Liver Frailty Index for Prediction of Short-Term Rehospitalization in Patients with Liver Cirrhosis
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. Liver Frailty Index
- Hand grip strength: the average of three trials, measured on the patient’s dominant hand using a hand dynamometer.
- Chair stands: measured as the number of seconds the patient needs to perform five chair stands with arms folded across the chest.
- Balance testing: measured as the number of seconds the patient manages to balance in three positions (feet placed side-to-side, semi tandem, and tandem) for a maximum time of 10 s each.
2.3. Ethics
2.4. Statistical Analysis
3. Results
3.1. Patient Characteristics at Baseline
3.2. Factors Associated with Non-Elective Rehospitalization within 30 Days
3.3. LFI Predicts 30-Days Rehospitalization of Patients with Liver Cirrhosis
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Chen, X.; Mao, G.; Leng, S.X. Frailty syndrome: An overview. Clin. Interv. Aging 2014, 9, 433. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xue, Q.L. The Frailty Syndrome: Definition and Natural History. Clin. Geriatr. Med. 2011, 27, 1. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fairhall, N.; Aggar, C.; Kurrle, S.E.; Sherrington, C.; Lord, S.; Lockwood, K.; Monaghan, N.; Cameron, I.D. Frailty Intervention Trial (FIT). BMC Geriatr. 2008, 8, 27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Friedman, S.L. Mechanisms of disease: Mechanisms of hepatic fibrosis and therapeutic implications. Nat. Clin. Pract. Gastroenterol. Hepatol. 2004, 1, 98–105. [Google Scholar] [CrossRef] [PubMed]
- Murphy, S.L.; Richardson, J.K.; Blackwood, J.; Martinez, B.; Tapper, E.B. Neurocognitive and Muscular Capacities Are Associated with Frailty in Adults with Cirrhosis. Dig. Dis. Sci. 2020, 65, 3734–3743. [Google Scholar] [CrossRef] [PubMed]
- Berry, K.; Duarte-Rojo, A.; Grab, J.D.; Dunn, M.A.; Boyarsky, B.J.; Verna, E.C.; Kappus, M.R.; Volk, M.L.; McAdams-DeMarco, M.; Segev, D.L.; et al. Cognitive Impairment and Physical Frailty in Patients with Cirrhosis. Hepatol. Commun. 2022, 6, 237–246. [Google Scholar] [CrossRef] [PubMed]
- Wiesner, R.H.; Mcdiarmid, S.V.; Kamath, P.S.; Edwards, E.B.; Malinchoc, M.; Kremers, W.K.; Krom, R.A.; Kim, W.R. MELD and PELD: Application of Survival Models to Liver Allocation. Liver Transplant. 2001, 7, 567–580. [Google Scholar] [CrossRef] [PubMed]
- Tapper, E.B.; Finkelstein, D.; Mittleman, M.A.; Piatkowski, G.; Lai, M. Standard assessments of frailty are validated predictors of mortality in hospitalized patients with cirrhosis. Hepatology 2015, 62, 584–590. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lai, J.C.; Covinsky, K.E.; Dodge, J.L.; Boscardin, W.J.; Segev, D.L.; Roberts, J.P.; Feng, S. Development of a novel frailty index to predict mortality in patients with end-stage liver disease. Hepatology 2017, 66, 564–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Whitlock, R.; Xu, C.; Taneja, S.; Singh, S.; Abraldes, J.G.; Burak, K.W.; Bailey, R.J.; Lai, J.C.; Tandon, P. Frailty is associated with increased risk of cirrhosis disease progression and death. Hepatology 2021, 75, 600–609. [Google Scholar] [CrossRef] [PubMed]
- Pugh, R.N.H.; Murray-Lyon, I.M.; Dawson, J.L.; Pietroni, M.C.; Williams, R. Transection of the oesophagus for bleeding oesophageal varices. Br. J. Surg. 1973, 60, 646–649. [Google Scholar] [CrossRef] [PubMed]
- Lai, J.C.; Rahimi, R.S.; Verna, E.C.; Kappus, M.R.; Dunn, M.A.; McAdams-DeMarco, M.; Haugen, C.E.; Volk, M.L.; Duarte-Rojo, A.; Ganger, D.R.; et al. Frailty Associated with Waitlist Mortality Independent of Ascites and Hepatic Encephalopathy in a Multicenter Study. Gastroenterology 2019, 156, 1675–1682. [Google Scholar] [CrossRef] [PubMed]
- Serper, M.; Tao, S.Y.; Kent, D.S.; Garren, P.; Burdzy, A.E.; Lai, J.C.; Gougol, A.; Bloomer, P.M.; Reddy, K.R.; Dunn, M.A.; et al. Inpatient Frailty Assessment Is Feasible and Predicts Nonhome Discharge and Mortality in Decompensated Cirrhosis. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2021, 27, 1711–1722. [Google Scholar] [CrossRef]
- Lai, J.C.; Tandon, P.; Bernal, W.; Tapper, E.B.; Ekong, U.; Dasarathy, S.; Carey, E.J. Malnutrition, Frailty, and Sarcopenia in Patients with Cirrhosis: 2021 Practice Guidance by the American Association for the Study of Liver Diseases. Hepatology 2021, 74, 1611–1644. [Google Scholar] [CrossRef] [PubMed]
- Mehta, M.; Louissaint, J.; Parikh, N.S.; Long, M.T.; Tapper, E.B. Cognitive Function, Sarcopenia, and Inflammation Are Strongly Associated with Frailty: A Framingham Cohort Study. Am. J. Med. 2021, 134, 1530–1538. [Google Scholar] [CrossRef]
- Kappus, M.R.; Rahimi, R.S.; Volk, M.L. Measuring the Toll of Acute Illness-Derived Frailty in Decompensated Cirrhosis. Liver Transplant. Off. Publ. Am. Assoc. Study Liver Dis. Int. Liver Transplant. Soc. 2021, 27, 1701–1703. [Google Scholar] [CrossRef] [PubMed]
- Plank, L.D.; Gane, E.J.; Peng, S.; Muthu, C.; Mathur, S.; Gillanders, L.; McIlroy, K.; Donaghy, A.J.; McCall, J.L. Nocturnal nutritional supplementation improves total body protein status of patients with liver cirrhosis: A randomized 12-month trial. Hepatology 2008, 48, 557–566. [Google Scholar] [CrossRef] [PubMed]
- Merli, M.; Berzigotti, A.; Zelber-Sagi, S.; Dasarathy, S.; Montagnese, S.; Genton, L.; Plauth, M.; Parés, A. EASL Clinical Practice Guidelines on nutrition in chronic liver disease. J. Hepatol. 2019, 70, 172–193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bischoff, S.C.; Bernal, W.; Dasarathy, S.; Merli, M.; Plank, L.D.; Schütz, T.; Plauth, M. ESPEN practical guideline: Clinical nutrition in liver disease. Clin. Nutr. 2020, 39, 3533–3562. [Google Scholar] [CrossRef] [PubMed]
- Hiraoka, A.; Michitaka, K.; Kiguchi, D.; Izumoto, H.; Ueki, H.; Kaneto, M.; Kitahata, S.; Aibiki, T.; Okudaira, T.; Tomida, H.; et al. Efficacy of branched-chain amino acid supplementation and walking exercise for preventing sarcopenia in patients with liver cirrhosis. Eur. J. Gastroenterol. Hepatol. 2017, 29, 1416–1423. [Google Scholar] [CrossRef] [PubMed]


| Variable | All Patients | Patients with Rehospitalization within 30 Days | Patients without Rehospitalization within 30 Days | p Value | |
|---|---|---|---|---|---|
| Total, n (%) | n = 83 (100%) | n = 26 (31%) | n = 57 (69%) | ||
| Age, y (IQR) | 60 (51; 67) | 60 (51; 69) | 60 (50; 67) | 0.36 | |
| Male gender, n (%) | 50 (60%) | 15 (57%) | 35 (63%) | 0.75 | |
| Charlson Comorbidity Index (IQR) | 5 (3; 6) | 5 (4; 7) | 5 (3; 6) | 0.50 | |
| Etiology | Alcohol, n (%) | 55 (66%) | 15 (57%) | 40 (70%) | 0.32 |
| Viral hepatitis, n (%) | 7 (8%) | 3 (12%) | 4 (7%) | ||
| NAFLD, n (%) | 9 (11%) | 3 (12%) | 6 (11%) | ||
| Cholestatic/Autoimmune, n (%) | 3 (4%) | 0 (0%) | 3 (5%) | ||
| Other/mixed, n (%) | 9 (11%) | 5 (19%) | 4 (7%) | ||
| Characteristics of liver cirrhosis | |||||
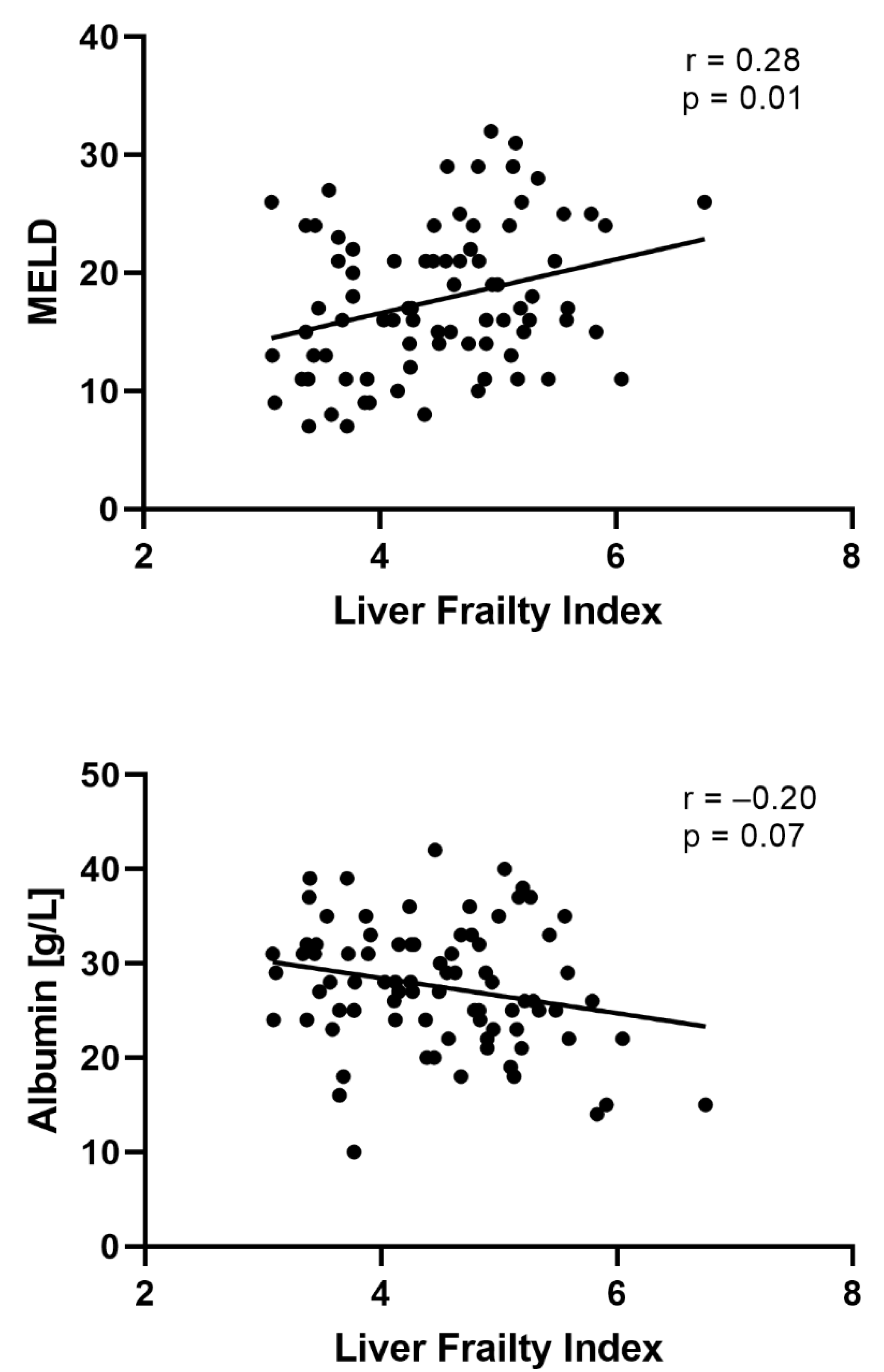
| MELD score (IQR) | 17 (13; 22) | 21 (18; 24) | 16 (11; 21) | <0.01 | |
| MELDNa score (IQR) | 17 (13; 24) | 22 (16; 27) | 14 (12; 23) | <0.01 | |
| Child-Pugh A/B/C, n (%) | 4/53/26 (5%/64%/31%) | 0/16/10 (0%/62%/38%) | 4/37/17 (7%/65%/28%) | 0.29 | |
| History of ascites, n (%) | 69 (83%) | 25 (96%) | 44 (75%) | 0.03 | |
| History of HE, n (%) | 29 (35%) | 13 (50%) | 16 (28%) | 0.05 | |
| History of SBP, n (%) | 17 (20%) | 7 (27%) | 10 (18%) | 0.33 | |
| Laboratory values | |||||
| Sodium, mmol/L (IQR) | 137 (133; 139) | 133 (131; 138) | 137 (134; 140) | 0.03 | |
| Albumin, g/L (IQR) | 28 (24; 32) | 25 (23; 28) | 29 (24; 33) | 0.04 | |
| Bilirubin, mg/dL (IQR) | 2.4 (1.4; 4.9) | 3.2 (1.7; 8.2) | 2.1 (1.4: 4.4) | 0.96 | |
| WBC, nL (IQR) | 5.2 (3.6; 7.8) | 4.3 (3.1; 6.9) | 5.8 (3.9; 8.1) | 0.64 | |
| CRP, mg/L (IQR) | 16 (5; 30) | 18 (8; 33) | 15 (5; 29) | 0.38 | |
| Hemoglobin, g/dL (IQR) | 10.1 (8.8; 12.1) | 9.7 (8.2; 10.8) | 10.2 (9.3; 12.3) | 0.73 | |
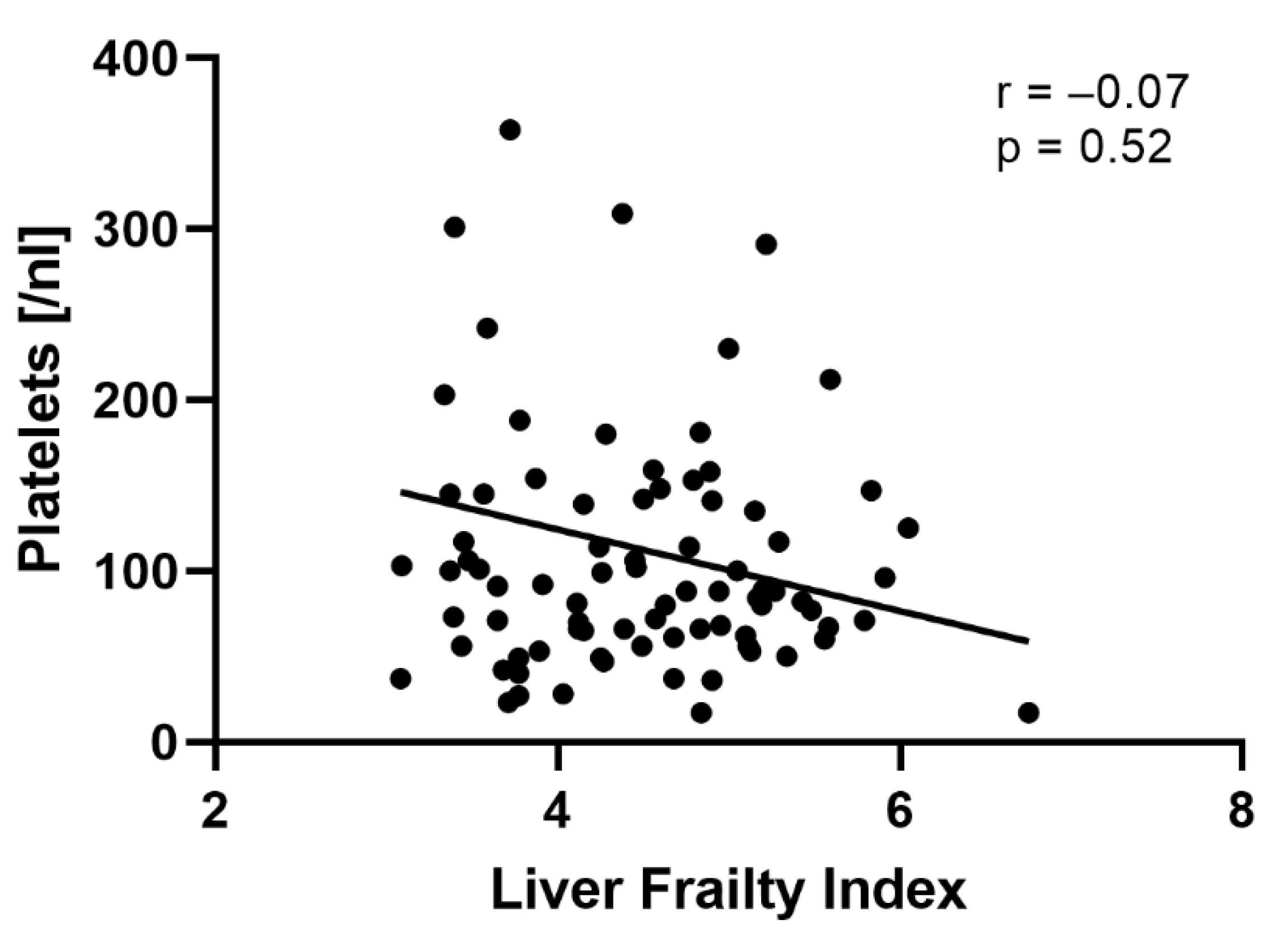
| Platelets, nL (IQR) | 88 (61; 142) | 69 (52; 90) | 103 (67; 156) | <0.01 | |
| Liver Frailty Index (LFI) | |||||
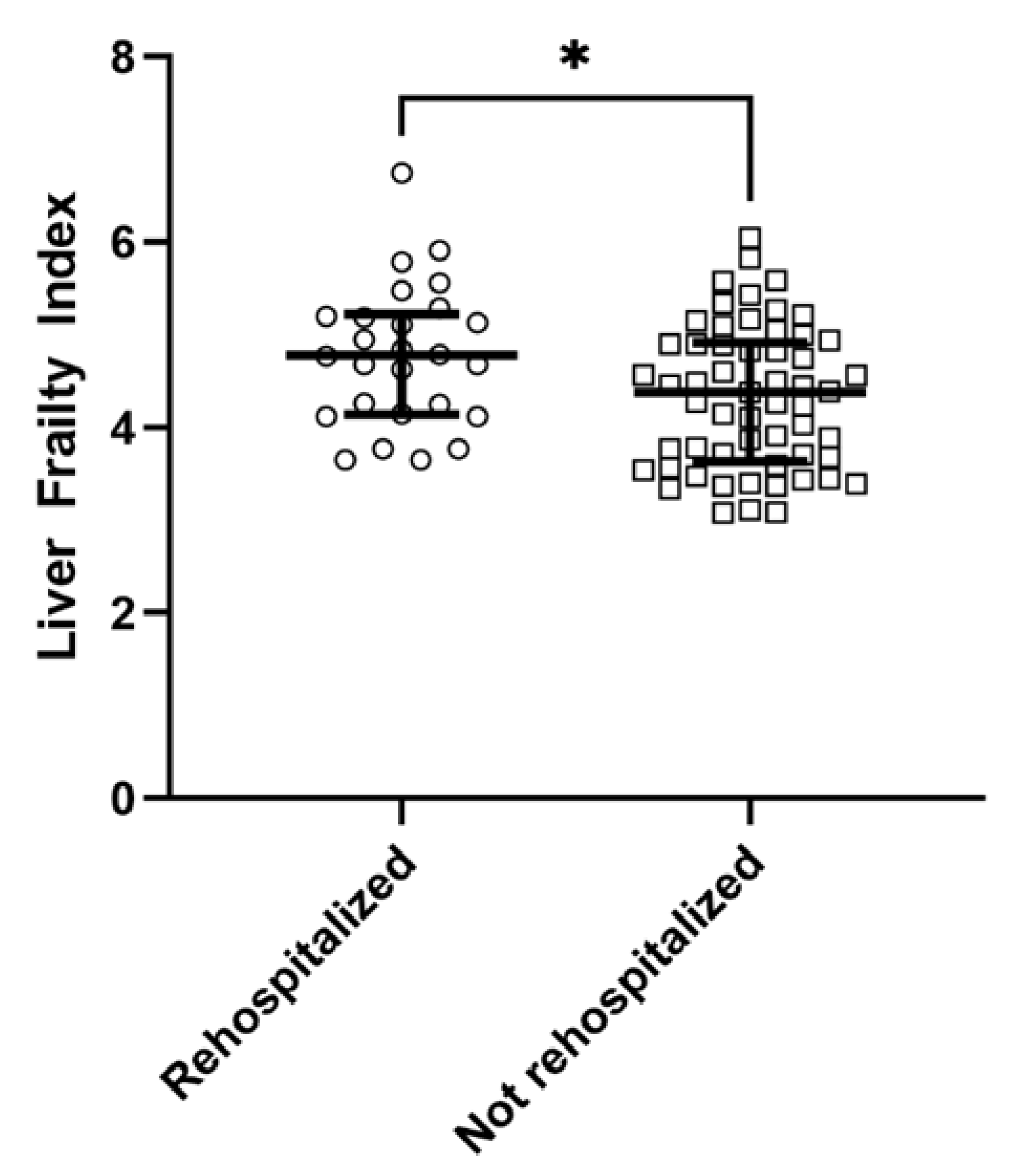
| LFI, (IQR) | 4.5 (3.8; 5.1) | 4.8 (4.2; 5.2) | 4.4 (3.6; 4.9) | 0.02 | |
| Frail, n (%) | 43 (52%) | 17 (65%) | 26 (46%) | 0.10 | |
| Dominant hand grip strength, kg (IQR) | 22.6 (16.8; 29.8) | 19.9 (16.7; 26.4) | 23.1 (17.9; 30.7) | 0.27 | |
| Chair stands, s (IQR) | 18.1 (11.9; 28.3) | 21.2 (16.5; 32.4) | 15.7 (10.4; 24.4) | 0.02 | |
| Balance | |||||
| Side (IQR) | 10 (10; 10) | 0.35 | |||
| Semi-Tandem (IQR) | 10 (5.4; 10) | 9.8 (4.8; 10) | 10 (5.8; 10) | 0.12 | |
| Tandem (IQR) | 5.9 (1.9; 10) | 4.1 (0; 8.7) | 6.7 (3.3; 10) | 0.09 | |
| Variable | Model 1 a | Model 2 b | Model 3 c | |||
|---|---|---|---|---|---|---|
| OR | p | OR | p | OR | p | |
| Platelets (95% CI) | 0.98 (0.97–0.99) | <0.01 | 0.98 (0.96–0.99) | 0.01 | 0.98 (0.96–0.99) | <0.01 |
| LFI (95% CI) | 2.36 (1.13–4.96) | 0.02 | 2.36 (1.13–4.96) | 0.02 | ||
| Sodium (95% CI) | 0.87 (0.77–0.98) | 0.03 | ||||
| Harrell’s C-index | 0.78 (0.68–0.88) | 0.78 (0.69–0.88) | 0.78 (0.68–0.88) | |||
| Subtests of the LFI | ||||||
|---|---|---|---|---|---|---|
| Variable | LFI | Chair Stands (s) | Tandem (s) | Semi-Tandem (s) | Side (s) | Hand Grip Strength (kg) |
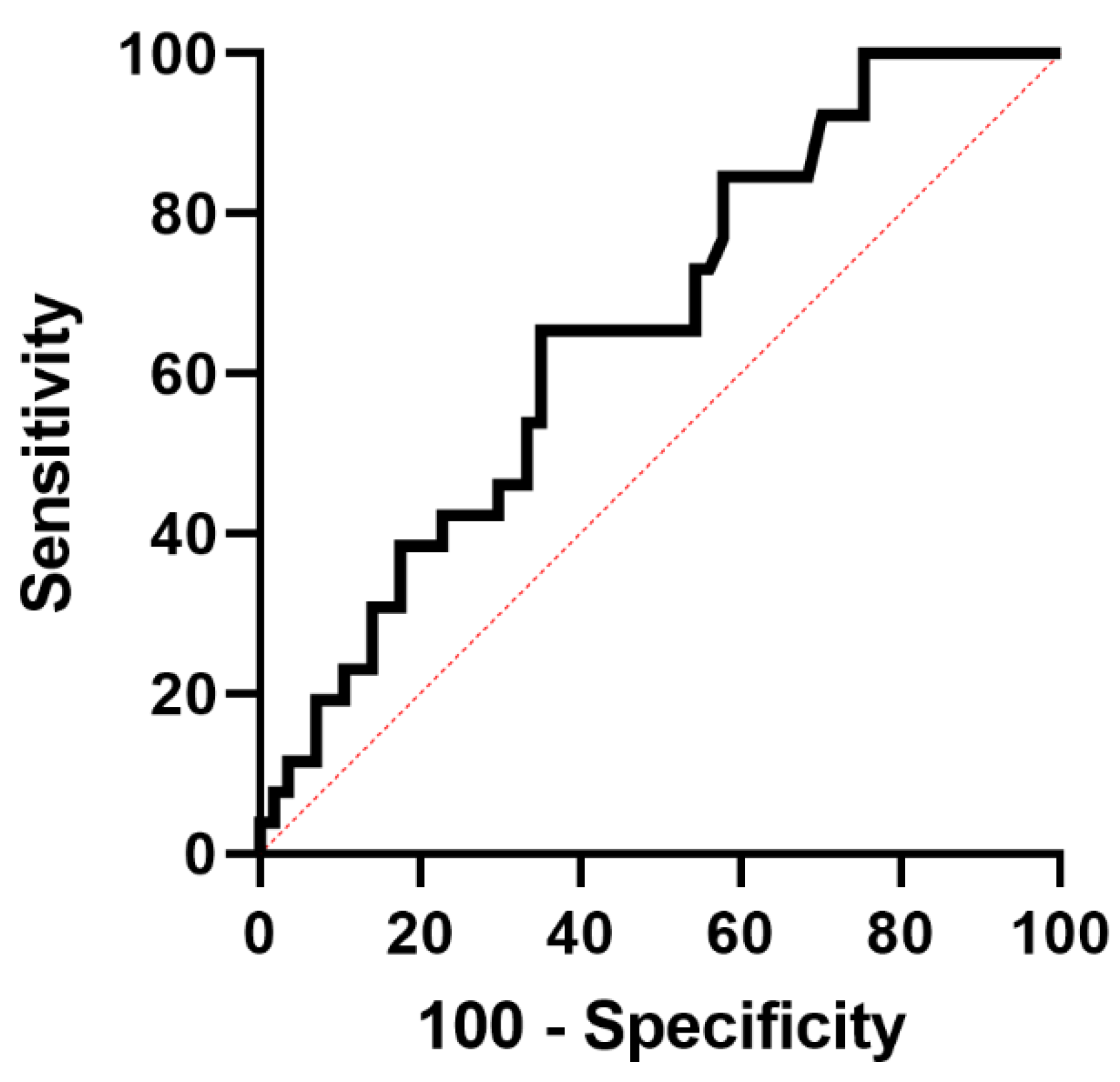
| AUC (95% CI) | 0.66 (0.54–0.78) | 0.67 (0.55–0.79) | 0.61 (0.48–0.75) | 0.59 (0.46–0.73) | 0.53 (0.40–0.67) | 0.58 (0.44–0.71) |
| Ideal cut-off | 4.62 | 19.23 | 5.74 | 9.88 | 9.84 | 21.89 |
| Sensitivity (95% CI) | 0.65 (0.44–0.82) | 0.57 (0.37–0.76) | 0.54 (0.34–0.73) | 0.54 (0.33–0.73) | 0.15 (0.05–0.36) | 0.57 (0.37–0.76) |
| Specificity (95% CI) | 0.65 (0.51–0.77) | 0.58 (0.44–0.71) | 0.54 (0.41–0.67) | 0.66 (0.53–0.78) | 0.91 (0.80–0.97) | 0.58 (0.44–0.71) |
| Positive Predictive Value (95% CI) | 0.46 (0.34–0.63) | 0.38 (0.24–0.55) | 0.35 (0.21–0.52) | 0.42 (0.26–0.60) | 0.44 (0.15–0.77) | 0.38 (0.24–0.55) |
| Negative Predictive Value (95% CI) | 0.80 (0.66–0.9) | 0.75 (0.59–0.86) | 0.72 (0.56–0.84) | 0.76 (0.62–0.86) | 0.70 (0.58–0.80) | 0.75 (0.59–0.86) |
| Positive likelihood ratio (95% CI) | 1.86 (1.19–2.92) | 1.37 (0.88–2.14) | 1.18 (0.74–1.86) | 1.62 (0.97–2.69) | 1.75 (0.51–6.00) | 1.37 (0.88–2.14) |
| Negative likelihood ratio (95% CI) | 0.53 (0.31–0.92) | 0.73 (0.45–1.17) | 0.84 (0.54–1.32) | 0.69 (0.45–1.07) | 0.93 (0.78–1.10) | 0.73 (0.45–1.17) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaps, L.; Lukac, L.; Michel, M.; Kremer, W.M.; Hilscher, M.; Gairing, S.J.; Galle, P.R.; Schattenberg, J.M.; Wörns, M.-A.; Nagel, M.; et al. Liver Frailty Index for Prediction of Short-Term Rehospitalization in Patients with Liver Cirrhosis. Diagnostics 2022, 12, 1069. https://doi.org/10.3390/diagnostics12051069
Kaps L, Lukac L, Michel M, Kremer WM, Hilscher M, Gairing SJ, Galle PR, Schattenberg JM, Wörns M-A, Nagel M, et al. Liver Frailty Index for Prediction of Short-Term Rehospitalization in Patients with Liver Cirrhosis. Diagnostics. 2022; 12(5):1069. https://doi.org/10.3390/diagnostics12051069
Chicago/Turabian StyleKaps, Leonard, Lejla Lukac, Maurice Michel, Wolfgang Maximilian Kremer, Max Hilscher, Simon Johannes Gairing, Peter R. Galle, Jörn M. Schattenberg, Marcus-Alexander Wörns, Michael Nagel, and et al. 2022. "Liver Frailty Index for Prediction of Short-Term Rehospitalization in Patients with Liver Cirrhosis" Diagnostics 12, no. 5: 1069. https://doi.org/10.3390/diagnostics12051069
APA StyleKaps, L., Lukac, L., Michel, M., Kremer, W. M., Hilscher, M., Gairing, S. J., Galle, P. R., Schattenberg, J. M., Wörns, M.-A., Nagel, M., & Labenz, C. (2022). Liver Frailty Index for Prediction of Short-Term Rehospitalization in Patients with Liver Cirrhosis. Diagnostics, 12(5), 1069. https://doi.org/10.3390/diagnostics12051069







