Uremic Pruritus: From Diagnosis to Treatment
Abstract
1. Introduction
2. Clinical Features
3. Diagnosis
4. Pathogenesis
5. Treatment
5.1. Moisturizers
5.2. Topical Calcineurin Inhibitor (Tacrolimus, Pimecrolimus)
5.3. Other Topical Agents
5.4. High Quality of Dialysis
5.5. Phototherapy
5.6. Antihistamine
5.7. Gabapentin, Pregabalin
5.8. Opioid Receptor Agonist/Antagonist
5.8.1. Naloxone
5.8.2. Nalfurafine
5.8.3. Difelikefalin
5.8.4. Nalbuphine
5.9. Mast Cell Stabilizer
5.10. Montelukast
5.11. Serotonin Receptor Antagonist: Ondansetron
5.12. Nemolizumab
5.13. Dupilumab
5.14. Acupuncture, Acupressure
5.15. Charcoal
5.16. Other Treatment
| Authors | Study Design | Participants | Enrollment | Intervention | Comparator | Efficacy |
|---|---|---|---|---|---|---|
| Duque et al. (2005) [52] | Randomized, double-blind, vehicle-controlled study | HD | N = 22 | 0.1% tacrolimus ointment twice daily for 4 weeks | Vehicle | No significant effect. |
| Ghorbani et al. (2011) [53] | Randomized double-blind study | Not mentioned | N = 60 | Pimecrolimus 1% twice daily for 8 weeks | Placebo | No significant effect. |
| Ko et al. (2011) [62] | Single-blind, randomized, controlled trial | HD, PD, CKD | N = 21 | NB-UVB phototherapy three times a week for 6 weeks | Long-wave UVA radiation | Significant and comparable improvement in the VAS scores in both groups. |
| Sherjeena et al. (2017) [65] | Controlled trial | CKD, stage IV, V | N = 30 | NBUVB phototherapy every 3 days for 15 sessions | Topical liquid paraffin, 10 mg oral cetirizine daily | Significant effect. VAS 9.13 to 1.9 at 3 months (NBUVB). VAS 9.1 to 8.8 at 3 months (control). |
| Mapar et al. (2015) [85] | Pilot randomized, triple-blind study | HD | N = 36 | Zinc sulfate 220 mg daily for 4 weeks | Placebo | No significant effect. |
| Mahmudpour et al. (2017) [88] | Randomized double-blind controlled trial | HD | N = 80 | Montelukast 10 mg daily for 30 days | Placebo | Reduction in VAS score was significantly greater in the montelukast group (2.73) compared to placebo group (5.47). |
| Amirkhanlou (2016) [70] | Double-blind randomized clinical trial | HD | N = 52 | Gabapentin 100 mg daily for 2 weeks | Ketotifen 1 mg twice daily for 2 weeks | Significant reduction in both groups (88.4% in gabapentin group vs. 76.9% in group ketotifen group). |
| Eusebio-Alpapara et al. (2020) [73] | Meta-analysis | HD | N = 315 | Gabapentin 100 mg daily, 100–400 mg 2–4 times per week | Antihistamine, pregabalin, placebo | Gabapentin decreased the pruritus severity compared to the placebo (n = 171). |
| Kumagai et al. (2010) [38] | Randomized, double-blind, placebo-controlled study | HD | N = 337 | Nalfurafine hydrochloride 5 μg, 2.5 μg for 14 days | Placebo | Significant reduction in VAS in both dosages of nalfurafine compared to placebo. |
| Mathur et al. (2017) [39] | Randomized, double-blind, placebo-controlled trial | HD | N = 373 | Nalbuphine 120 mg, 60 mg twice daily for 8 weeks | Placebo | Significant effect with nalbuphine 120 mg, but not with nalbuphine 60 mg. |
| Fishbane et al. (2020) [40] | Double-blind, placebo-controlled, phase III trial | HD | N = 378 | Intravenous difelikefalin 0.5 μg per kilogram per week for 12 weeks | Placebo | A decrease of at least 3 points (WI-NRS score) in 49.1% of the difelikefalin group (27.9% of the placebo group). |
| Kinugasa et al. (2021) [91] | Randomized, double-blind, placebo-controlled clinical study | HD | N = 69 | Nemolizumab 0.125, 0.5, 2.0 mg/kg on day 1 | Placebo | No significant effect. |
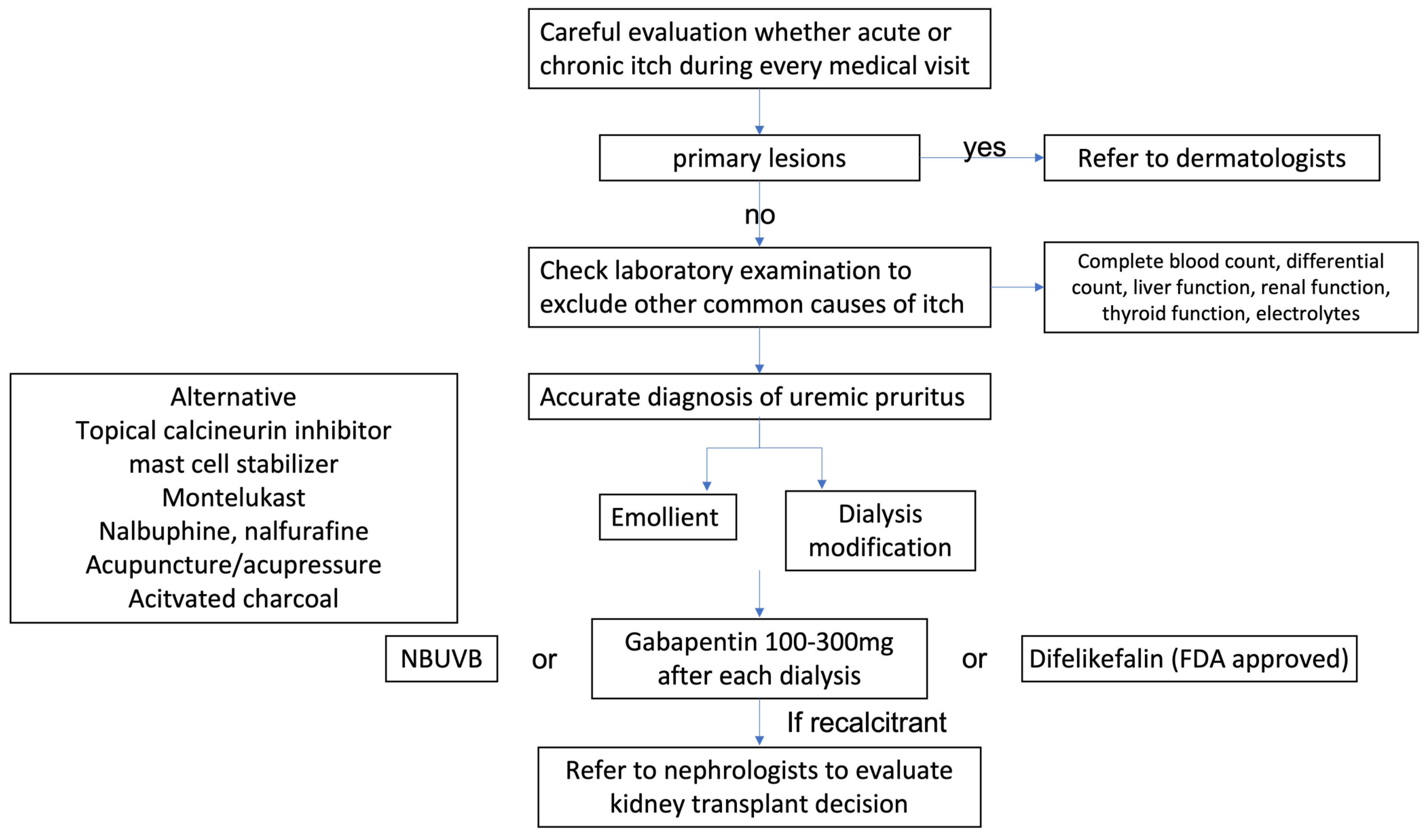
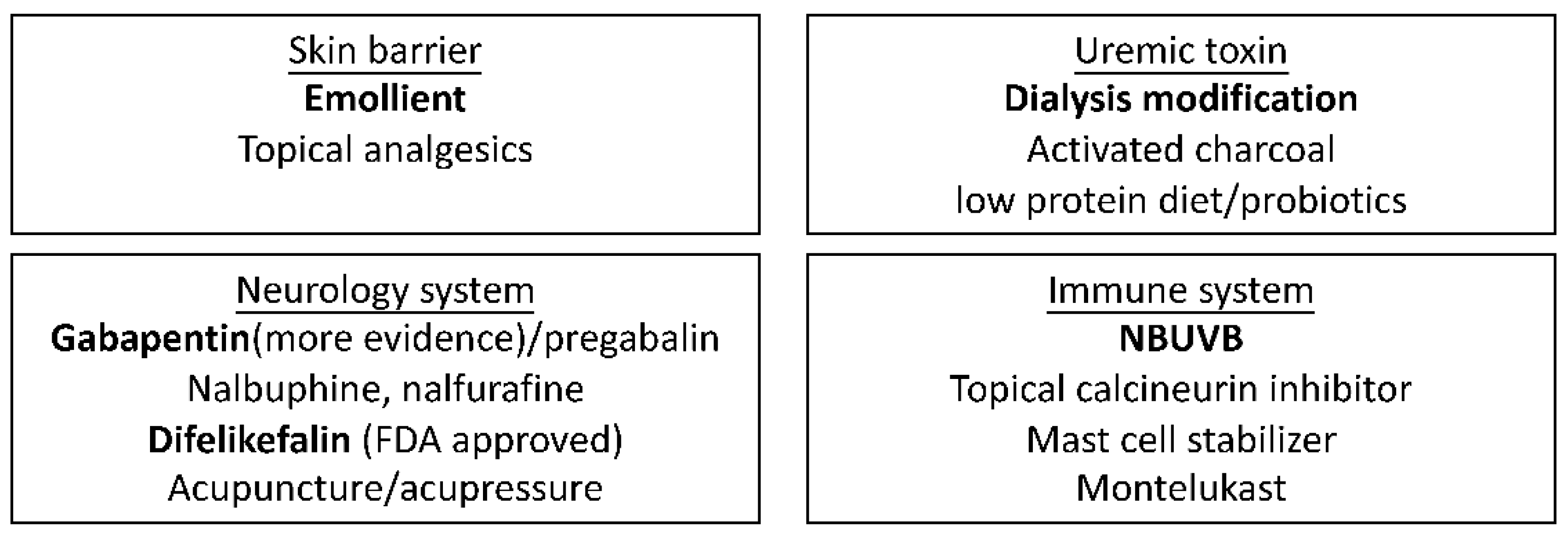
6. Proposed Algorithm from Diagnosis to Treatment
7. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sukul, N.; Speyer, E.; Tu, C.; Bieber, B.A.; Li, Y.; Lopes, A.A.; Asahi, K.; Mariani, L.; Laville, M.; Rayner, H.C.; et al. Pruritus and Patient Reported Outcomes in Non-Dialysis CKD. Clin. J. Am. Soc. Nephrol. 2019, 14, 673–681. [Google Scholar] [CrossRef]
- Pisoni, R.L.; Wikström, B.; Elder, S.J.; Akizawa, T.; Asano, Y.; Keen, M.L.; Saran, R.; Mendelssohn, D.C.; Young, E.W.; Port, F.K. Pruritus in haemodialysis patients: International results from the Dialysis Outcomes and Practice Patterns Study (DOPPS). Nephrol. Dial. Transpl. 2006, 21, 3495–3505. [Google Scholar] [CrossRef]
- Rayner, H.C.; Larkina, M.; Wang, M.; Graham-Brown, M.; van der Veer, S.N.; Ecder, T.; Hasegawa, T.; Kleophas, W.; Bieber, B.A.; Tentori, F.; et al. International Comparisons of Prevalence, Awareness, and Treatment of Pruritus in People on Hemodialysis. Clin. J. Am. Soc. Nephrol. 2017, 12, 2000–2007. [Google Scholar] [CrossRef]
- Kim, D.; Pollock, C. Epidemiology and burden of chronic kidney disease-associated pruritus. Clin. Kidney J. 2021, 14, i1–i7. [Google Scholar] [CrossRef]
- Ramakrishnan, K.; Bond, T.C.; Claxton, A.; Sood, V.C.; Kootsikas, M.; Agnese, W.; Sibbel, S. Clinical characteristics and outcomes of end-stage renal disease patients with self-reported pruritus symptoms. Int. J. Nephrol. Renovasc. Dis. 2013, 7, 1–12. [Google Scholar] [CrossRef]
- Weiss, M.; Mettang, T.; Tschulena, U.; Passlick-Deetjen, J.; Weisshaar, E. Prevalence of chronic itch and associated factors in haemodialysis patients: A representative cross-sectional study. Acta Derm. Venereol. 2015, 95, 816–821. [Google Scholar] [CrossRef]
- Weisshaar, E.; Weiss, M.; Passlick-Deetjen, J.; Tschulena, U.; Maleki, K.; Mettang, T. Laboratory and dialysis characteristics in hemodialysis patients suffering from chronic itch--results from a representative cross-sectional study. BMC Nephrol. 2015, 16, 184. [Google Scholar] [CrossRef]
- Mathur, V.S.; Lindberg, J.; Germain, M.; Block, G.; Tumlin, J.; Smith, M.; Grewal, M.; McGuire, D. A longitudinal study of uremic pruritus in hemodialysis patients. Clin. J. Am. Soc. Nephrol. 2010, 5, 1410–1419. [Google Scholar] [CrossRef]
- Kimata, N.; Fuller, D.S.; Saito, A.; Akizawa, T.; Fukuhara, S.; Pisoni, R.L.; Robinson, B.M.; Akiba, T. Pruritus in hemodialysis patients: Results from the Japanese Dialysis Outcomes and Practice Patterns Study (JDOPPS). Hemodial. Int. 2014, 18, 657–667. [Google Scholar] [CrossRef]
- Ting, S.W.; Fan, P.C.; Lin, Y.S.; Lin, M.S.; Lee, C.C.; Kuo, G.; Chang, C.H. Uremic pruritus and long-term morbidities in the dialysis population. PLoS ONE 2020, 15, e0241088. [Google Scholar] [CrossRef]
- Ting, S.W.; Fan, P.C.; Lin, Y.S.; Lin, M.S.; Lee, C.C.; Kuo, G.; Chang, C.H. Association between uremic pruritus and long-term outcomes in patients undergoing dialysis. J. Am. Acad. Dermatol. 2020, 83, 924–925. [Google Scholar] [CrossRef]
- Mettang, T.; Kremer, A.E. Uremic pruritus. Kidney Int. 2015, 87, 685–691. [Google Scholar] [CrossRef]
- Hu, X.; Sang, Y.; Yang, M.; Chen, X.; Tang, W. Prevalence of chronic kidney disease-associated pruritus among adult dialysis patients: A meta-analysis of cross-sectional studies. Medicine 2018, 97, e10633. [Google Scholar] [CrossRef]
- Min, J.W.; Kim, S.H.; Kim, Y.O.; Jin, D.C.; Song, H.C.; Choi, E.J.; Kim, Y.L.; Kim, Y.S.; Kang, S.W.; Kim, N.H.; et al. Comparison of uremic pruritus between patients undergoing hemodialysis and peritoneal dialysis. Kidney Res. Clin. Pract. 2016, 35, 107–113. [Google Scholar] [CrossRef]
- Wu, H.Y.; Huang, J.W.; Tsai, W.C.; Peng, Y.S.; Chen, H.Y.; Yang, J.Y.; Hsu, S.P.; Pai, M.F.; Ko, M.J.; Hung, K.Y.; et al. Prognostic importance and determinants of uremic pruritus in patients receiving peritoneal dialysis: A prospective cohort study. PLoS ONE 2018, 13, e0203474. [Google Scholar] [CrossRef]
- Schricker, S.; Weisshaar, E.; Kupfer, J.; Mettang, T. Prevalence of Pruritus in a Single Cohort of Long-term Kidney Transplant Recipients. Acta Derm. Venereol. 2020, 100, adv00066. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Krajewska, M.; Szepietowski, J.C. Pruritus in renal transplant recipients: Current state of knowledge. Adv Clin Exp Med. 2020, 29, 769–772. [Google Scholar] [CrossRef]
- Krajewski, P.K.; Olczyk, P.; Krajewska, M.; Krajewski, W.; Szepietowski, J.C. Clinical Characteristics of Itch in Renal Transplant Recipients. Front. Med. 2021, 7, 615334. [Google Scholar] [CrossRef]
- Verduzco, H.A.; Shirazian, S. CKD-Associated Pruritus: New Insights into Diagnosis, Pathogenesis, and Management. Kidney Int. Rep. 2020, 5, 1387–1402. [Google Scholar] [CrossRef]
- Roh, Y.S.; Choi, J.; Sutaria, N.; Kwatra, S.G. Itch: Epidemiology, clinical presentation, and diagnostic workup. J. Am. Acad Dermatol. 2022, 86, 1–14. [Google Scholar] [CrossRef]
- Manenti, L.; Leuci, E. Do you feel itchy? A guide towards diagnosis and measurement of chronic kidney disease-associated pruritus in dialysis patients. Clin. Kidney J. 2021, 14, i8–i15. [Google Scholar] [CrossRef]
- Sutaria, N.; Adawi, W.; Goldberg, R.; Roh, Y.S.; Choi, J.; Kwatra, S.G. Itch: Pathogenesis and treatment. J. Am. Acad. Dermatol. 2022, 86, 17–34. [Google Scholar] [CrossRef]
- Oweis, A.O.; Al-Qarqaz, F.; Bodoor, K.; Heis, L.; Alfaqih, M.A.; Almomani, R.; Obeidat, M.A.; Alshelleh, S.A. Elevated interleukin 31 serum levels in hemodialysis patients are associated with uremic pruritus. Cytokine 2021, 138, 155369. [Google Scholar] [CrossRef]
- Fallahzadeh, M.K.; Roozbeh, J.; Geramizadeh, B.; Namazi, M.R. Interleukin-2 serum levels are elevated in patients with uremic pruritus: A novel finding with practical implications. Nephrol. Dial. Transpl. 2011, 26, 3338–3344. [Google Scholar] [CrossRef]
- Kimmel, M.; Alscher, D.M.; Dunst, R.; Braun, N.; Machleidt, C.; Kiefer, T.; Stülten, C.; van der Kuip, H.; Pauli-Magnus, C.; Raub, U.; et al. The role of micro-inflammation in the pathogenesis of uraemic pruritus in haemodialysis patients. Nephrol. Dial. Transpl. 2006, 21, 749–755. [Google Scholar] [CrossRef]
- Schricker, S.; Kimmel, M. Unravelling the pathophysiology of chronic kidney disease-associated pruritus. Clin. Kidney J. 2021, 14, i23–i31. [Google Scholar] [CrossRef]
- Melo, H.; Basso, L.; Iftinca, M.; MacNaughton, W.K.; Hollenberg, M.D.; McKay, D.M.; Altier, C. Itch induced by peripheral mu opioid receptors is dependent on TRPV1-expressing neurons and alleviated by channel activation. Sci. Rep. 2018, 8, 15551. [Google Scholar] [CrossRef]
- Yatzidis, H.; Digenis, P.; Fountas, P. Hypervitaminosis A accompanying advanced chronic renal failure. Br. Med. J. 1975, 3, 352–353. [Google Scholar] [CrossRef][Green Version]
- Dugas-Breit, S.; Schöpf, P.; Dugas, M.; Schiffl, H.; Ruëff, F.; Przybilla, B. Baseline serum levels of mast cell tryptase are raised in hemodialysis patients and associated with severity of pruritus. J. Dtsch. Dermatol. Ges. 2005, 3, 343–347. [Google Scholar] [CrossRef]
- Bencini, P.L.; Pravettoni, G.; Crosti, C. Cutaneous divalent ions and uremic pruritus. G. Ital. Dermatol. Venereol. 1988, 123, 143–145. [Google Scholar]
- Ståhle-Bäckdahl, M.; Hägermark, O.; Lins, L.E.; Törring, O.; Hilliges, M.; Johansson, O. Experimental and immunohistochemical studies on the possible role of parathyroid hormone in uraemic pruritus. J. Intern. Med. 1989, 225, 411–415. [Google Scholar] [CrossRef]
- Vanholder, R.; De Smet, R.; Glorieux, G.; Argilés, A.; Baurmeister, U.; Brunet, P.; Clark, W.; Cohen, G.; De Deyn, P.P.; Deppisch, R.; et al. Review on uremic toxins: Classification, concentration, and interindividual variability. Kidney Int. 2003, 63, 1934–1943. [Google Scholar] [CrossRef]
- Vanholder, R.; Schepers, E.; Pletinck, A.; Nagler, E.V.; Glorieux, G. The uremic toxicity of indoxyl sulfate and p-cresyl sulfate: A systematic review. J. Am. Soc. Nephrol. 2014, 25, 1897–1907. [Google Scholar] [CrossRef]
- Bolanos, C.G.; Pham, N.M.; Mair, R.D.; Meyer, T.W.; Sirich, T.L. Metabolomic analysis of uremic pruritus in patients on hemodialysis. PLoS ONE 2021, 16, e0246765. [Google Scholar] [CrossRef]
- Ko, M.J.; Peng, Y.S.; Chen, H.Y.; Hsu, S.P.; Pai, M.F.; Yang, J.Y.; Wen, S.Y.; Jee, S.H.; Wu, H.Y.; Chiu, H.C. Interleukin-31 is associated with uremic pruritus in patients receiving hemodialysis. J. Am. Acad. Dermatol. 2014, 71, 1151–1159.e1. [Google Scholar] [CrossRef]
- Arzhan, S.; Roumelioti, M.E.; Unruh, M.L. Itch and Ache on Dialysis: New Approaches to Manage Uremic Pruritus and Restless Legs. Blood Purif. 2020, 49, 222–227. [Google Scholar] [CrossRef]
- Fantini, F.; Baraldi, A.; Sevignani, C.; Spattini, A.; Pincelli, C.; Giannetti, A. Cutaneous innervation in chronic renal failure patients. An immunohistochemical study. Acta Derm. Venereol. 1992, 72, 102–105. [Google Scholar]
- Kumagai, H.; Ebata, T.; Takamori, K.; Muramatsu, T.; Nakamoto, H.; Suzuki, H. Effect of a novel kappa-receptor agonist, nalfurafine hydrochloride, on severe itch in 337 haemodialysis patients: A Phase III, randomized, double-blind, placebo-controlled study. Nephrol. Dial. Transpl. 2009, 25, 1251–1257. [Google Scholar] [CrossRef]
- Mathur, V.S.; Kumar, J.; Crawford, P.W.; Hait, H.; Sciascia, T. A Multicenter, Randomized, Double-Blind, Placebo-Controlled Trial of Nalbuphine ER Tablets for Uremic Pruritus. Am. J. Nephrol. 2017, 46, 450–458. [Google Scholar] [CrossRef]
- Fishbane, S.; Jamal, A.; Munera, C.; Wen, W.; Menzaghi, F. A Phase 3 Trial of Difelikefalin in Hemodialysis Patients with Pruritus. N. Engl. J. Med. 2020, 382, 222–232. [Google Scholar] [CrossRef]
- Deeks, E.D. Difelikefalin: First Approval. Drugs 2021, 81, 1937–1944. [Google Scholar] [CrossRef]
- Lipman, Z.M.; Yosipovitch, G. An evaluation of difelikefalin as a treatment option for moderate-to-severe pruritus in end stage renal disease. Expert Opin. Pharmacother. 2021, 22, 549–555. [Google Scholar] [CrossRef]
- Szepietowski, J.C.; Reich, A.; Schwartz, R.A. Uraemic xerosis. Nephrol. Dial. Transpl. 2004, 19, 2709–2712. [Google Scholar] [CrossRef]
- Balaskas, E.; Szepietowski, J.C.; Bessis, D.; Ioannides, D.; Ponticelli, C.; Ghienne, C.; Taberly, A.; Dupuy, P. Randomized, double-blind study with glycerol and paraffin in uremic xerosis. Clin. J. Am. Soc. Nephrol. 2011, 6, 748–752. [Google Scholar] [CrossRef]
- Szepietowski, J.C.; Szepietowski, T.; Reich, A. Efficacy and tolerance of the cream containing structured physiological lipids with endocannabinoids in the treatment of uremic pruritus: A preliminary study. Acta Dermatovenerol. Croat. 2005, 13, 97–103. [Google Scholar]
- Okada, K.; Matsumoto, K. Effect of skin care with an emollient containing a high water content on mild uremic pruritus. Ther. Apher. Dial. 2004, 8, 419–422. [Google Scholar] [CrossRef]
- Morton, C.A.; Lafferty, M.; Hau, C.; Henderson, I.; Jones, M.; Lowe, J.G. Pruritus and skin hydration during dialysis. Nephrol. Dial. Transpl. 1996, 11, 2031–2036. [Google Scholar] [CrossRef]
- Singh, V.S.; Vinayadev, V. Effectiveness of Baby Oil Therapy for Uremic Pruritus in Hemodialysis Patients. Saudi J. Kidney Dis. Transpl. 2021, 32, 163–169. [Google Scholar] [CrossRef]
- Rodrigues, M.; Ezzedine, K.; Hamzavi, I.; Pandya, A.G.; Harris, J.E. Current and emerging treatments for vitiligo. J. Am. Acad Dermatol. 2017, 77, 17–29. [Google Scholar] [CrossRef]
- Langan, S.M.; Irvine, A.D.; Weidinger, S. Atopic dermatitis. Lancet 2020, 396, 345–360. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Klumpp, S.; Alscher, D.M.; Kuhlmann, U.; Mettang, T. Short-term efficacy of tacrolimus ointment in severe uremic pruritus. Perit. Dial. Int. 2000, 20, 802–803. [Google Scholar] [CrossRef]
- Duque, M.I.; Yosipovitch, G.; Fleischer, A.B., Jr.; Willard, J.; Freedman, B.I. Lack of efficacy of tacrolimus ointment 0.1% for treatment of hemodialysis-related pruritus: A randomized, double-blind, vehicle-controlled study. J. Am. Acad. Dermatol. 2005, 52, 519–521. [Google Scholar] [CrossRef]
- Ghorbani, A.R.; Feily, A.; Khalili, A.; Dormanesh, B. Lack of efficacy of topical calcineurin inhibitor pimecrolimus 1% on pruritus of severely uremic patients: A randomized double-blind study in 60 patients. Dermatitis 2011, 22, 167–168. [Google Scholar] [CrossRef]
- Blair, H.A. Capsaicin 8% Dermal Patch: A Review in Peripheral Neuropathic Pain. Drugs 2018, 78, 1489–1500. [Google Scholar] [CrossRef]
- Gooding, S.M.; Canter, P.H.; Coelho, H.F.; Boddy, K.; Ernst, E. Systematic review of topical capsaicin in the treatment of pruritus. Int. J. Dermatol. 2010, 49, 858–865. [Google Scholar] [CrossRef]
- Young, T.A.; Patel, T.S.; Camacho, F.; Clark, A.; Freedman, B.I.; Kaur, M.; Fountain, J.; Williams, L.L.; Yosipovitch, G.; Fleischer, A.B., Jr. A pramoxine-based anti-itch lotion is more effective than a control lotion for the treatment of uremic pruritus in adult hemodialysis patients. J. Dermatol. Treat. 2009, 20, 76–81. [Google Scholar] [CrossRef]
- Hiroshige, K.; Kabashima, N.; Takasugi, M.; Kuroiwa, A. Optimal dialysis improves uremic pruritus. Am. J. Kidney Dis. 1995, 25, 413–419. [Google Scholar] [CrossRef]
- Jiang, X.; Ji, F.; Chen, Z.W.; Huang, Q.L. Comparison of high-flux hemodialysis with hemodialysis filtration in treatment of uraemic pruritus: A randomized controlled trial. Int. Urol. Nephrol. 2016, 48, 1533–1541. [Google Scholar] [CrossRef]
- Zhang, J.; Yuan, Y.; An, X.; Ouyang, C.; Ren, H.; Yang, G.; Yu, X.; Lv, X.; Zhang, B.; Wang, N.; et al. Comparison of combined blood purification techniques in treatment of dialysis patients with uraemic pruritus. Int. J. Clin. Exp. Med. 2016, 9, 8563–8568. [Google Scholar]
- Chen, Z.J.; Cao, G.; Tang, W.X.; Lv, X.Y.; Huang, S.M.; Qin, W.; Ping, F.; Ye, T. A randomized controlled trial of high-permeability haemodialysis against conventional haemodialysis in the treatment of uraemic pruritus. Clin. Exp. Dermatol. 2009, 34, 679–683. [Google Scholar] [CrossRef]
- Armstrong, A.W.; Read, C. Pathophysiology, Clinical Presentation, and Treatment of Psoriasis: A Review. JAMA 2020, 323, 1945–1960. [Google Scholar] [CrossRef]
- Ko, M.J.; Yang, J.Y.; Wu, H.Y.; Hu, F.C.; Chen, S.I.; Tsai, P.J.; Jee, S.H.; Chiu, H.C. Narrowband ultraviolet B phototherapy for patients with refractory uraemic pruritus: A randomized controlled trial. Br. J. Dermatol. 2011, 165, 633–639. [Google Scholar] [CrossRef]
- Garssen, J.; Vandebriel, R.J.; De Gruijl, F.R.; Wolvers, D.A.; Van Dijk, M.; Fluitman, A.; Van Loveren, H. UVB exposure-induced systemic modulation of Th1- and Th2-mediated immune responses. Immunology 1999, 97, 506–514. [Google Scholar] [CrossRef]
- Gilchrest, B.A.; Rowe, J.W.; Brown, R.S.; Steinman, T.I.; Arndt, K.A. Relief of uremic pruritus with ultraviolet phototherapy. N. Engl. J. Med. 1977, 297, 136–138. [Google Scholar] [CrossRef]
- Sherjeena, P.B.; Binitha, M.P.; Rajan, U.; Sreelatha, M.; Sarita, S.; Nirmal, C.; Deepthi, N.S. A controlled trial of narrowband ultraviolet B phototherapy for the treatment of uremic pruritus. Indian J. Dermatol. Venereol. Leprol. 2017, 83, 247–249. [Google Scholar] [CrossRef]
- Weisshaar, E.; Dunker, N.; Röhl, F.W.; Gollnick, H. Antipruritic effects of two different 5-HT3 receptor antagonists and an antihistamine in haemodialysis patients. Exp. Dermatol. 2004, 13, 298–304. [Google Scholar] [CrossRef]
- Robertson, K.; Marshman, L.A.G.; Plummer, D.; Downs, E. Effect of Gabapentin vs Pregabalin on Pain Intensity in Adults With Chronic Sciatica: A Randomized Clinical Trial. JAMA Neurol. 2019, 76, 28–34. [Google Scholar] [CrossRef]
- Gunal, A.I.; Ozalp, G.; Yoldas, T.K.; Gunal, S.Y.; Kirciman, E.; Celiker, H. Gabapentin therapy for pruritus in haemodialysis patients: A randomized, placebo-controlled, double-blind trial. Nephrol. Dial. Transpl. 2004, 19, 3137–3139. [Google Scholar] [CrossRef]
- Foroutan, N.; Etminan, A.; Nikvarz, N.; Shojai Shahrokh Abadi, M. Comparison of pregabalin with doxepin in the management of uremic pruritus: A randomized single blind clinical trial. Hemodial. Int. 2017, 21, 63–71. [Google Scholar] [CrossRef]
- Amirkhanlou, S.; Rashedi, A.; Taherian, J.; Hafezi, A.A.; Parsaei, S. Comparison of Gabapentin and Ketotifen in Treatment of Uremic Pruritus in Hemodialysis Patients. Pak. J. Med. Sci. 2016, 32, 22–26. [Google Scholar] [CrossRef]
- Nofal, E.; Farag, F.; Nofal, A.; Eldesouky, F.; Alkot, R.; Abdelkhalik, Z. Gabapentin: A promising therapy for uremic pruritus in hemodialysis patients: A randomized-controlled trial and review of literature. J. Dermatol. Treat. 2016, 27, 515–519. [Google Scholar] [CrossRef]
- Solak, Y.; Biyik, Z.; Atalay, H.; Gaipov, A.; Guney, F.; Turk, S.; Covic, A.; Goldsmith, D.; Kanbay, M. Pregabalin versus gabapentin in the treatment of neuropathic pruritus in maintenance haemodialysis patients: A prospective, crossover study. Nephrology 2012, 17, 710–717. [Google Scholar] [CrossRef]
- Eusebio-Alpapara, K.M.V.; Castillo, R.L.; Dofitas, B.L. Gabapentin for uremic pruritus: A systematic review of randomized controlled trials. Int. J. Dermatol. 2020, 59, 412–422. [Google Scholar] [CrossRef]
- Rayner, H.; Baharani, J.; Smith, S.; Suresh, V.; Dasgupta, I. Uraemic pruritus: Relief of itching by gabapentin and pregabalin. Nephron Clin. Pract. 2012, 122, 75–79. [Google Scholar] [CrossRef]
- Simonsen, E.; Komenda, P.; Lerner, B.; Askin, N.; Bohm, C.; Shaw, J.; Tangri, N.; Rigatto, C. Treatment of Uremic Pruritus: A Systematic Review. Am. J. Kidney Dis. 2017, 70, 638–655. [Google Scholar] [CrossRef]
- Andersen, L.W.; Friedberg, M.; Lokkegaard, N. Naloxone in the treatment of uremic pruritus: A case history. Clin. Nephrol. 1984, 21, 355–356. [Google Scholar]
- Peer, G.; Kivity, S.; Agami, O.; Fireman, E.; Silverberg, D.; Blum, M.; Laina, A. Randomised crossover trial of naltrexone in uraemic pruritus. Lancet 1996, 348, 1552–1554. [Google Scholar] [CrossRef]
- Pauli-Magnus, C.; Mikus, G.; Alscher, D.M.; Kirschner, T.; Nagel, W.; Gugeler, N.; Risler, T.; Berger, E.D.; Kuhlmann, U.; Mettang, T. Naltrexone Does Not Relieve Uremic Pruritus: Results of a randomized, double-blind, placebo-control crossover study. J. Am. Soc. Nephrol. 2000, 11, 514–519. [Google Scholar] [CrossRef]
- Legroux-Crespel, E.; Clèdes, J.; Misery, L. A comparative study on the effects of naltrexone and loratadine on uremic pruritus. Dermatology 2004, 208, 326–330. [Google Scholar] [CrossRef]
- Wikström, B.; Gellert, R.; Ladefoged, S.D.; Danda, Y.; Akai, M.; Ide, K.; Ogasawara, M.; Kawashima, Y.; Ueno, K.; Mori, A.; et al. Kappa-opioid system in uremic pruritus: Multicenter, randomized, double-blind, placebo-controlled clinical studies. J. Am. Soc. Nephrol. 2005, 16, 3742–3747. [Google Scholar] [CrossRef]
- Liao, C.C.; Chang, C.S.; Tseng, C.H.; Sheen, M.J.; Tsai, S.C.; Chang, Y.L.; Wong, S.Y. Efficacy of intramuscular nalbuphine versus diphenhydramine for the prevention of epidural morphine-induced pruritus after cesarean delivery. Chang. Gung Med. J. 2011, 34, 172–178. [Google Scholar]
- Feily, A.; Dormanesh, B.; Ghorbani, A.R.; Moosavi, Z.; Kouchak, M.; Cheraghian, B.; Mousavi, S.S.; Mehrabian, A.; Ranjbari, N. Efficacy of topical cromolyn sodium 4% on pruritus in uremic nephrogenic patients: A randomized double-blind study in 60 patients. Int. J. Clin. Pharmacol. Ther. 2012, 50, 510–513. [Google Scholar] [CrossRef]
- Rosner, M.H. Cromolyn sodium: A potential therapy for uremic pruritus? Hemodial. Int. 2006, 10, 189–192. [Google Scholar] [CrossRef]
- Najafabadi, M.M.; Faghihi, G.; Emami, A.; Monghad, M.; Moeenzadeh, F.; Sharif, N.; Davarpanah Jazi, A.H. Zinc sulfate for relief of pruritus in patients on maintenance hemodialysis. Ther. Apher. Dial. 2012, 16, 142–145. [Google Scholar] [CrossRef]
- Mapar, M.A.; Pazyar, N.; Siahpoosh, A.; Latifi, S.M.; Beladi Mousavi, S.S.; Khazanee, A. Comparison of the efficacy and safety of zinc sulfate vs. placebo in the treatment of pruritus of hemodialytic patients: A pilot randomized, triple-blind study. G. Ital. Dermatol. Venereol. 2015, 150, 351–355. [Google Scholar]
- Kremer, A.E.; Feramisco, J.; Reeh, P.W.; Beuers, U.; Oude Elferink, R.P. Receptors, cells and circuits involved in pruritus of systemic disorders. Biochim. Biophys. Acta 2014, 1842, 869–892. [Google Scholar] [CrossRef]
- Toyama, S.; Tominaga, M.; Takamori, K. Connections between Immune-Derived Mediators and Sensory Nerves for Itch Sensation. Int. J. Mol. Sci. 2021, 22, 2365. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Raiss Jalali, G.A.; Pakfetrat, M.; Ezzat Zadegan, S.; Sagheb, M.M. Therapeutic Effect of Montelukast for Treatment of Uremic Pruritus in Hemodialysis Patients. Iran J. Kidney Dis. 2017, 11, 50–55. [Google Scholar]
- Wang, W.; Zhou, L.; Sun, L. Ondansetron for neuraxial morphine-induced pruritus: A meta-analysis of randomized controlled trials. J. Clin. Pharm. Ther. 2017, 42, 383–393. [Google Scholar] [CrossRef]
- To, T.H.; Clark, K.; Lam, L.; Shelby-James, T.; Currow, D.C. The role of ondansetron in the management of cholestatic or uremic pruritus—A systematic review. J. Pain Symptom Manag. 2012, 44, 725–730. [Google Scholar] [CrossRef]
- Kinugasa, E.; Igawa, K.; Shimada, H.; Kondo, M.; Funakoshi, S.; Imada, N.; Itami, N.; Fukazawa, N.; Takubo, R.; Kawata, Y.; et al. Anti-pruritic effect of nemolizumab in hemodialysis patients with uremic pruritus: A phase II, randomized, double-blind, placebo-controlled clinical study. Clin. Exp. Nephrol. 2021, 25, 875–884. [Google Scholar] [CrossRef]
- Silverberg, J.I.; Brieva, J. A successful case of dupilumab treatment for severe uremic pruritus. JAAD Case Rep. 2019, 5, 339–341. [Google Scholar] [CrossRef]
- Zhai, L.L.; Savage, K.T.; Qiu, C.C.; Jin, A.; Valdes-Rodriguez, R.; Mollanazar, N.K. Chronic Pruritus Responding to Dupilumab-A Case Series. Medicines 2019, 6, 72. [Google Scholar] [CrossRef]
- Kim, K.H.; Lee, M.S.; Choi, S.M. Acupuncture for treating uremic pruritus in patients with end-stage renal disease: A systematic review. J. Pain Symptom Manag. 2010, 40, 117–125. [Google Scholar] [CrossRef]
- Chen, T.; Zhang, W.W.; Chu, Y.X.; Wang, Y.Q. Acupuncture for Pain Management: Molecular Mechanisms of Action. Am. J. Chin. Med. 2020, 48, 793–811. [Google Scholar] [CrossRef]
- Badiee Aval, S.; Ravanshad, Y.; Azarfar, A.; Mehrad-Majd, H.; Torabi, S.; Ravanshad, S. A Systematic Review and Meta-analysis of Using Acupuncture and Acupressure for Uremic Pruritus. Iran J. Kidney Dis. 2018, 12, 78–83. [Google Scholar]
- Rehman, I.U.; Wu, D.B.; Ahmed, R.; Khan, N.A.; Rahman, A.U.; Munib, S.; Lee, L.H.; Chan, K.G.; Khan, T.M. A randomized controlled trial for effectiveness of zolpidem versus acupressure on sleep in hemodialysis patients having chronic kidney disease-associated pruritus. Medicine 2018, 97, e10764. [Google Scholar] [CrossRef]
- Rehman, I.U.; Ahmed, R.; Rahman, A.U.; Wu, D.B.C.; Munib, S.; Shah, Y.; Khan, N.A.; Rehman, A.U.; Lee, L.H.; Chan, K.G.; et al. Effectiveness and safety profiling of zolpidem and acupressure in CKD associated pruritus: An interventional study. Medicine 2021, 100, e25995. [Google Scholar] [CrossRef]
- Pederson, J.A.; Matter, B.J.; Czerwinski, A.W.; Llach, F. Relief of idiopathic generalized pruritus in dialysis patients treated with activated oral charcoal. Ann. Intern. Med. 1980, 93, 446–448. [Google Scholar] [CrossRef]
- Morachiello, P.; Landini, S.; Fracasso, A.; Righetto, F.; Scanferla, F.; Toffoletto, P.; Genchi, R.; Bazzato, G. Combined hemodialysis-hemoperfusion in the treatment of secondary hyperparathyroidism of uremic patients. Blood Purif. 1991, 9, 148–152. [Google Scholar] [CrossRef]
- Cupisti, A.; Piccoli, G.B.; Gallieni, M. Charcoal for the management of pruritus and uremic toxins in patients with chronic kidney disease. Curr. Opin. Nephrol. Hypertens. 2020, 29, 71–79. [Google Scholar] [CrossRef]
- Massry, S.G.; Popovtzer, M.M.; Coburn, J.W.; Makoff, D.L.; Maxwell, M.H.; Kleeman, C.R. Intractable pruritus as a manifestation of secondary hyperparathyroidism in uremia. Disappearance of itching after subtotal parathyroidectomy. N. Engl. J. Med. 1968, 279, 697–700. [Google Scholar] [CrossRef]
- Chou, F.F.; Ho, J.C.; Huang, S.C.; Sheen-Chen, S.M. A study on pruritus after parathyroidectomy for secondary hyperparathyroidism. J. Am. Coll. Surg. 2000, 190, 65–70. [Google Scholar] [CrossRef]
- Sharma, D.; Kwatra, S.G. Thalidomide for the treatment of chronic refractory pruritus. J. Am. Acad. Dermatol. 2016, 74, 363–369. [Google Scholar] [CrossRef]
- Silva, S.R.; Viana, P.C.; Lugon, N.V.; Hoette, M.; Ruzany, F.; Lugon, J.R. Thalidomide for the treatment of uremic pruritus: A crossover randomized double-blind trial. Nephron 1994, 67, 270–273. [Google Scholar] [CrossRef]
- Chen, Y.C.; Chiu, W.T.; Wu, M.S. Therapeutic effect of topical gamma-linolenic acid on refractory uremic pruritus. Am. J. Kidney Dis. 2006, 48, 69–76. [Google Scholar] [CrossRef]
- Avila, C.; Massick, S.; Kaffenberger, B.H.; Kwatra, S.G.; Bechtel, M. Cannabinoids for the treatment of chronic pruritus: A review. J. Am. Acad. Dermatol. 2020, 82, 1205–1212. [Google Scholar] [CrossRef]
- Rein, J.L.; Wyatt, C.M. Marijuana and Cannabinoids in ESRD and Earlier Stages of CKD. Am. J. Kidney Dis. 2018, 71, 267–274. [Google Scholar] [CrossRef]
- Mettang, T. Uremic Itch Management. Curr. Probl. Dermatol. 2016, 50, 133–141. [Google Scholar] [CrossRef]
- Forouhari, A.; Moghtaderi, M.; Raeisi, S.; Shahidi, S.; Parin Hedayati, Z.; Zaboliyan, J.; Ani, S.; Moeinzadeh, F.; Mortazavi, M. Pruritus-reducing effects of omega-3 fatty acids in hemodialysis patients: A cross-over randomized clinical trial. Hemodial. Int. 2022. online ahead of print. [Google Scholar] [CrossRef]
- Lipman, Z.M.; Paramasivam, V.; Yosipovitch, G.; Germain, M.J. Clinical management of chronic kidney disease-associated pruritus: Current treatment options and future approaches. Clin. Kidney J. 2021, 14, i16–i22. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cheng, A.-Y.; Wong, L.-S. Uremic Pruritus: From Diagnosis to Treatment. Diagnostics 2022, 12, 1108. https://doi.org/10.3390/diagnostics12051108
Cheng A-Y, Wong L-S. Uremic Pruritus: From Diagnosis to Treatment. Diagnostics. 2022; 12(5):1108. https://doi.org/10.3390/diagnostics12051108
Chicago/Turabian StyleCheng, An-Yu, and Lai-San Wong. 2022. "Uremic Pruritus: From Diagnosis to Treatment" Diagnostics 12, no. 5: 1108. https://doi.org/10.3390/diagnostics12051108
APA StyleCheng, A.-Y., & Wong, L.-S. (2022). Uremic Pruritus: From Diagnosis to Treatment. Diagnostics, 12(5), 1108. https://doi.org/10.3390/diagnostics12051108






