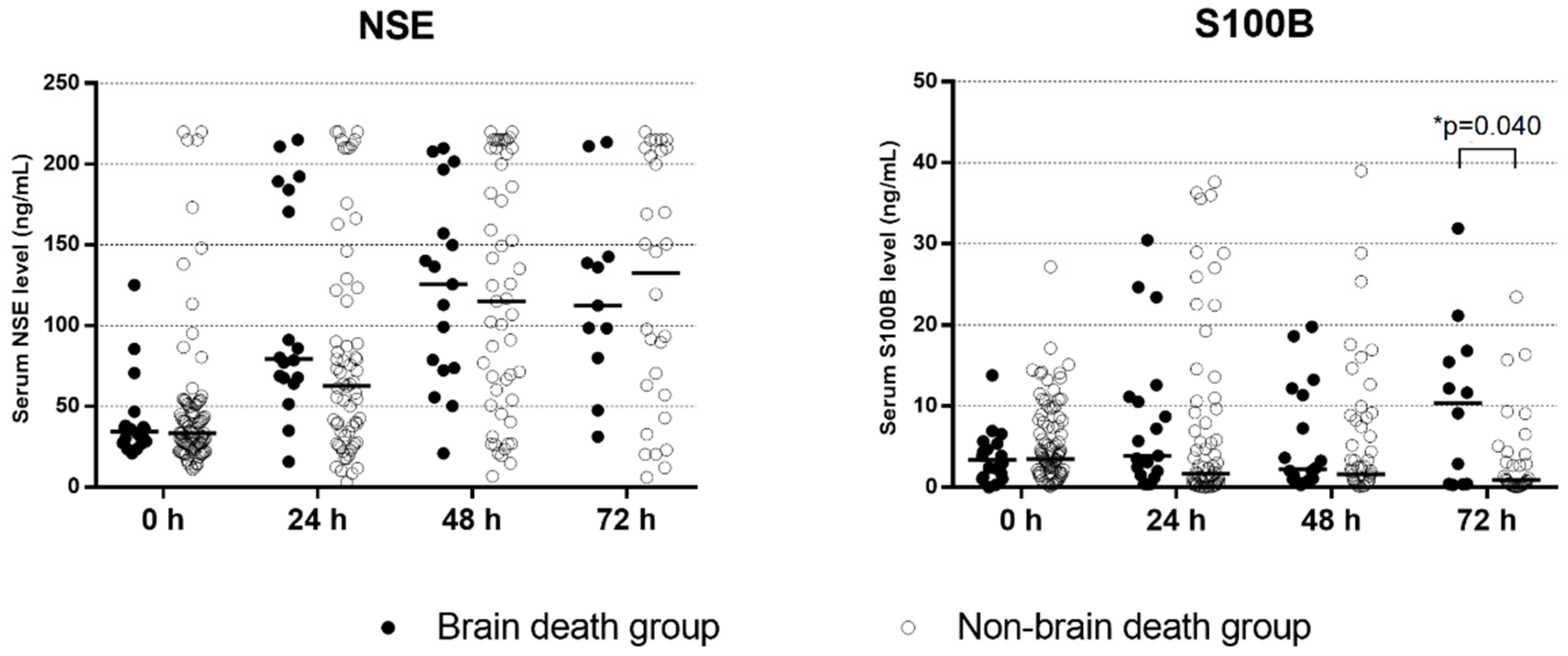
3.3. Results of other Prognostic Tests
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Dankiewicz, J.; Cronberg, T.; Lilja, G.; Jakobsen, J.C.; Levin, H.; Ullen, S.; Rylander, C.; Wise, M.P.; Oddo, M.; Cariou, A.; et al. Hypothermia versus normothermia after out-of-hospital cardiac arrest. N. Engl. J. Med. 2021, 384, 2283–2294. [Google Scholar] [CrossRef]
- Jentzer, J.C.; Scutella, M.; Pike, F.; Fitzgibbon, J.; Krehel, N.M.; Kowalski, L.; Callaway, C.W.; Rittenberger, J.C.; Reynolds, J.C.; Barsness, G.W.; et al. Early coronary angiography and percutaneous coronary intervention are associated with improved outcomes after out of hospital cardiac arrest. Resuscitation 2018, 123, 15–21. [Google Scholar] [CrossRef]
- Fugate, J.E.; Brinjikji, W.; Mandrekar, J.N.; Cloft, H.J.; White, R.D.; Wijdicks, E.F.M.; Rabinstein, A.A. Post-cardiac arrest mortality is declining a study of the US national inpatient sample 2001 to 2009. Circulation 2012, 126, 546–550. [Google Scholar] [CrossRef] [Green Version]
- Nolan, J.P.; Laver, S.R.; Welch, C.A.; Harrison, D.A.; Gupta, V.; Rowan, K. Outcome following admission to UK intensive care units after cardiac arrest: A secondary analysis of the ICNARC Case Mix Programme Database. Anaesthesia 2007, 62, 1207–1216. [Google Scholar] [CrossRef]
- Witten, L.; Gardner, R.; Holmberg, M.J.; Wiberg, S.; Moskowitz, A.; Mehta, S.; Grossestreuer, A.V.; Yankama, T.; Donnino, M.W.; Berg, K.M. Reasons for death in patients successfully resuscitated from out-of-hospital and in-hospital cardiac arrest. Resuscitation 2019, 136, 93–99. [Google Scholar] [CrossRef] [PubMed]
- Lee, B.K.; Park, K.N.; Kang, G.H.; Kim, K.H.; Kim, G.; Kim, W.Y.; Min, J.H.; Park, Y.; Park, J.B.; Suh, G.J.; et al. Outcome and current status of therapeutic hypothermia after out-of-hospital cardiac arrest in Korea using data from the Korea Hypothermia Network registry. Clin. Exp. Emerg. Med. 2014, 1, 19–27. [Google Scholar] [CrossRef] [PubMed]
- Elmer, J.; Callaway, C.W. The Brain after cardiac arrest. Semin. Neurol. 2017, 37, 19–24. [Google Scholar] [CrossRef] [Green Version]
- Jang, J.; Oh, S.H.; Nam, Y.; Lee, K.; Choi, H.S.; Jung, S.L.; Ahn, K.J.; Park, K.N.; Kim, B.S. Prognostic value of phase information of 2D T2*-weighted gradient echo brain imaging in cardiac arrest survivors: A preliminary study. Resuscitation 2019, 140, 142–149. [Google Scholar] [CrossRef]
- Sandroni, C.; D’Arrigo, S.; Callaway, C.W.; Cariou, A.; Dragancea, I.; Taccone, F.S.; Antonelli, M. The rate of brain death and organ donation in patients resuscitated from cardiac arrest: A systematic review and meta-analysis. Intensive Care Med. 2016, 42, 1661–1671. [Google Scholar] [CrossRef]
- Orioles, A.; Morrison, W.E.; Rossano, J.W.; Shore, P.M.; Hasz, R.D.; Martiner, A.C.; Berg, R.A.; Nadkarni, V.M. An under-recognized benefit of cardiopulmonary resuscitation: Organ transplantation. Crit. Care Med. 2013, 41, 2794–2799. [Google Scholar] [CrossRef]
- Faucher, A.; Savary, D.; Jund, J.; Dorez, D.; Debaty, G.; Gaillard, A.; Atchabahian, A.; Tazarourte, K. Out-of-hospital traumatic cardiac arrest: An underrecognized source of organ donors. Transpl. Int. 2014, 27, 42–48. [Google Scholar] [CrossRef] [PubMed]
- West, S.; Soar, J.; Callaway, C.W. The viability of transplanting organs from donors who underwent cardiopulmonary resuscitation: A systematic review. Resuscitation 2016, 108, 27–33. [Google Scholar] [CrossRef] [PubMed]
- Callaway, C.W.; Donnino, M.W.; Fink, E.L.; Geocadin, R.G.; Golan, E.; Kern, K.B.; Leary, M.; Meurer, W.J.; Peberdy, M.A.; Thompson, T.M.; et al. Part 8: Post-cardiac arrest care: 2015 American Heart Association guidelines update for cardiopulmonary resuscitation and emergency cardiovascular care. Circulation 2015, 132, S465–S482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nolan, J.P.; Sandroni, C.; Böttiger, B.W.; Cariou, A.; Cronberg, T.; Friberg, H.; Genbrugge, C.; Haywood, K.; Lilja, G.; Moulaert, V.R.M.; et al. European Resuscitation Council and European Society of Intensive Care Medicine guidelines 2021: Post-resuscitation care. Intensive Care Med. 2021, 47, 369–421. [Google Scholar] [CrossRef] [PubMed]
- Serri, K.; Marsolais, P. End-of-life issues in cardiac critical care: The option of organ donation. Can. J. Cardiol. 2017, 33, 128–134. [Google Scholar] [CrossRef]
- Adrie, C.; Haouache, H.; Saleh, M.; Memain, N.; Laurent, I.; Thuong, M.; Darques, L.; Guerrini, P.; Monchi, M. An underrecognized source of organ donors: Patients with brain death after successfully resuscitated cardiac arrest. Intensive Care Med. 2008, 34, 132–137. [Google Scholar] [CrossRef]
- Scarpino, M.; Lanzo, G.; Lolli, F.; Moretti, M.; Carrai, R.; Migliaccio, M.L.; Spalletti, M.; Bonizzoli, M.; Peris, A.; Amantini, A.; et al. Is brain computed tomography combined with somatosensory evoked potentials useful in the prediction of brain death after cardiac arrest? Neurophysiol. Clin. 2017, 47, 327–335. [Google Scholar] [CrossRef]
- Cour, M.; Turc, J.; Madelaine, T.; Argaud, L. Risk factors for progression toward brain death after out-of-hospital cardiac arrest. Ann. Intensive Care 2019, 9, 45. [Google Scholar] [CrossRef]
- Jouffroy, R.; Lamhaut, L.; Guyard, A.; Philippe, P.; An, K.; Spaulding, C.; Baud, F.; Carli, P.; Vivien, B. Early detection of brain death using the Bispectral Index (BIS) in patients treated by extracorporeal cardiopulmonary resuscitation (E-CPR) for refractory cardiac arrest. Resuscitation 2017, 120, 8–13. [Google Scholar] [CrossRef]
- Lee, S.Y.; Kim, W.J.; Kim, J.M.; Kim, J.; Park, S.; The Korean Society of Clinical Neurophysiology Education Committee. Electroencephalography for the diagnosis of brain death. Ann. Clin. Neurophysiol. 2017, 19, 118–124. [Google Scholar] [CrossRef] [Green Version]
- Vincent, J.L.; Moreno, R.; Takala, J.; Willatts, S.; DeMendonca, A.; Bruining, H.; Reinhart, C.K.; Suter, P.M.; Thijs, L.G. The SOFA (sepsis-related organ failure assessment) score to describe organ dysfunction/failure. Intensive Care Med. 1996, 22, 707–710. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.H.; Choi, S.P.; Park, K.N.; Youn, C.S.; Oh, S.H.; Choi, S.M. Early brain computed tomography findings are associated with outcome in patients treated with therapeutic hypothermia after out-of-hospital cardiac arrest. Scand. J. Trauma Resusc. Emerg. Med. 2013, 21, 57. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cronberg, T.; Rundgren, M.; Westhall, E.; Englund, E.; Siemund, R.; Rosen, I.; Widner, H.; Friberg, H. Neuron-specific enolase correlates with other prognostic markers after cardiac arrest. Neurology 2011, 77, 623–630. [Google Scholar] [CrossRef] [PubMed]
- Oh, S.H.; Park, K.N.; Choi, S.P.; Oh, J.S.; Youn, C.S.; Jeong, W.J.; Ryoo, S.M.; Lee, D.H.; Lee, K.H.; KORHN Investigators. Prognostic value of somatosensory evoked potential in cardiac arrest patients without withdrawal of life-sustaining therapy. Resuscitation 2020, 150, 154–161. [Google Scholar] [CrossRef] [PubMed]
- Greer, D.M.; Shemie, S.D.; Lewis, A.; Torrance, S.; Varelas, P.; Goldenberg, F.D.; Bernat, J.L.; Souter, M.; Topcuoglu, M.A.; Alexandrov, A.W.; et al. Determination of brain death/death by neurologic criteria: The world brain death project. JAMA 2020, 324, 1078–1097. [Google Scholar] [CrossRef]
- Park, J.; Kim, C.J. Recent decrease in organ donation from brain-dead potential organ donors in Korea and possible causes. J. Korean Med. Sci. 2020, 35, e94. [Google Scholar] [CrossRef] [Green Version]
- Nobile, L.; Taccone, F.S.; Szakmany, T.; Sakr, Y.; Jakob, S.M.; Pellis, T.; Antonelli, M.; Leone, M.; Wittebole, X.; Pickkers, P.; et al. The impact of extracerebral organ failure on outcome of patients after cardiac arrest: An observational study from the ICON database. Crit. Care 2016, 20, 368. [Google Scholar] [CrossRef] [Green Version]
- Sandroni, C.; Dell’anna, A.M.; Tujjar, O.; Geri, G.; Cariou, A.; Taccone, F.S. Acute kidney injury after cardiac arrest: A systematic review and meta-analysis of clinical studies. Minerva Anestesiol. 2016, 82, 989–999. [Google Scholar]
- De Groot, Y.J.; Jansen, N.E.; Bakker, J.; Kuiper, M.A.; Aerdts, S.; Maas, A.I.; Wijdicks, E.F.; van Leiden, H.A.; Hoitsma, A.J.; Kremer, B.H.; et al. Imminent brain death: Point of departure for potential heart-beating organ donor recognition. Intensive Care Med. 2010, 36, 1488–1494. [Google Scholar] [CrossRef] [Green Version]
- Bro-Jeppesen, J.; Annborn, M.; Hassager, C.; Wise, M.P.; Pelosi, P.; Nielsen, N.; Erlinge, D.; Wanscher, M.; Friberg, H.; Kjaergaard, J.; et al. Hemodynamics and vasopressor support during targeted temperature management at 33 °C versus 36 °C after out-of-hospital cardiac arrest: A post hoc study of the target temperature management trial. Crit. Care Med. 2015, 43, 318–327. [Google Scholar] [CrossRef] [Green Version]
- Bro-Jeppesen, J.; Kjaergaard, J.; Soholm, H.; Wanscher, M.; Lippert, F.K.; Moller, J.E.; Kober, L.; Hassager, C. Hemodynamics and vasopressor support in therapeutic hypothermia after cardiac arrest: Prognostic implications. Resuscitation 2014, 85, 664–670. [Google Scholar] [CrossRef] [PubMed]
- Heppekcan, D.; Ekin, S.; Qivi, M.; Tok, D.A. Impact of secondary insults in brain death after traumatic brain injury. Transpl. Proc. 2019, 51, 2186–2188. [Google Scholar] [CrossRef] [PubMed]
- Lascarrou, J.B.; Merdji, H.; Le Gouge, A.; Colin, G.; Grillet, G.; Girardie, P.; Coupez, E.; Dequin, P.F.; Cariou, A.; Boulain, T.; et al. Targeted temperature management for cardiac arrest with nonshockable rhythm. N. Engl. J. Med. 2019, 381, 2327–2337. [Google Scholar] [CrossRef]
- Hsu, C.H.; Haac, B.E.; Drake, M.; Bernard, A.C.; Aiolfi, A.; Inaba, K.; Hinson, H.E.; Agarwal, C.; Galante, J.; Tibbits, E.M.; et al. EAST Multicenter Trial on targeted temperature management for hanging-induced cardiac arrest. J. Trauma Acute Care 2018, 85, 37–47. [Google Scholar] [CrossRef]
- Sekhon, M.S.; Ainslie, P.N.; Griesdale, D.E. Clinical pathophysiology of hypoxic ischemic brain injury after cardiac arrest: A “two-hit” model. Crit. Care 2017, 21, 90. [Google Scholar] [CrossRef] [Green Version]
- Casadio, M.C.; Coppo, A.; Vargiolu, A.; Villa, J.; Rota, M.; Avalli, L.; Citerio, G. Organ donation in cardiac arrest patients treated with extracorporeal CPR: A single centre observational study. Resuscitation 2017, 118, 133–139. [Google Scholar] [CrossRef]
- Madelaine, T.; Cour, M.; Roy, P.; Vivien, B.; Charpentier, J.; Dumas, F.; Deye, N.; Bonnefoy, E.; Gueugniaud, P.Y.; Coste, J.; et al. Prediction of brain death after out-of-hospital cardiac arrest: Development and validation of the brain death after cardiac arrest score. Chest 2021, 160, 139–147. [Google Scholar] [CrossRef]
- Egea-Guerrero, J.J.; Revuelto-Rey, J.; Gordillo-Escobar, E.; Rodriguez-Rodriguez, A.; Enamorado-Enamorado, J.; Ruiz de Azua Lopez, Z.; Aldabo-Pallas, T.; Leon-Justel, A.; Murillo-Cabezas, F.; Vilches-Arenas, A. Serologic behavior of S100B protein in patients who are brain dead: Preliminary results. Transplant. Proc. 2013, 45, 3569–3572. [Google Scholar] [CrossRef]
- Krzych, L.J.; Czempik, P.F.; Saucha, W.; Kokocinska, D.; Knapik, P. Serum S100B protein concentration in brain-dead organ donors: A pilot study. Anaesthesiol. Intensive Ther. 2015, 47, 320–323. [Google Scholar] [CrossRef]
- Bottiger, B.W.; Mobes, S.; Glatzer, R.; Bauer, H.; Gries, A.; Bartsch, P.; Motsch, J.; Martin, E. Astroglial protein S-100 is an early and sensitive marker of hypoxic brain damage and outcome after cardiac arrest in humans. Circulation 2001, 103, 2694–2698. [Google Scholar] [CrossRef] [Green Version]
- Chae, M.K.; Lee, J.H.; Lee, T.R.; Yoon, H.; Hwang, S.Y.; Cha, W.C.; Shin, T.G.; Sim, M.S.; Jo, I.J.; Song, K.J.; et al. Early central diabetes insipidus: An ominous sign in post-cardiac arrest patients. J. Crit. Care 2016, 32, 63–67. [Google Scholar] [CrossRef] [PubMed]

| Survivors (n = 130) | In-Hospital Deaths (n = 121) | p-Value | In-Hospital Deaths (n = 121) | |||
|---|---|---|---|---|---|---|
| BD (n = 19) | Non-BD (n = 102) | p-Value | ||||
| Male, n (%) | 90 (69.2) | 91 (75.2) | 0.291 | 13 (68.4) | 78 (76.5) | 0.563 |
| Age, years, mean ± SD or median (IQR) | 52.9 ± 14.5 | 60.7 ± 17.4 | <0.001 | 45.0 (33.0–54.0) | 68.9 (54.0–75.5) | <0.001 |
| Comorbidities, n (%) | ||||||
| Hypertension | 38 (29.2) | 51 (42.1) | 0.033 | 0 (0.0) | 51 (50.0) | <0.001 |
| Diabetes mellitus | 25 (19.2) | 38 (31.4) | 0.026 | 3 (15.8) | 35 (34.3) | 0.177 |
| COPD | 5 (3.8) | 17 (14.0) | 0.004 | 1 (5.3) | 16 (15.7) | 0.305 |
| Ischemic heart disease | 19 (14.6) | 14 (11.6) | 0.476 | 0 (0.0) | 14 (13.7) | 0.123 |
| Chronic renal failure | 6 (4.6) | 13 (10.7) | 0.067 | 1 (5.3) | 12 (11.8) | 0.690 |
| Presumed cause, n (%) | <0.001 | <0.001 | ||||
| Cardiac | 102 (78.5) | 56 (46.3) | 3 (15.8) | 53 (52.0) | ||
| Submersion | 0 (0.0) | 5 (4.1) | 0 (0.0) | 5 (4.9) | ||
| Drug | 0 (0.0) | 2 (1.7) | 0 (0.0) | 2 (2.0) | ||
| Asphyxia | 20 (15.4) | 32 (26.4) | 13 (68.4) | 19 (18.6) | ||
| Other-medical | 8 (6.2) | 26 (21.5) | 3 (15.8) | 23 (22.5) | ||
| Witnessed, n (%) | 99 (76.2) | 74 (61.2) | 0.010 | 6 (31.6) | 68 (66.7) | 0.009 |
| Initial shockable rhythm, n (%) | 64 (49.2) | 26 (21.5) | <0.001 | 2 (10.5) | 24 (23.5) | 0.360 |
| Bystander CPR, n (%) | 86 (66.2) | 72 (59.5) | 0.276 | 12 (63.2) | 60 (58.8) | 0.803 |
| Arrest time *, min, mean ± SD | 27.4 ± 15.9 | 40.9 ± 22.2 | <0.001 | 41.0 (33.0–54.0) | 39.0 (28.0–51.0) | 0.117 |
| LOS, day, median (IQR) | 13.0 (8.3–21.0) | 5.0 (2.2–8.0) | <0.001 | 6.0 (5.0–7.0) | 4.5 (2.0–8.3) | 0.158 |
| BD (n = 19) | Non-BD (n = 102) | p-Value | |
|---|---|---|---|
| GWR at basal ganglia, n = 96 | 1.12 ± 0.08, n = 18 | 1.18 ± 0.07, n = 78 | 0.003 |
| Pupillary light reflex at 3 days, n = 76 | 0.005 | ||
| Present reflex | 2 (11.1) | 28 (48.3) | |
| Absent reflex | 16 (88.9) | 30 (51.7) | |
| NSE at 72 h, ng/mL, n = 41 | 112.4 (IQR, 79.8–142.7), n = 11 | 132.5 (IQR, 53.5–208.8), n = 30 | 0.860 |
| S100B at 72 h, ng/mL, n = 44 | 10.4 (IQR, 0.4–16.5), n = 12 | 0.9 (IQR, 0.3–4.3), n = 32 | 0.040 |
| Diffusion-weighted imaging, n = 43 | 0.307 | ||
| No lesion | 0 (0.0) | 2 (7.1) | |
| Isolated cortex or deep gray matter lesion | 0 (0.0) | 2 (7.1) | |
| Multifocal or global lesion | 15 (100.0) | 24 (85.7) | |
| Somatosensory evoked potential, n = 65 | 0.104 | ||
| Present N20 | 0 (0.0) | 12 (23.1) | |
| Absent N20 | 13 (0.0) | 40 (76.9) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Song, H.; Oh, S.H.; Woo, H.R.; on behalf of CROWN Investigators. Brain Death and Its Prediction in Out-of-Hospital Cardiac Arrest Patients Treated with Targeted Temperature Management. Diagnostics 2022, 12, 1190. https://doi.org/10.3390/diagnostics12051190
Song H, Oh SH, Woo HR, on behalf of CROWN Investigators. Brain Death and Its Prediction in Out-of-Hospital Cardiac Arrest Patients Treated with Targeted Temperature Management. Diagnostics. 2022; 12(5):1190. https://doi.org/10.3390/diagnostics12051190
Chicago/Turabian StyleSong, Hwan, Sang Hoon Oh, Hye Rim Woo, and on behalf of CROWN Investigators. 2022. "Brain Death and Its Prediction in Out-of-Hospital Cardiac Arrest Patients Treated with Targeted Temperature Management" Diagnostics 12, no. 5: 1190. https://doi.org/10.3390/diagnostics12051190
APA StyleSong, H., Oh, S. H., Woo, H. R., & on behalf of CROWN Investigators. (2022). Brain Death and Its Prediction in Out-of-Hospital Cardiac Arrest Patients Treated with Targeted Temperature Management. Diagnostics, 12(5), 1190. https://doi.org/10.3390/diagnostics12051190







