Role of AS-OCT in Managing Corneal Disorders
Abstract
1. Introduction
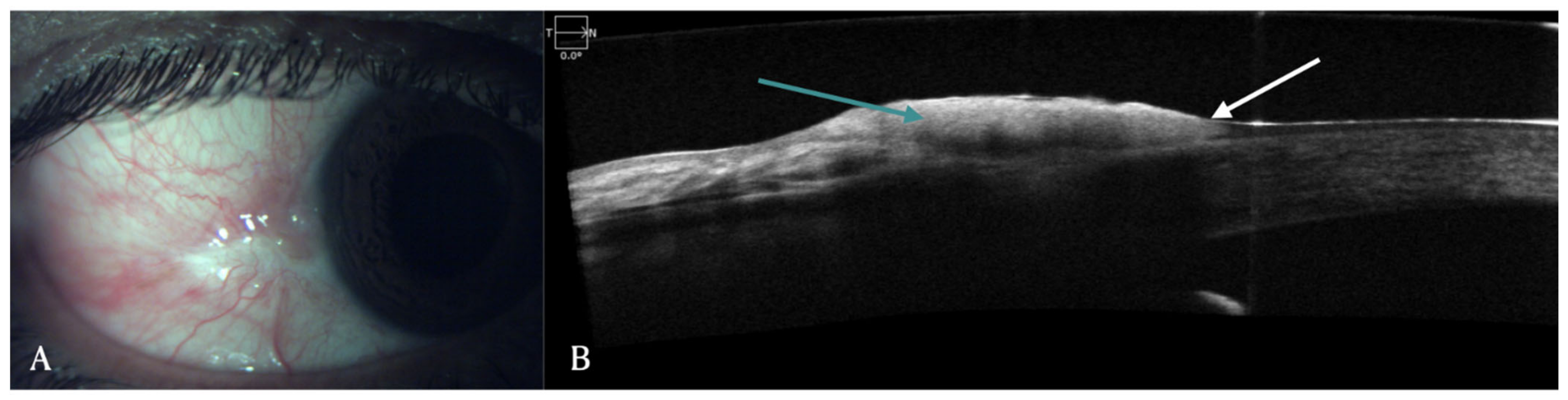
2. Conjunctival Diseases
3. Limbal Stem Cell Deficiency (LSCD)
4. Ocular Chemical Burns (OCBs)
5. Dry Eye Disease (DED)
6. Keratoconus
7. Corneal Dystrophy
8. Infective Keratitis
9. Corneal Transplantation
10. Refractive Surgery
11. Pediatric Corneal Disease
12. Corneal Trauma and Foreign Body
13. AS-OCT in Animal Experiments
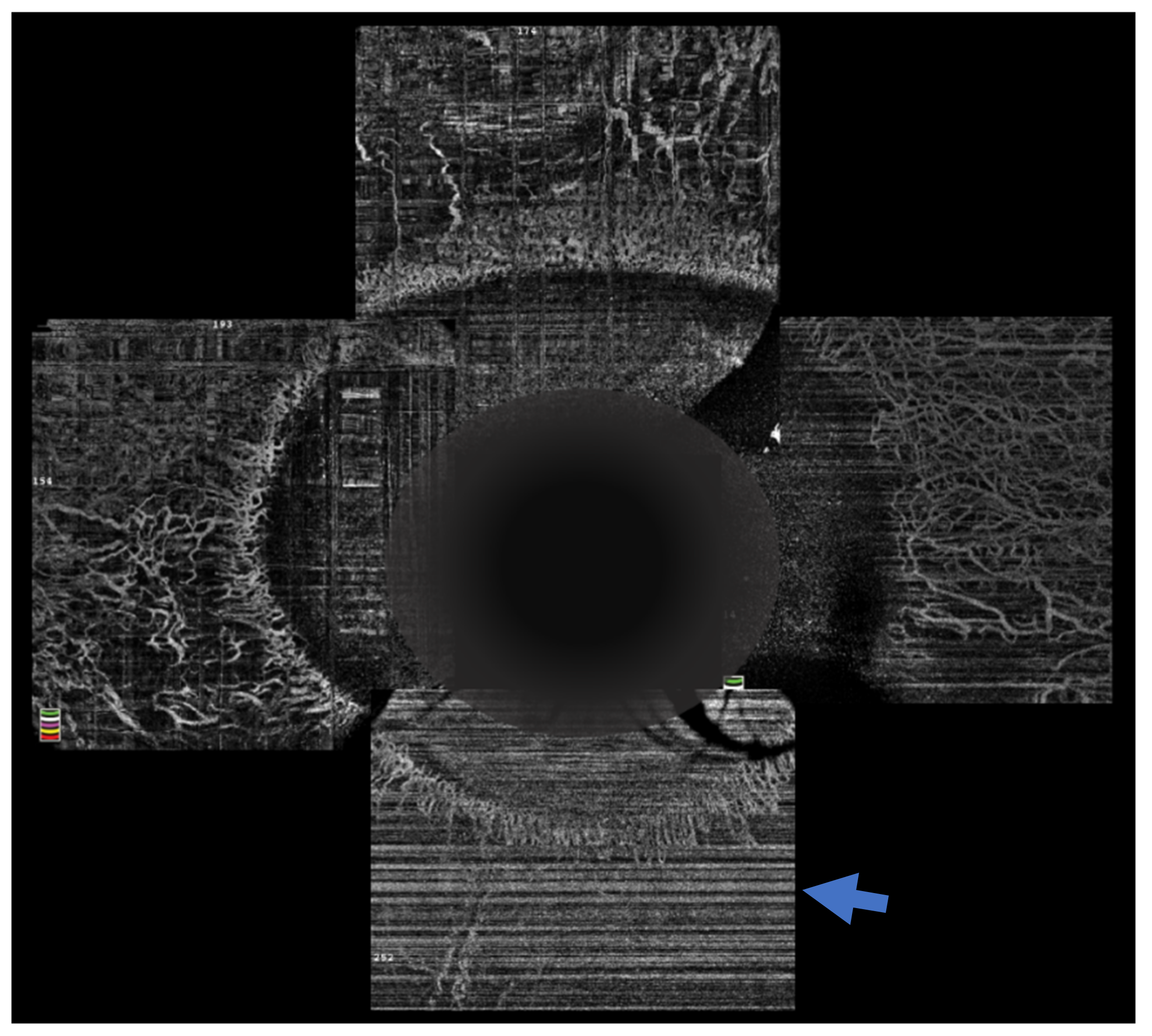
14. AS-OCT Angiography
15. Conclusions
Funding
Data Availability Statement
Conflicts of Interest
References
- Gasser, T.; Romano, V.; Seifarth, C.; Bechrakis, N.E.; Kaye, S.B.; Steger, B. Morphometric characterisation of pterygium associated with corneal stromal scarring using high-resolution anterior segment optical coherence tomography. Br. J. Ophthalmol. 2017, 101, 660–664. [Google Scholar] [CrossRef] [PubMed]
- Soliman, W.; Mohamed, T.A. Spectral domain anterior segment optical coherence tomography assessment of pterygium and pinguecula. Acta Ophthalmol. 2012, 90, 461–465. [Google Scholar] [CrossRef] [PubMed]
- Nanji, A.A.; Sayyad, F.E.; Galor, A.; Dubovy, S.; Karp, C.L. High-Resolution Optical Coherence Tomography as an Adjunctive Tool in the Diagnosis of Corneal and Conjunctival Pathology. Ocul. Surf. 2015, 13, 226–235. [Google Scholar] [CrossRef] [PubMed]
- Thomas, B.J.; Galor, A.; Nanji, A.A.; El Sayyad, F.; Wang, J.; Dubovy, S.R.; Joag, M.G.; Karp, C.L. Ultra high-resolution anterior segment optical coherence tomography in the diagnosis and management of ocular surface squamous neoplasia. Ocul. Surf. 2014, 12, 46–58. [Google Scholar] [CrossRef]
- Shousha, M.A.; Karp, C.L.; Canto, A.P.; Hodson, K.; Oellers, P.; Kao, A.A.; Bielory, B.; Matthews, J.; Dubovy, S.R.; Perez, V.L.; et al. Diagnosis of ocular surface lesions using ultra-high-resolution optical coherence tomography. Ophthalmology 2013, 120, 883–891. [Google Scholar] [CrossRef]
- Singh, S.; Mittal, R.; Ghosh, A.; Tripathy, D.; Rath, S. High-Resolution Anterior Segment Optical Coherence Tomography in Intraepithelial Versus Invasive Ocular Surface Squamous Neoplasia. Cornea 2018, 37, 1292–1298. [Google Scholar] [CrossRef]
- Kieval, J.Z.; Karp, C.L.; Abou Shousha, M.; Galor, A.; Hoffman, R.A.; Dubovy, S.R.; Wang, J. Ultra-high resolution optical coherence tomography for differentiation of ocular surface squamous neoplasia and pterygia. Ophthalmology 2012, 119, 481–486. [Google Scholar] [CrossRef]
- Shousha, M.A.; Karp, C.L.; Perez, V.L.; Hoffmann, R.; Ventura, R.; Chang, V.; Dubovy, S.R.; Wang, J. Diagnosis and management of conjunctival and corneal intraepithelial neoplasia using ultra high-resolution optical coherence tomography. Ophthalmology 2011, 118, 1531–1537. [Google Scholar] [CrossRef]
- Tran, A.Q.; Venkateswaran, N.; Galor, A.; Karp, C.L. Utility of high-resolution anterior segment optical coherence tomography in the diagnosis and management of sub-clinical ocular surface squamous neoplasia. Eye Vis. 2019, 6, 27. [Google Scholar] [CrossRef]
- Vempuluru, V.S.; Jakati, S.; Godbole, A.; Mishra, D.K.; Mohamed, A.; Kaliki, S. Spectrum of AS-OCT features of ocular surface tumors and correlation of clinico-tomographic features with histopathology: A study of 70 lesions. Int. Ophthalmol. 2021, 41, 3571–3586. [Google Scholar] [CrossRef]
- Sun, Y.; Hua, R. Ocular surface squamous neoplasia: Angiographic characteristics and response to subconjunctival/perilesional 5-fluorouracil injections. Drug Des. Devel. Ther. 2019, 13, 1323–1334. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Karp, C.L.; Galor, A.; Al Bayyat, G.J.; Jiang, H.; Wang, J. Role of optical coherence tomography angiography in the characterization of vascular network patterns of ocular surface squamous neoplasia. Ocul. Surf. 2020, 18, 926–935. [Google Scholar] [CrossRef]
- Wong, J.R.; Nanji, A.A.; Galor, A.; Karp, C.L. Management of conjunctival malignant melanoma: A review and update. Expert Rev. Ophthalmol. 2014, 9, 185–204. [Google Scholar] [CrossRef] [PubMed]
- Banayan, N.; Georgeon, C.; Grieve, K.; Ghoubay, D.; Baudouin, F.; Borderie, V. In vivo confocal microscopy and optical coherence tomography as innovative tools for the diagnosis of limbal stem cell deficiency. J. Fr. Ophtalmol. 2018, 41, e395–e406. [Google Scholar] [CrossRef] [PubMed]
- Liang, Q.; Le, Q.; Cordova, D.W.; Tseng, C.H.; Deng, S.X. Corneal Epithelial Thickness Measured Using Anterior Segment Optical Coherence Tomography as a Diagnostic Parameter for Limbal Stem Cell Deficiency. Am. J. Ophthalmol. 2020, 216, 132–139. [Google Scholar] [CrossRef] [PubMed]
- Haagdorens, M.; Behaegel, J.; Rozema, J.; Van Gerwen, V.; Michiels, S.; Ni Dhubhghaill, S.; Tassignon, M.J.; Zakaria, N. A method for quantifying limbal stem cell niches using OCT imaging. Br. J. Ophthalmol. 2017, 101, 1250–1255. [Google Scholar] [CrossRef]
- Varma, S.; Shanbhag, S.S.; Donthineni, P.R.; Mishra, D.K.; Singh, V.; Basu, S. High-Resolution Optical Coherence Tomography Angiography Characteristics of Limbal Stem Cell Deficiency. Diagnostics 2021, 11, 1130. [Google Scholar] [CrossRef]
- Shanbhag, S.S.; Patel, C.N.; Goyal, R.; Donthineni, P.R.; Singh, V.; Basu, S. Simple limbal epithelial transplantation (SLET): Review of indications, surgical technique, mechanism, outcomes, limitations, and impact. Indian J. Ophthalmol. 2019, 67, 1265–1277. [Google Scholar] [CrossRef]
- Kate, A.; Basu, S. Mini-conjunctival autograft combined with deep anterior lamellar keratoplasty for chronic sequelae of severe unilateral chemical burn: A case report. Int. J. Surg. Case Rep. 2021, 88, 106508. [Google Scholar] [CrossRef]
- Kate, A.; Mudgil, T.; Basu, S. Longitudinal Changes in Corneal Epithelial Thickness and Reflectivity following Simple Limbal Epithelial Transplantation: An Optical Coherence Tomography-Based Study. Curr. Eye Res. 2021, 1–7. [Google Scholar] [CrossRef]
- Kam, K.W.; Patel, C.N.; Nikpoor, N.; Yu, M.; Basu, S. Limbal ischemia: Reliability of clinical assessment and implications in the management of ocular burns. Indian J. Ophthalmol. 2019, 67, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Luisi, J.; Kraft, E.R.; Giannos, S.A.; Patel, K.; Schmitz-Brown, M.E.; Reffatto, V.; Merkley, K.H.; Gupta, P.K. Longitudinal Assessment of Alkali Injury on Mouse Cornea Using Anterior Segment Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Tey, K.Y.; Gan, J.; Foo, V.; Tan, B.; Ke, M.Y.; Schmetterer, L.; Mehta, J.S.; Ang, M. Role of anterior segment optical coherence tomography angiography in the assessment of acute chemical ocular injury: A pilot animal model study. Sci. Rep. 2021, 11, 16625. [Google Scholar] [CrossRef] [PubMed]
- Fung, S.S.M.; Stewart, R.M.K.; Dhallu, S.K.; Sim, D.A.; Keane, P.A.; Wilkins, M.R.; Tuft, S.J. Anterior Segment Optical Coherence Tomographic Angiography Assessment of Acute Chemical Injury. Am. J. Ophthalmol. 2019, 205, 165–174. [Google Scholar] [CrossRef] [PubMed]
- Kate, A.; Basu, S. Role of Anterior Segment-Optical Coherence Tomography Angiography in Acute Ocular Burns. Diagnostics 2022, 12, 607. [Google Scholar] [CrossRef] [PubMed]
- Czajkowski, G.; Kaluzny, B.J.; Laudencka, A.; Malukiewicz, G.; Kaluzny, J.J. Tear meniscus measurement by spectral optical coherence tomography. Optom. Vis. Sci. 2012, 89, 336–342. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Gong, L.; Lu, Y.; Jin, H.; Robitaille, M. The diagnostic significance of Fourier-domain optical coherence tomography in Sjogren syndrome, aqueous tear deficiency and lipid tear deficiency patients. Acta Ophthalmol. 2012, 90, e359–e366. [Google Scholar] [CrossRef]
- Li, N.; Deng, X.G.; He, M.F. Comparison of the Schirmer I test with and without topical anesthesia for diagnosing dry eye. Int. J. Ophthalmol. 2012, 5, 478–481. [Google Scholar] [CrossRef]
- Veres, A.; Tapaszto, B.; Kosina-Hagyo, K.; Somfai, G.M.; Nemeth, J. Imaging lid-parallel conjunctival folds with OCT and comparing its grading with the slit lamp classification in dry eye patients and normal subjects. Investig. Ophthalmol. Vis. Sci. 2011, 52, 2945–2951. [Google Scholar] [CrossRef][Green Version]
- Han, S.B.; Liu, Y.C.; Noriega, K.M.; Mehta, J.S. Applications of Anterior Segment Optical Coherence Tomography in Cornea and Ocular Surface Diseases. J. Ophthalmol. 2016, 2016, 4971572. [Google Scholar] [CrossRef]
- Hwang, E.S.; Schallhorn, J.M.; Randleman, J.B. Utility of regional epithelial thickness measurements in corneal evaluations. Surv. Ophthalmol. 2020, 65, 187–204. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Chamberlain, W.; Tan, O.; Brass, R.; Weiss, J.L.; Huang, D. Subclinical keratoconus detection by pattern analysis of corneal and epithelial thickness maps with optical coherence tomography. J. Cataract Refract. Surg. 2016, 42, 284–295. [Google Scholar] [CrossRef] [PubMed]
- Gokul, A.; Vellara, H.R.; Patel, D.V. Advanced anterior segment imaging in keratoconus: A review. Clin. Exp. Ophthalmol. 2018, 46, 122–132. [Google Scholar] [CrossRef] [PubMed]
- Belin, M.W.; Jang, H.S.; Borgstrom, M. Keratoconus: Diagnosis and Staging. Cornea 2022, 41, 1–11. [Google Scholar] [CrossRef]
- Kymionis, G.D.; Grentzelos, M.A.; Plaka, A.D.; Tsoulnaras, K.I.; Diakonis, V.F.; Liakopoulos, D.A.; Kankariya, V.P.; Pallikaris, A.I. Correlation of the corneal collagen cross-linking demarcation line using confocal microscopy and anterior segment optical coherence tomography in keratoconic patients. Am. J. Ophthalmol. 2014, 157, 110–115.e111. [Google Scholar] [CrossRef]
- Sridhar, M.S.; Martin, R. Anterior segment optical coherence tomography for evaluation of cornea and ocular surface. Indian J. Ophthalmol. 2018, 66, 367–372. [Google Scholar] [CrossRef]
- Shahhoseini, S.; Hashemi, H.; Asgari, S. Intracorneal ring segment depth in keratoconus patients: A long-term follow-up study. Int. Ophthalmol. 2018, 38, 1379–1383. [Google Scholar] [CrossRef]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha Dos Santos, V.; Garhofer, G.; Mehta, J.S.; Schmetterer, L. Anterior segment optical coherence tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef]
- Siebelmann, S.; Scholz, P.; Sonnenschein, S.; Bachmann, B.; Matthaei, M.; Cursiefen, C.; Heindl, L.M. Anterior segment optical coherence tomography for the diagnosis of corneal dystrophies according to the IC3D classification. Surv. Ophthalmol. 2018, 63, 365–380. [Google Scholar] [CrossRef]
- Repp, D.J.; Hodge, D.O.; Baratz, K.H.; McLaren, J.W.; Patel, S.V. Fuchs’ endothelial corneal dystrophy: Subjective grading versus objective grading based on the central-to-peripheral thickness ratio. Ophthalmology 2013, 120, 687–694. [Google Scholar] [CrossRef]
- Konstantopoulos, A.; Kuo, J.; Anderson, D.; Hossain, P. Assessment of the use of anterior segment optical coherence tomography in microbial keratitis. Am. J. Ophthalmol. 2008, 146, 534–542. [Google Scholar] [CrossRef] [PubMed]
- Sharma, N.; Singhal, D.; Maharana, P.K.; Agarwal, T.; Sinha, R.; Satpathy, G.; Singh Bageshwar, L.M.; Titiyal, J.S. Spectral Domain Anterior Segment Optical Coherence Tomography in Fungal Keratitis. Cornea 2018, 37, 1388–1394. [Google Scholar] [CrossRef] [PubMed]
- Bonnet, C.; Debillon, L.; Al-Hashimi, S.; Hoogewoud, F.; Monnet, D.; Bourges, J.L.; Brezin, A. Anterior segment optical coherence tomography imaging in peripheral ulcerative keratitis, a corneal structural description. BMC Ophthalmol. 2020, 20, 205. [Google Scholar] [CrossRef] [PubMed]
- Cohen, S.; Tucker, Y.; Guttman, S.; Bubis, E.; Rubinstein, Y.; Skaat, A.; Sher, I.; Rotenstreich, Y. Anterior-Segment Optical Coherence Tomography-Guided Measurement Of A Melting Ulcer For Follow-Up Of Corneoscleral Thinning Progression. Int. Med. Case Rep. J. 2019, 12, 335–338. [Google Scholar] [CrossRef]
- Yamazaki, N.; Kobayashi, A.; Yokogawa, H.; Ishibashi, Y.; Oikawa, Y.; Tokoro, M.; Sugiyama, K. In vivo imaging of radial keratoneuritis in patients with Acanthamoeba keratitis by anterior-segment optical coherence tomography. Ophthalmology 2014, 121, 2153–2158. [Google Scholar] [CrossRef]
- Soliman, W.; Nassr, M.A.; Abdelazeem, K.; Al-Hussaini, A.K. Appearance of herpes simplex keratitis on anterior segment optical coherence tomography. Int. Ophthalmol. 2019, 39, 2923–2928. [Google Scholar] [CrossRef]
- Yokogawa, H.; Kobayashi, A.; Yamazaki, N.; Sugiyama, K. In vivo imaging of coin-shaped lesions in cytomegalovirus corneal endotheliitis by anterior segment optical coherence tomography. Cornea 2014, 33, 1332–1335. [Google Scholar] [CrossRef]
- Sridhar, M.S.; Shaik, B. Anterior segment optical coherence tomography of microsporidial keratoconjunctivitis. Indian J. Ophthalmol. 2018, 66, 691–692. [Google Scholar] [CrossRef]
- Romano, V.; Tey, A.; Hill, N.M.; Ahmad, S.; Britten, C.; Batterbury, M.; Willoughby, C.; Kaye, S.B. Influence of graft size on graft survival following Descemet stripping automated endothelial keratoplasty. Br. J. Ophthalmol. 2015, 99, 784–788. [Google Scholar] [CrossRef]
- Romano, V.; Steger, B.; Myneni, J.; Batterbury, M.; Willoughby, C.E.; Kaye, S.B. Preparation of ultrathin grafts for Descemet-stripping endothelial keratoplasty with a single microkeratome pass. J. Cataract Refract. Surg. 2017, 43, 12–15. [Google Scholar] [CrossRef]
- Ruzza, A.; Parekh, M.; Avoni, L.; Wojcik, G.; Ferrari, S.; Desneux, L.; Ponzin, D.; Levis, H.J.; Romano, V. Ultra-thin DSAEK using an innovative artificial anterior chamber pressuriser: A proof-of-concept study. Graefe’s Arch. Clin. Exp. Ophthalmol. 2021, 259, 1871–1877. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Ruzza, A.; Steger, B.; Willoughby, C.E.; Rehman, S.; Ferrari, S.; Ponzin, D.; Kaye, S.B.; Romano, V. Cross-Country Transportation Efficacy and Clinical Outcomes of Preloaded Large-Diameter Ultra-Thin Descemet Stripping Automated Endothelial Keratoplasty Grafts. Cornea 2019, 38, 30–34. [Google Scholar] [CrossRef] [PubMed]
- Parekh, M.; Leon, P.; Ruzza, A.; Borroni, D.; Ferrari, S.; Ponzin, D.; Romano, V. Graft detachment and rebubbling rate in Descemet membrane endothelial keratoplasty. Surv. Ophthalmol. 2018, 63, 245–250. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.Y.; Wang, C.X.; Cai, X.Y.; Liu, Y.Z. Anterior segment OCT-based diagnosis and management of Descemet’s membrane detachment. Ophthalmologica 2012, 227, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Price, M.O.; Giebel, A.W.; Fairchild, K.M.; Price, F.W., Jr. Descemet’s membrane endothelial keratoplasty: Prospective multicenter study of visual and refractive outcomes and endothelial survival. Ophthalmology 2009, 116, 2361–2368. [Google Scholar] [CrossRef]
- Moutsouris, K.; Dapena, I.; Ham, L.; Balachandran, C.; Oellerich, S.; Melles, G.R. Optical coherence tomography, Scheimpflug imaging, and slit-lamp biomicroscopy in the early detection of graft detachment after Descemet membrane endothelial keratoplasty. Cornea 2011, 30, 1369–1375. [Google Scholar] [CrossRef]
- Huang, Y.; Lan, J.; Zang, X.; Huan, Y.; Xie, L. Optical coherence tomography-guided intracameral air injection for treatment of extensive Descemet’s membrane detachment. Br. J. Ophthalmol. 2012, 96, 1441–1443. [Google Scholar] [CrossRef]
- Gatzioufas, Z.; Schirra, F.; Low, U.; Walter, S.; Lang, M.; Seitz, B. Spontaneous bilateral late-onset Descemet membrane detachment after successful cataract surgery. J. Cataract Refract. Surg. 2009, 35, 778–781. [Google Scholar] [CrossRef]
- Pagano, L.; Gadhvi, K.A.; Coco, G.; Fenech, M.; Titley, M.; Levis, H.J.; Ruzza, A.; Ferrari, S.; Kaye, S.B.; Parekh, M.; et al. Rebubbling rate in preloaded versus surgeon prepared DSAEK. Eur. J. Ophthalmol. 2021, 32, 11206721211014380. [Google Scholar] [CrossRef]
- Romano, V.; Kazaili, A.; Pagano, L.; Gadhvi, K.A.; Titley, M.; Steger, B.; Fernandez-Vega-Cueto, L.; Meana, A.; Merayo-Lloves, J.; Diego, P.; et al. Eye bank versus surgeon prepared DMEK tissues: Influence on adhesion and re-bubbling rate. Br. J. Ophthalmol. 2022, 106, 177–183. [Google Scholar] [CrossRef]
- Scorcia, V.; Busin, M.; Lucisano, A.; Beltz, J.; Carta, A.; Scorcia, G. Anterior segment optical coherence tomography-guided big-bubble technique. Ophthalmology 2013, 120, 471–476. [Google Scholar] [CrossRef] [PubMed]
- De Benito-Llopis, L.; Mehta, J.S.; Angunawela, R.I.; Ang, M.; Tan, D.T. Intraoperative anterior segment optical coherence tomography: A novel assessment tool during deep anterior lamellar keratoplasty. Am. J. Ophthalmol. 2014, 157, 334–341.e333. [Google Scholar] [CrossRef] [PubMed]
- Bleriot, A.; Martin, E.; Lebranchu, P.; Zimmerman, K.; Libeau, L.; Weber, M.; Vabres, B.; Orignac, I. Comparison of 12-month anatomic and functional results between Z6 femtosecond laser-assisted and manual trephination in deep anterior lamellar keratoplasty for advanced keratoconus. J. Fr. Ophtalmol. 2017, 40, e193–e200. [Google Scholar] [CrossRef]
- Gadhvi, K.A.; Romano, V.; Fernandez-Vega Cueto, L.; Aiello, F.; Day, A.C.; Gore, D.M.; Allan, B.D. Femtosecond Laser-Assisted Deep Anterior Lamellar Keratoplasty for Keratoconus: Multi-surgeon Results. Am. J. Ophthalmol. 2020, 220, 191–202. [Google Scholar] [CrossRef] [PubMed]
- Singh, B.; Sharma, S.; Bharti, N.; Bharti, S. A novel method of tunnel creation using intraoperative optical coherence tomography-guided deep anterior lamellar keratoplasty. Indian J. Ophthalmol. 2021, 69, 3743–3744. [Google Scholar] [CrossRef] [PubMed]
- Doors, M.; Cals, D.W.; Berendschot, T.T.; de Brabander, J.; Hendrikse, F.; Webers, C.A.; Nuijts, R.M. Influence of anterior chamber morphometrics on endothelial cell changes after phakic intraocular lens implantation. J. Cataract Refract. Surg. 2008, 34, 2110–2118. [Google Scholar] [CrossRef] [PubMed]
- Baikoff, G.; Bourgeon, G.; Jodai, H.J.; Fontaine, A.; Lellis, F.V.; Trinquet, L. Pigment dispersion and Artisan phakic intraocular lenses: Crystalline lens rise as a safety criterion. J. Cataract Refract. Surg. 2005, 31, 674–680. [Google Scholar] [CrossRef]
- Izquierdo, L., Jr.; Henriquez, M.A.; Zakrzewski, P.A. Detection of an abnormally thick LASIK flap with anterior segment OCT imaging prior to planned LASIK retreatment surgery. J. Refract. Surg. 2008, 24, 197–199. [Google Scholar] [CrossRef]
- Zhang, Y.; Chen, Y.G.; Xia, Y.J. Comparison of corneal flap morphology using AS-OCT in LASIK with the WaveLight FS200 femtosecond laser versus a mechanical microkeratome. J. Refract. Surg. 2013, 29, 320–324. [Google Scholar] [CrossRef]
- Assayag, E.; Smadja, D.; Roditi, E.; Zadok, D.; Abulafia, A.; Weill, Y. Interface Fluid Syndrome 2 Decades After Laser-Assisted In situ Keratomileusis. Eye Contact Lens 2021, 47, 381–382. [Google Scholar] [CrossRef]
- Senthil, S.; Rathi, V.; Garudadri, C. Misleading Goldmann applanation tonometry in a post-LASIK eye with interface fluid syndrome. Indian J. Ophthalmol. 2010, 58, 333–335. [Google Scholar] [CrossRef] [PubMed]
- Majander, A.S.; Lindahl, P.M.; Vasara, L.K.; Krootila, K. Anterior segment optical coherence tomography in congenital corneal opacities. Ophthalmology 2012, 119, 2450–2457. [Google Scholar] [CrossRef]
- Nischal, K.K.; Lathrop, K.L. The Palisades of Vogt in Congenital Corneal Opacification (An American Ophthalmological Society Thesis). Trans. Am. Ophthalmol. Soc. 2016, 114, T8. [Google Scholar] [PubMed]
- Ramappa, M.; Chaurasia, S.; Jalali, S. Keratoplasty in congenital primary aphakia. Indian J. Ophthalmol. 2018, 66, 341–342. [Google Scholar] [CrossRef] [PubMed]
- Ramappa, M.; Mohamed, A.; Achanta, D.S.R.; Tumati, C.S.K.; Chaurasia, S.; Edward, D.P. Descemet Stripping Automated Endothelial Keratoplasty in Pediatric Age Group: A Decade of Our Experience. Cornea 2021, 40, 1571–1580. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.A. Primary cysts of the iris. Trans. Am. Ophthalmol. Soc. 1981, 79, 771–809. [Google Scholar]
- Asif, M.I.; Bafna, R.K.; Sharma, N.; Kaginalkar, A.; Sinha, R.; Agarwal, T.; Maharana, P.K.; Kaur, M.; Taank, P.; Titiyal, J.S. Microscope Integrated Optical Coherence Tomography Guided Descemet Stripping Automated Endothelial Keratoplasty in Congenital Hereditary Endothelial Dystrophy. Clin. Ophthalmol. 2021, 15, 3173–3181. [Google Scholar] [CrossRef]
- Ryan, D.S.; Sia, R.K.; Colyer, M.; Stutzman, R.D.; Wroblewski, K.J.; Mines, M.J.; Bower, K.S. Anterior segment imaging in combat ocular trauma. J. Ophthalmol. 2013, 2013, 308259. [Google Scholar] [CrossRef]
- Zheng, K.; Huang, H.; Peng, K.; Cai, J.; Jhanji, V.; Chen, H. Change of Optical Intensity during Healing Process of Corneal Wound on Anterior Segment Optical Coherence Tomography. Sci. Rep. 2016, 6, 32352. [Google Scholar] [CrossRef]
- Zheng, K.K.; Cai, J.; Rong, S.S.; Peng, K.; Xia, H.; Jin, C.; Lu, X.; Liu, X.; Chen, H.; Jhanji, V. Longitudinal Evaluation of Wound Healing after Penetrating Corneal Injury: Anterior Segment Optical Coherence Tomography Study. Curr. Eye Res. 2017, 42, 982–986. [Google Scholar] [CrossRef]
- Armarnik, S.; Mimouni, M.; Goldenberg, D.; Segev, F.; Meshi, A.; Segal, O.; Geffen, N. Characterization of deeply embedded corneal foreign bodies with anterior segment optical coherence tomography. Graefe’s Arch. Clin. Exp. Ophthalmol. 2019, 257, 1247–1252. [Google Scholar] [CrossRef] [PubMed]
- Akbas, E.; Barut Selver, O.; Palamar, M. Retrospective Evaluation of Corneal Foreign Bodies with Anterior Segment Optical Coherence Tomography. Turk. J. Ophthalmol. 2021, 51, 265–268. [Google Scholar] [CrossRef] [PubMed]
- Al-Ghadeer, H.A.; Al-Assiri, A. Identification and localization of multiple intrastromal foreign bodies with anterior segment optical coherence tomography and ocular Pentacam. Int. Ophthalmol. 2014, 34, 355–358. [Google Scholar] [CrossRef]
- Mahmoud, A.; Messaoud, R.; Abid, F.; Ksiaa, I.; Bouzayene, M.; Khairallah, M. Anterior segment optical coherence tomography and retained vegetal intraocular foreign body masquerading as chronic anterior uveitis. J. Ophthalmic Inflamm. Infect. 2017, 7, 13. [Google Scholar] [CrossRef] [PubMed]
- Joshi, V.P.; Vaishnavi, K.S.; Ojha, S.K.; Singh, V.; Basu, S. A reliable animal model of corneal stromal opacity: Development and validation using in vivo imaging. Ocul. Surf. 2020, 18, 681–688. [Google Scholar] [CrossRef] [PubMed]
- Han, S.B.; Ang, H.; Balehosur, D.; Peh, G.; Chaurasia, S.S.; Tan, D.T.; Mehta, J.S. A mouse model of corneal endothelial decompensation using cryoinjury. Mol. Vis. 2013, 19, 1222–1230. [Google Scholar] [PubMed]
- Jiao, H.; Hill, L.J.; Downie, L.E.; Chinnery, H.R. Anterior segment optical coherence tomography: Its application in clinical practice and experimental models of disease. Clin. Exp. Optom. 2019, 102, 208–217. [Google Scholar] [CrossRef]
- Shirzaei Sani, E.; Kheirkhah, A.; Rana, D.; Sun, Z.; Foulsham, W.; Sheikhi, A.; Khademhosseini, A.; Dana, R.; Annabi, N. Sutureless repair of corneal injuries using naturally derived bioadhesive hydrogels. Sci. Adv. 2019, 5, eaav1281. [Google Scholar] [CrossRef]
- Shen, L.; Sun, P.; Zhang, C.; Yang, L.; Du, L.; Wu, X. Therapy of corneal endothelial dysfunction with corneal endothelial cell-like cells derived from skin-derived precursors. Sci. Rep. 2017, 7, 13400. [Google Scholar] [CrossRef]
- Pantalon, A.; Pfister, M.; Aranha Dos Santos, V.; Sapeta, S.; Unterhuber, A.; Pircher, N.; Schmidinger, G.; Garhofer, G.; Schmidl, D.; Schmetterer, L.; et al. Ultrahigh-resolution anterior segment optical coherence tomography for analysis of corneal microarchitecture during wound healing. Acta Ophthalmol. 2019, 97, e761–e771. [Google Scholar] [CrossRef]
- Ang, M.; Devarajan, K.; Das, S.; Yam, G.H.F.; Htoon, H.M.; Chen, S.; Liu, X.; Liu, L.; Girard, M.; Mehta, J.S. Novel application of In Vivo Micro-Optical Coherence Tomography to assess Cornea scarring in an Animal Model. Sci. Rep. 2018, 8, 11483. [Google Scholar] [CrossRef] [PubMed]
- Sharif, Z.; Sharif, W. Corneal neovascularization: Updates on pathophysiology, investigations & management. Rom. J. Ophthalmol. 2019, 63, 15–22. [Google Scholar] [PubMed]
- Roshandel, D.; Eslani, M.; Baradaran-Rafii, A.; Cheung, A.Y.; Kurji, K.; Jabbehdari, S.; Maiz, A.; Jalali, S.; Djalilian, A.R.; Holland, E.J. Current and emerging therapies for corneal neovascularization. Ocul. Surf. 2018, 16, 398–414. [Google Scholar] [CrossRef] [PubMed]
- Martin, R. Cornea and anterior eye assessment with slit lamp biomicroscopy, specular microscopy, confocal microscopy, and ultrasound biomicroscopy. Indian J. Ophthalmol. 2018, 66, 195–201. [Google Scholar] [CrossRef]
- Ang, M.; Sim, D.A.; Keane, P.A.; Sng, C.C.; Egan, C.A.; Tufail, A.; Wilkins, M.R. Optical Coherence Tomography Angiography for Anterior Segment Vasculature Imaging. Ophthalmology 2015, 122, 1740–1747. [Google Scholar] [CrossRef]
- Lee, W.D.; Devarajan, K.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. Optical coherence tomography angiography for the anterior segment. Eye Vis. 2019, 6, 4. [Google Scholar] [CrossRef]
- Roberts, P.K.; Goldstein, D.A.; Fawzi, A.A. Anterior Segment Optical Coherence Tomography Angiography for Identification of Iris Vasculature and Staging of Iris Neovascularization: A Pilot Study. Curr. Eye Res. 2017, 42, 1136–1142. [Google Scholar] [CrossRef]
- Akagi, T.; Uji, A.; Huang, A.S.; Weinreb, R.N.; Yamada, T.; Miyata, M.; Kameda, T.; Ikeda, H.O.; Tsujikawa, A. Conjunctival and Intrascleral Vasculatures Assessed Using Anterior Segment Optical Coherence Tomography Angiography in Normal Eyes. Am. J. Ophthalmol. 2018, 196, 1–9. [Google Scholar] [CrossRef]
- Ong, H.S.; Tey, K.Y.; Ke, M.; Tan, B.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. A pilot study investigating anterior segment optical coherence tomography angiography as a non-invasive tool in evaluating corneal vascularisation. Sci. Rep. 2021, 11, 1212. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gupta, N.; Varshney, A.; Ramappa, M.; Basu, S.; Romano, V.; Acharya, M.; Gaur, A.; Kapur, N.; Singh, A.; Shah, G.; et al. Role of AS-OCT in Managing Corneal Disorders. Diagnostics 2022, 12, 918. https://doi.org/10.3390/diagnostics12040918
Gupta N, Varshney A, Ramappa M, Basu S, Romano V, Acharya M, Gaur A, Kapur N, Singh A, Shah G, et al. Role of AS-OCT in Managing Corneal Disorders. Diagnostics. 2022; 12(4):918. https://doi.org/10.3390/diagnostics12040918
Chicago/Turabian StyleGupta, Nidhi, Akhil Varshney, Muralidhar Ramappa, Sayan Basu, Vito Romano, Manisha Acharya, Abha Gaur, Neha Kapur, Aastha Singh, Gaurav Shah, and et al. 2022. "Role of AS-OCT in Managing Corneal Disorders" Diagnostics 12, no. 4: 918. https://doi.org/10.3390/diagnostics12040918
APA StyleGupta, N., Varshney, A., Ramappa, M., Basu, S., Romano, V., Acharya, M., Gaur, A., Kapur, N., Singh, A., Shah, G., Chaudhary, I., Patel, N., Tiwari, A., Kate, A., Sangwan, V., & Mathur, U. (2022). Role of AS-OCT in Managing Corneal Disorders. Diagnostics, 12(4), 918. https://doi.org/10.3390/diagnostics12040918






