Diagnosis and Treatment of Lumbar Giant Cell Tumor of the Spine: Update on Current Management Strategies
Abstract
1. Introduction
2. Materials and Methods
3. Results
4. Discussion
4.1. Epidemiology
4.2. Diagnosis
4.2.1. Clinical Presentation
4.2.2. Imaging
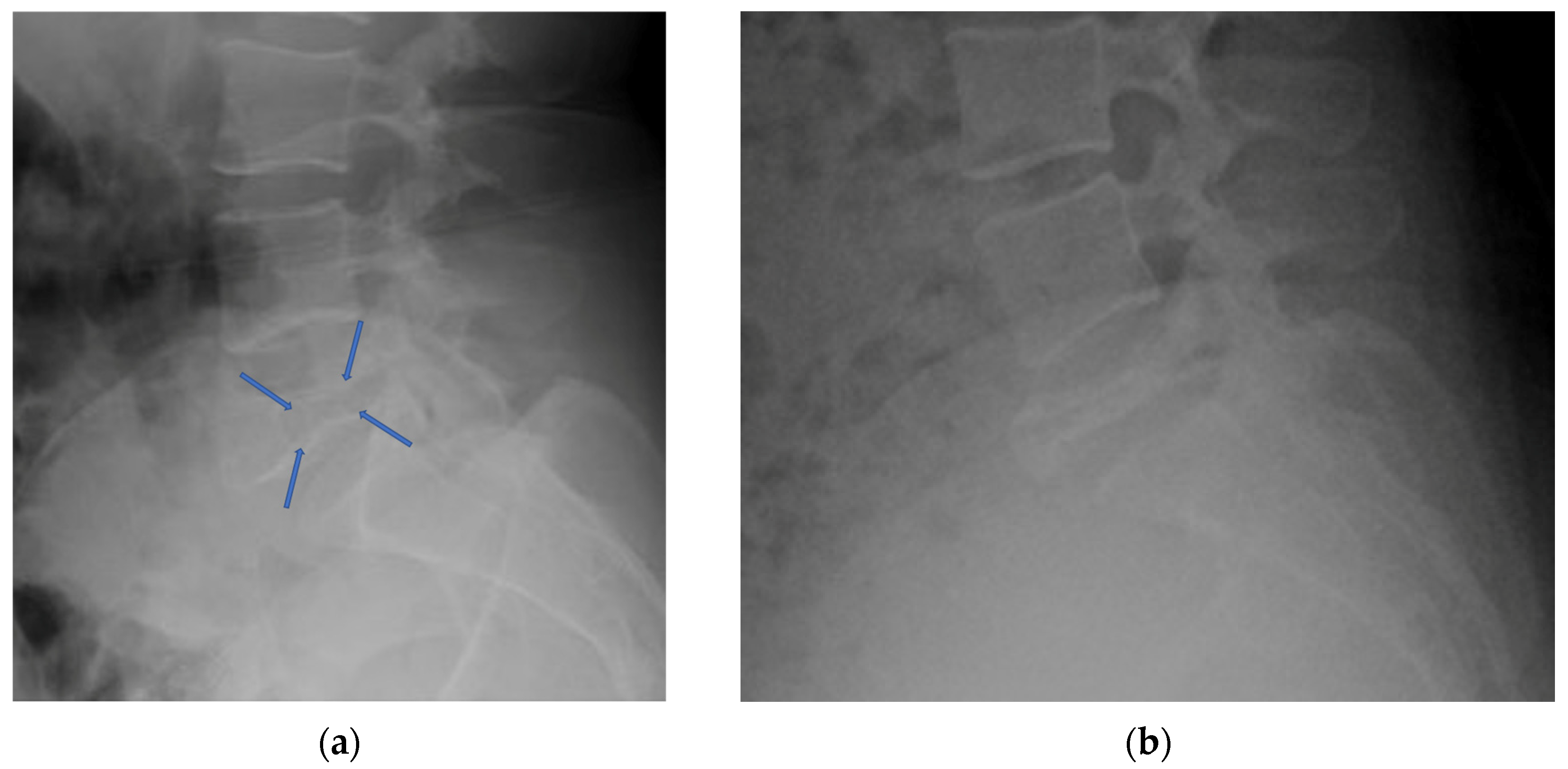
4.2.3. Plain Radiograph
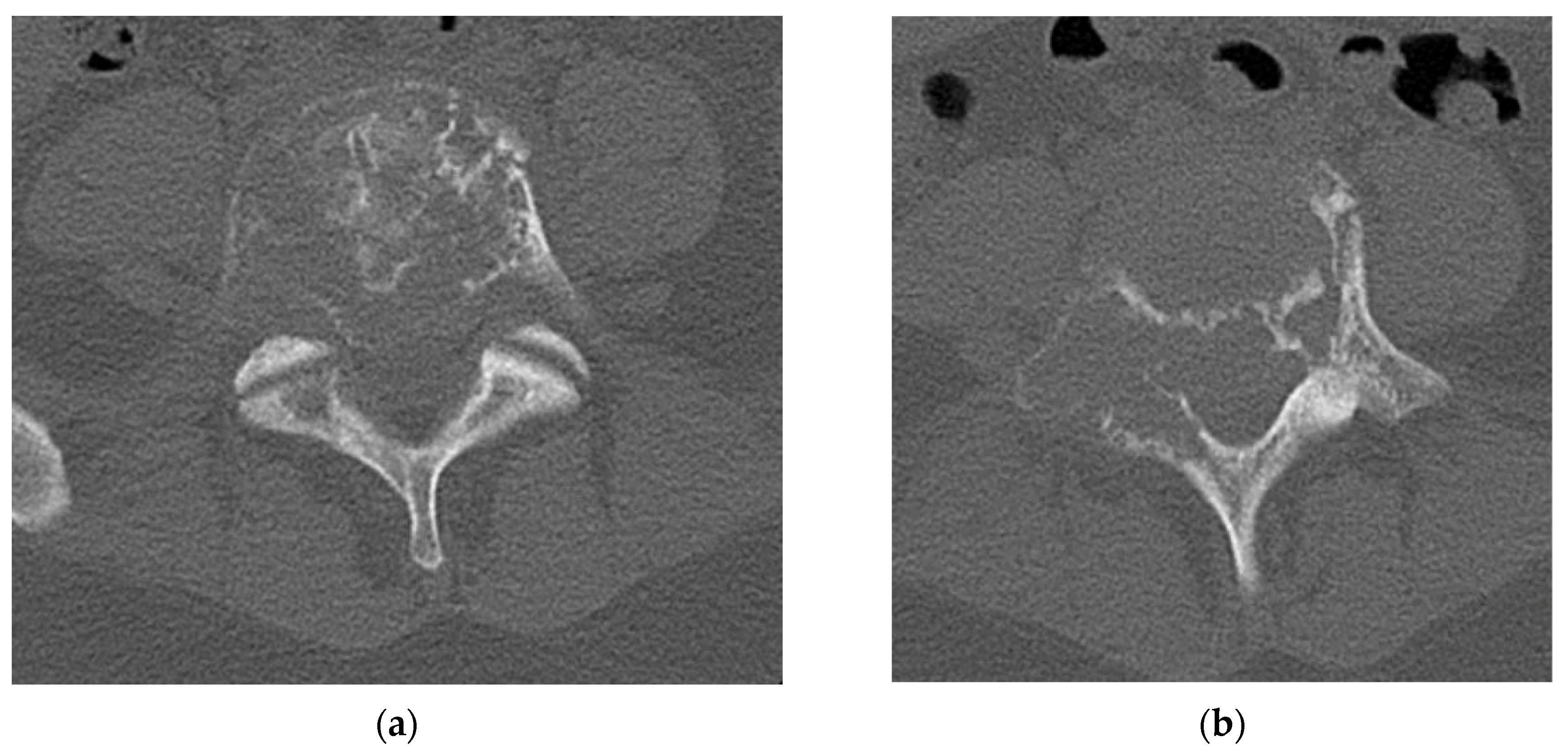
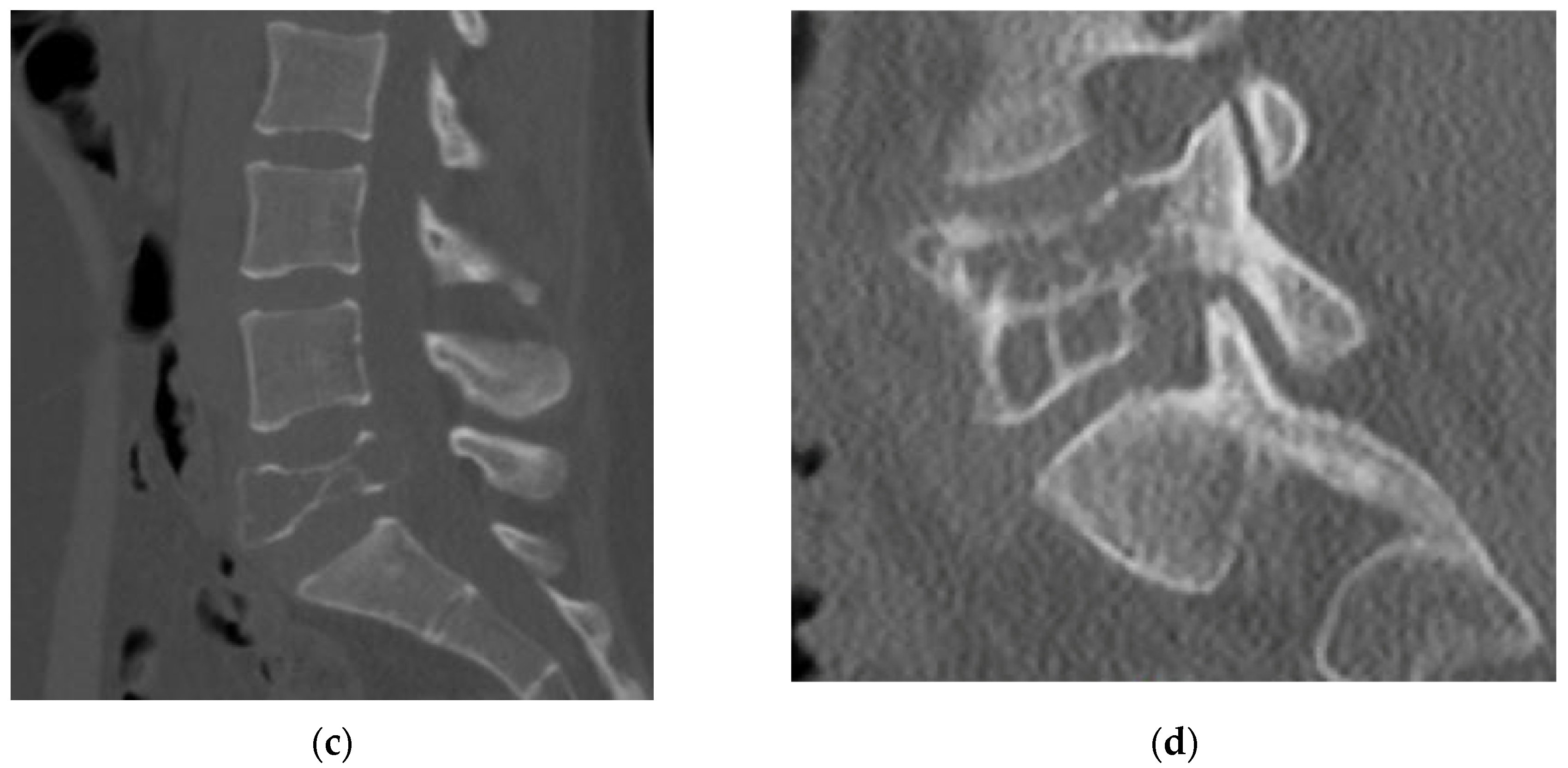
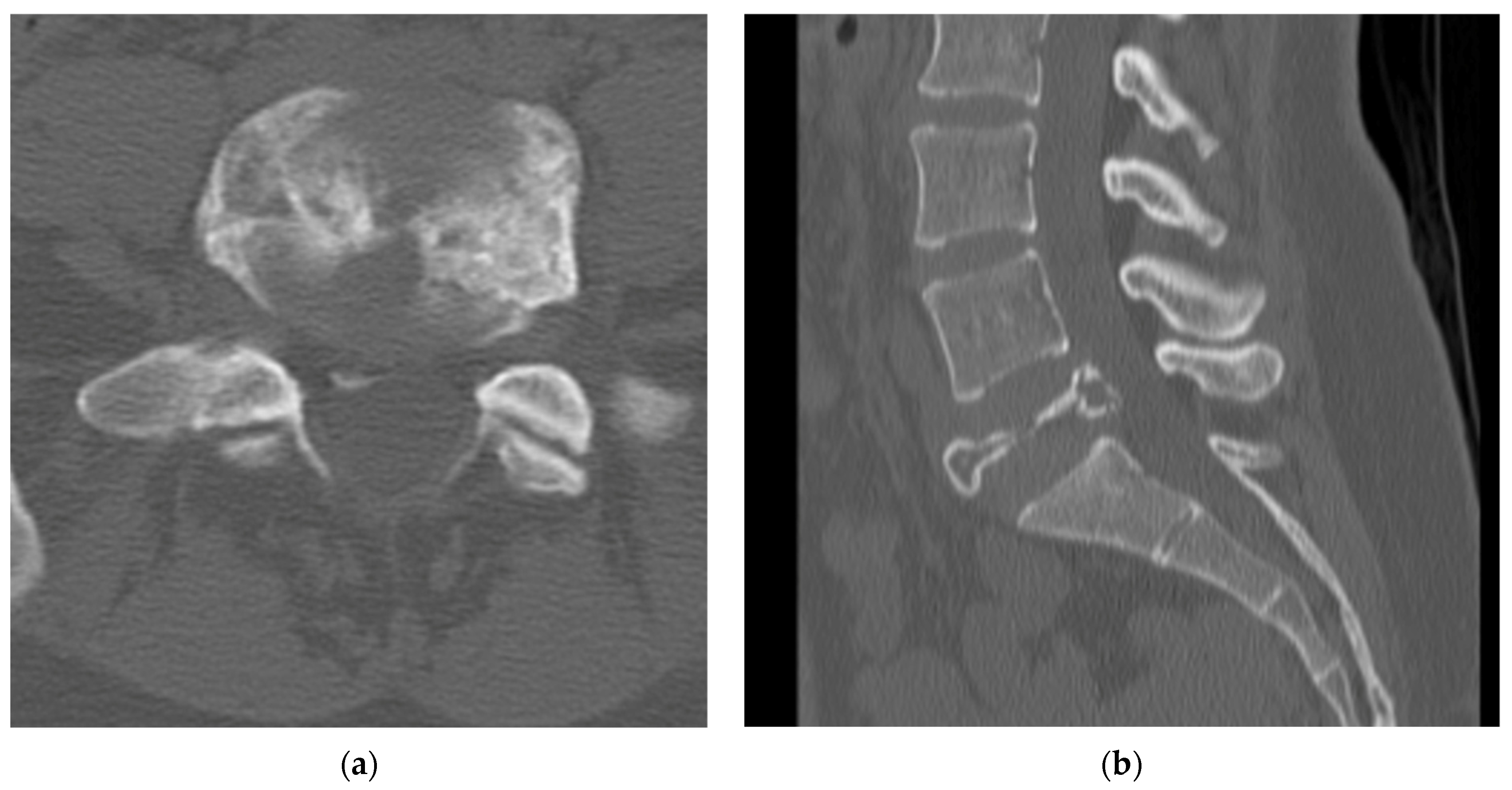
4.2.4. Computerized Tomography Scan
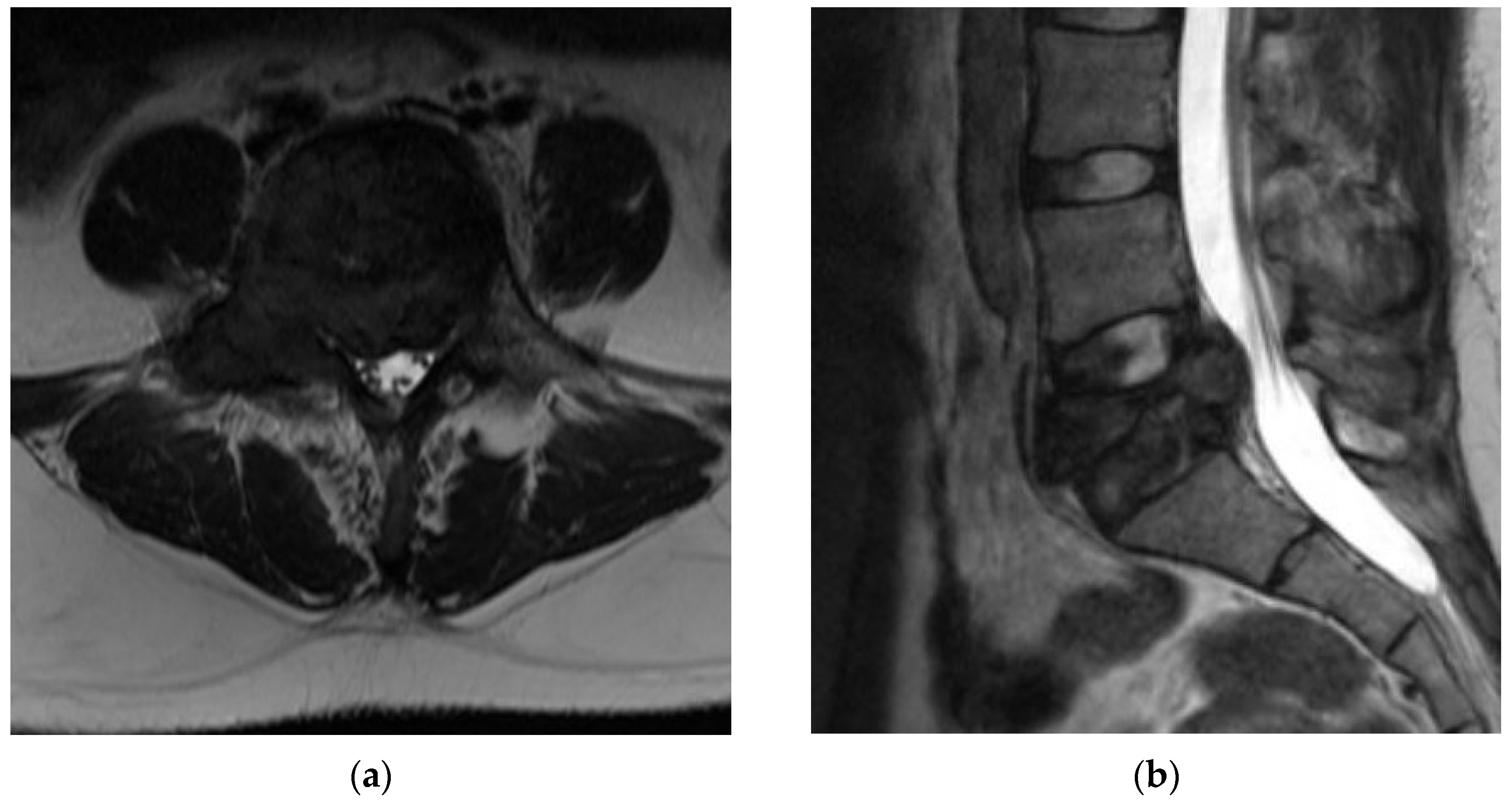
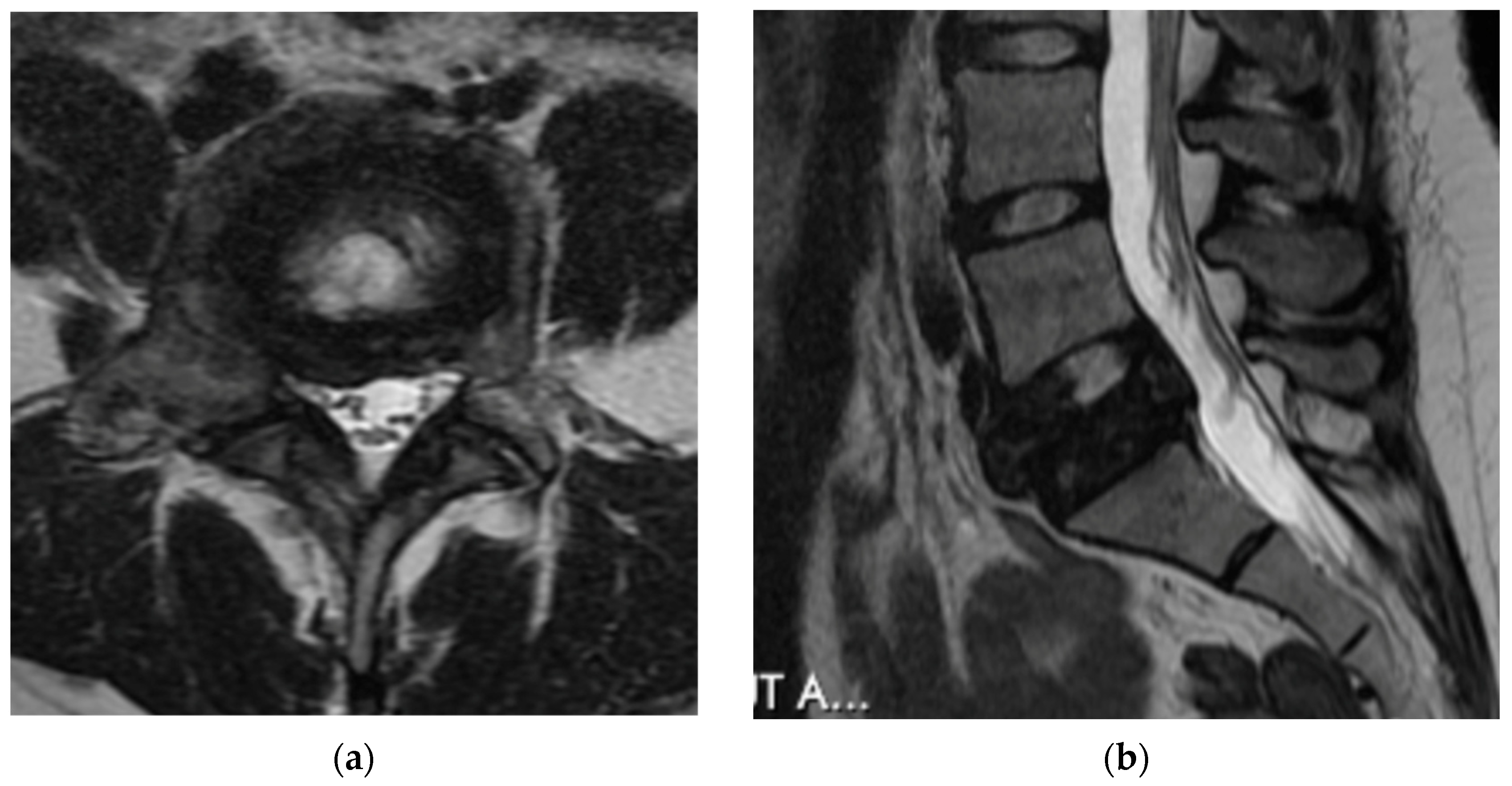
4.2.5. MRI
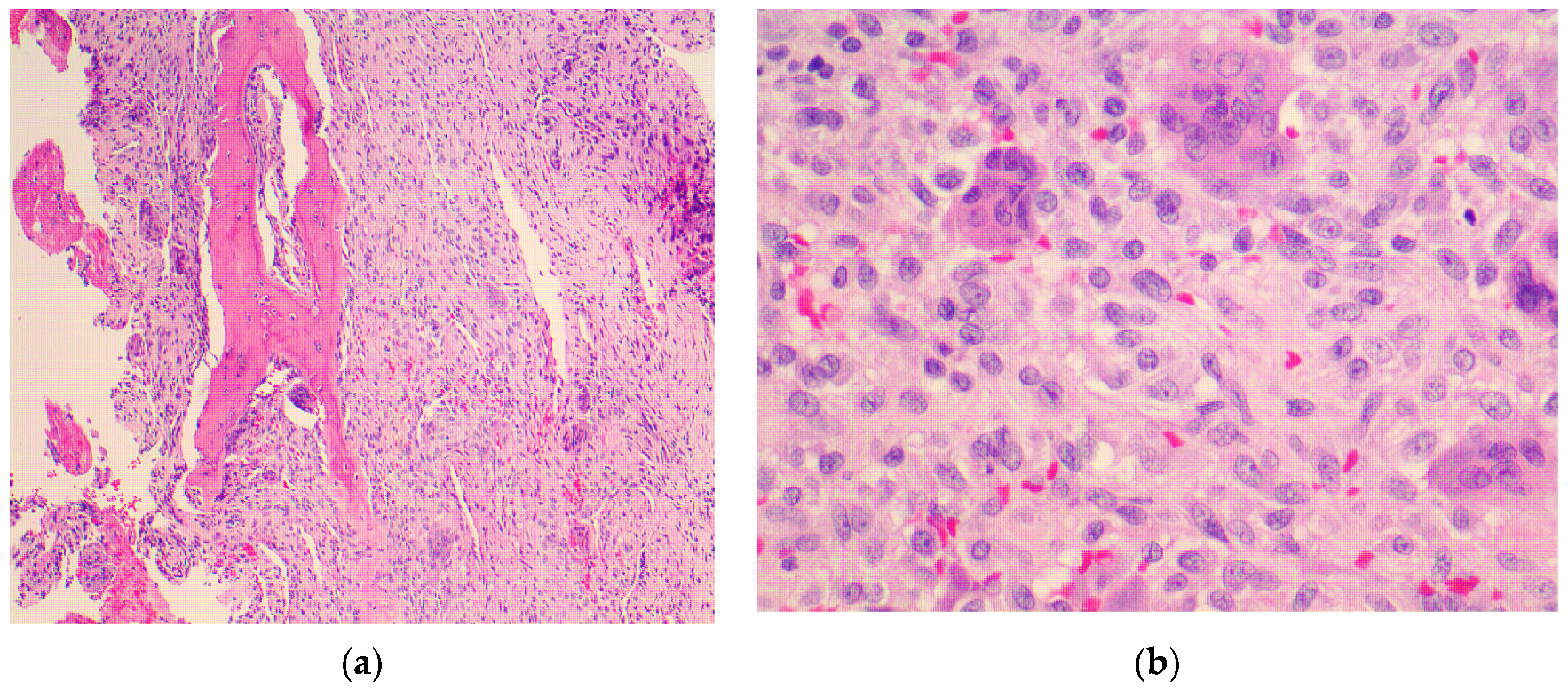
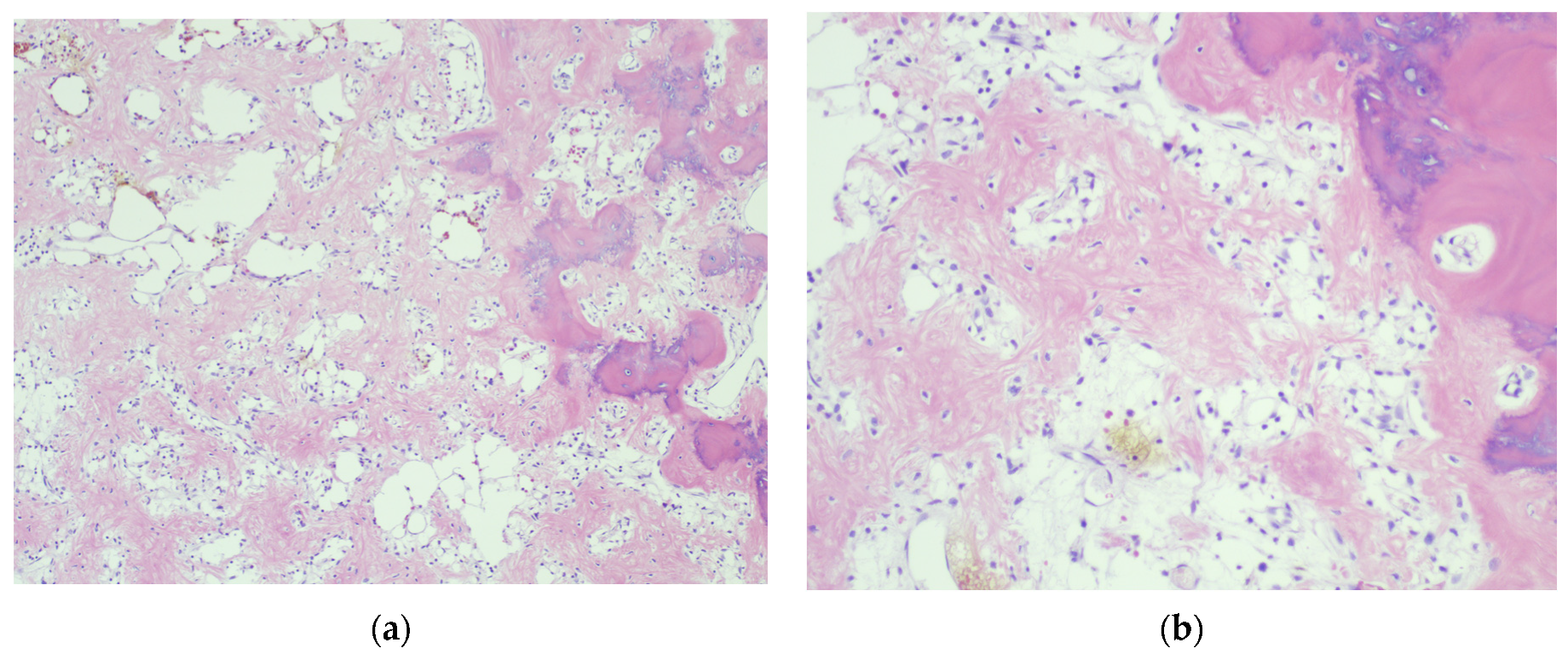
4.3. Final Diagnosis
4.4. Classification and Staging
4.4.1. Lung Metastases and Malignant Transformation
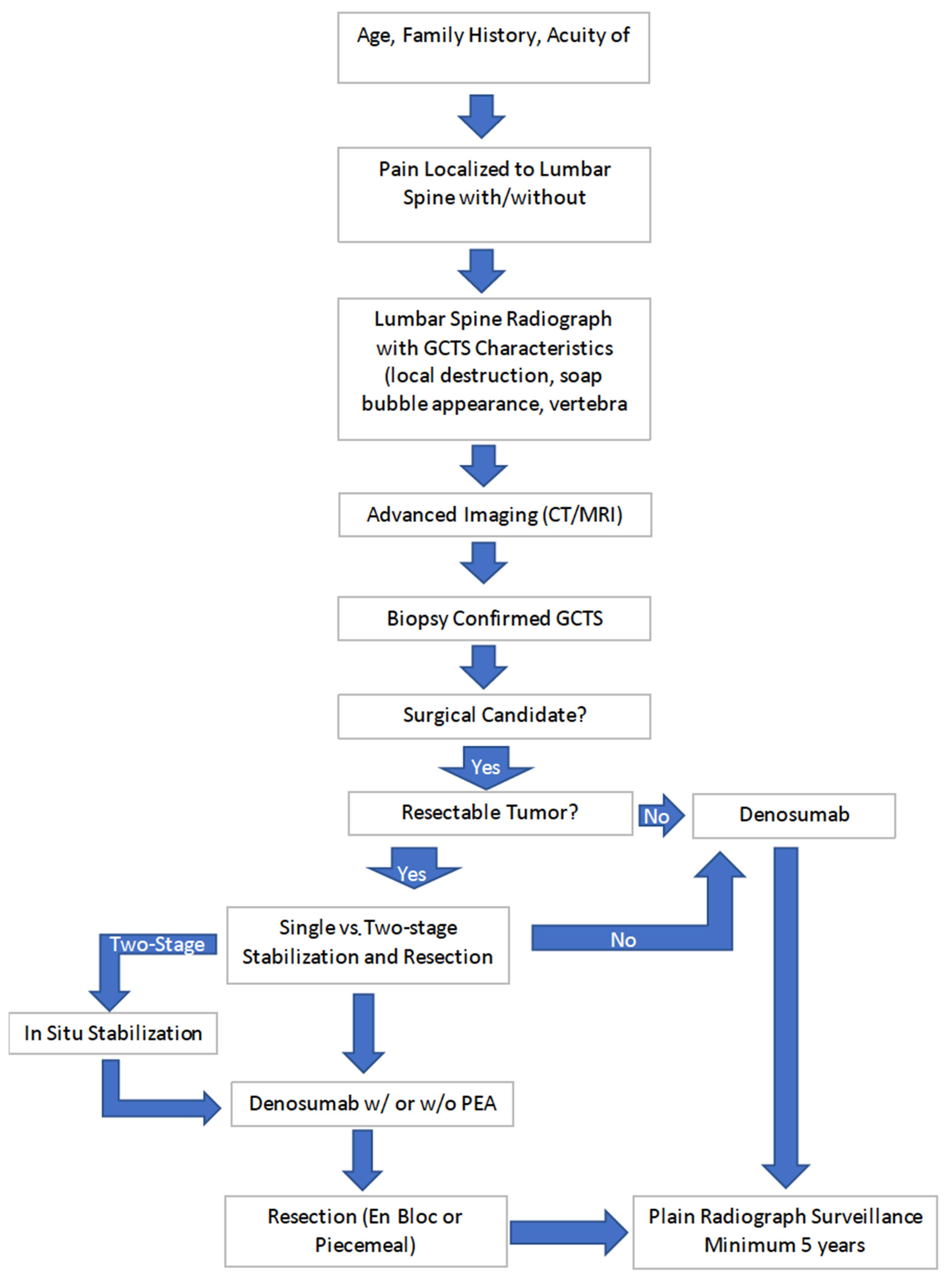
4.4.2. Management of Giant Cell Tumor of the Lumbar Spine
4.5. Denosumab
4.5.1. Radiation Therapy
4.5.2. Surgical Treatment of GCTS of the Lumbar Spine
4.5.3. Secondary Aneurysmal Bone Cysts
4.5.4. Diagnosis and Management of Recurrent Giant Cell Tumor
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
Appendix A
| Type | Reference # | Author | Publication Year | Title | Publishing Journal | Article Type | Level of Evidence |
|---|---|---|---|---|---|---|---|
| Journal Article | [40] | T. L. Aghaloo; A. L. Felsenfeld; S. Tetradis | 2010 | Osteonecrosis of the Jaw in a Patient on Denosumab | Journal of Oral and Maxillofacial Surgery | Case Report | 4 |
| Journal Article | [1] | P. Anract; G. De Pinieux; P. Cottias; P. Pouillart; M. Forest; B. Tomeno | 1998 | Malignant giant-cell tumours of bone | International Orthopaedics | Retrospective Case Series | 3 |
| Journal Article | [29] | J. Aoki; H. Tanikawa; K. Ishii; G. S. Seo; O. Karakida; S. Sone; T. Ichikawa; K. Kachi | 1996 | MR findings indicative of hemosiderin in giant-cell tumor of bone: frequency, cause, and diagnostic significance | AJR Am J Roentgenol | Retrospective Case Series | 3 |
| Journal Article | [5] | S. Boriani; S. Bandiera; R. Casadei; L. Boriani; R. Donthineni; A. Gasbarrini; E. Pignotti; R. Biagini; J. H. Schwab | 2012 | Giant cell tumor of the mobile spine: a review of 49 cases | Spine (Phila Pa 1976) | Retrospective Case Series | 3 |
| Journal Article | [11] | S. Boriani; R. Cecchinato; F. Cuzzocrea; S. Bandiera; M. Gambarotti; A. Gasbarrini | 2020 | Denosumab in the treatment of giant cell tumor of the spine. Preliminary report, review of the literature and protocol proposal | European Spine Journal | Retrospective Case Series | 3 |
| Journal Article | [4] | S. Boriani; J. N. Weinstein; R. Biagini | 1997 | Primary bone tumors of the spine. Terminology and surgical staging | Spine (Phila Pa 1976) | Literature Update | N/A |
| Journal Article | [23] | M. Campanacci; N. Baldini; S. Boriani; A. Sudanese | 1987 | Giant-cell tumor of bone | JBJS | Retrospective Case Series | 3 |
| Journal Article | [43] | A. Chakravarti; I. J. Spiro; E. B. Hug; H. J. Mankin; J. T. Efird; H. D. Suit | 1999 | Megavoltage radiation therapy for axial and inoperable giant-cell tumor of bone | J Bone Joint Surg Am | Retrospective Case Series | 3 |
| Journal Article | [47] | B. Z. Chin; T. Ji; X. Tang; R. Yang; W. Guo | 2019 | Three-Level Lumbar En Bloc Spondylectomy with Three-Dimensional−Printed Vertebrae Reconstruction for Recurrent Giant Cell Tumor | World Neurosurgery | Case Report | 4 |
| Journal Article | [48] | R. A. de Carvalho Cavalcante; R. A. Silva Marques; V. G. dos Santos; E. Sabino; J. A. C. Fraga; V. A. Zaccariotti; J. B. Arruda; Y. B. Fernandes | 2016 | Spondylectomy for Giant Cell Tumor After Denosumab Therapy | Spine (Philadelphia, Pa. 1976) | Case Report | 4 |
| Journal Article | [32] | M. Dominkus; P. Ruggieri; F. Bertoni; A. Briccoli; P. Picci; M. Rocca; M. Mercuri | 2006 | Histologically verified lung metastases in benign giant cell tumours--14 cases from a single institution | Int Orthop | Retrospective Case Series | 3 |
| Journal Article | [24] | R. Donthineni; L. Boriani; O. Ofluoglu; S. Bandiera | 2009 | Metastatic behaviour of giant cell tumour of the spine | International Orthopaedics | Retrospective Case Series | 3 |
| Journal Article | [38] | T. Goldschlager; N. Dea; M. Boyd; J. Reynolds; S. Patel; L. D. Rhines; E. Mendel; M. Pacheco; E. Ramos; T. A. Mattei; C. G. Fisher | 2015 | Giant cell tumors of the spine: has denosumab changed the treatment paradigm? | Journal of Neurosurgery: Spine SPI | Prospective Case Series | 2 |
| Journal Article | [33] | R. Gupta; V. Seethalakshmi; N. A. Jambhekar; S. Prabhudesai; N. Merchant; A. Puri; M. Agarwal | 2008 | Clinicopathologic profile of 470 giant cell tumors of bone from a cancer hospital in western India | Ann Diagn Pathol | Retrospective Case Series | 3 |
| Journal Article | [6] | R. A. Hart; S. Boriani; R. Biagini; B. Currier; J. N. Weinstein | 1997 | A system for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine | Spine (Phila Pa 1976) | Retrospective Case Series | 3 |
| Journal Article | [22] | Q. Jia; G. Chen; J. Cao; X. Yang; Z. Zhou; H. Wei; T. Liu; J. Xiao | 2019 | Clinical features and prognostic factors of pediatric spine giant cell tumors: report of 31 clinical cases in a single center | The spine journal | Retrospective Case Series | 3 |
| Journal Article | [41] | D. C. Khan; S. Malhotra; R. E. Stevens; A. D. Steinfeld | 1999 | Radiotherapy for the treatment of giant cell tumor of the spine: a report of six cases and review of the literature | Cancer Invest | Retrospective Case Series | 3 |
| Journal Article | [49] | H. Kinoshita; S. Orita; T. Yonemoto; T. Ishii; S. Iwata; H. Kamoda; T. Tsukanishi; K. Inage; K. Abe; M. Inoue; M. Norimoto; T. Umimura; K. Fujimoto; Y. Shiga; H. Kanamoto; T. Furuya; K. Takahashi; S. Ohtori | 2019 | Successful total en bloc spondylectomy of the L3 vertebra with a paravertebral giant cell tumor following preoperative treatment with denosumab: a case report | Journal of Medical Case Reports | Case Report | 4 |
| Journal Article | [2] | J. W. Kwon; H. W. Chung; E. Y. Cho; S. H. Hong; S. H. Choi; Y. C. Yoon; S. K. Yi | 2007 | MRI findings of giant cell tumors of the spine | AJR Am J Roentgenol | Retrospective Case Series | 3 |
| Journal Article | [54] | R. Lador; G. Regev; K. Salame; M. Khashan; Z. Lidar | 2020 | Use of 3-Dimensional Printing Technology in Complex Spine Surgeries | World Neurosurgery | Retrospective Case Series | 3 |
| Journal Article | [45] | R. E. Leggon; R. Zlotecki; J. Reith; M. T. Scarborough | 2004 | Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature | Clin Orthop Relat Res | Retrospective Case Series | 3 |
| Journal Article | [39] | T. A. Mattei; E. Ramos; A. A. Rehman; A. Shaw; S. R. Patel; E. Mendel | 2014 | Sustained long-term complete regression of a giant cell tumor of the spine after treatment with denosumab | The Spine Journal | Case Report | 4 |
| Journal Article | [18] | W. M. Mendenhall; R. A. Zlotecki; M. T. Scarborough; C. P. Gibbs; N. P. Mendenhall | 2006 | Giant cell tumor of bone | Am J Clin Oncol | Literature Review | N/A |
| Journal Article | [50] | K. Minato; T. Hirano; H. Kawashima; T. Yamagishi; K. Watanabe; M. Ohashi; A. Ogose; N. Endo | 2021 | Minimally Invasive Spinal Stabilization with Denosumab before Total Spondylectomy for a Collapsing Lower Lumbar Spinal Giant Cell Tumor | Acta medica Okayama | Case Report | 4 |
| Journal Article | [16] | M. D. Murphey; C. L. Andrews; D. J. Flemming; H. T. Temple; W. S. Smith; J. G. Smirniotopoulos | 1996 | From the archives of the AFIP. Primary tumors of the spine: radiologic pathologic correlation | Radiographics | Literature Review | N/A |
| Journal Article | [28] | M. D. Murphey; G. C. Nomikos; D. J. Flemming; F. H. Gannon; H. T. Temple; M. J. Kransdorf | 2001 | From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: radiologic-pathologic correlation | Radiographics | Literature Review | N/A |
| Journal Article | [19] | S. Orguc; R. Arkun | 2014 | Primary tumors of the spine | Semin Musculoskelet Radiol | Literature Review | N/A |
| Journal Article | [53] | E. Palmerini; E. L. Staals; L. B. Jones; D. M. Donati; A. Longhi; R. L. Randall | 2020 | Role of (Neo)adjuvant Denosumab for Giant Cell Tumor of Bone | Current Treatment Options in Oncology | Case Report | 4 |
| Journal Article | [26] | S. S. Randhawa; A. K. N. Kwan; C. K. Chiu; C. Y. W. Chan; M. K. Kwan | 2016 | Neurological Recovery in Two Patients with Cauda Equina Syndrome Secondary to L5 Lumbar Spine Giant Cell Tumour after Treatment with Denosumab without Surgery | Asian Spine J | Retrospective Case Series | 3 |
| Journal Article | [15] | Yonezawa N, Murakami H, Demura S, et al, | 2019 | Morphologic Changes After Denosumab Therapy in Patients with Giant Cell Tumor of the Spine: Report of Four Cases and a Review of the Literature | World Neurosurgery | Retrospective Case Series | 3 |
| Journal Article | [36] | M. Rock | 1990 | Curettage of giant cell tumor of bone. Factors influencing local recurrences and metastasis | Chir Organi Mov | Retrospective Case Series | 3 |
| Journal Article | [34] | M. G. Rock; D. J. Pritchard; K. K. Unni | 1984 | Metastases from histologically benign giant-cell tumor of bone | J Bone Joint Surg Am | Retrospective Case Series | 3 |
| Journal Article | [46] | F. Roeder; C. Timke; F. Zwicker; C. Thieke; M. Bischof; J. Debus; P. E. Huber | 2010 | Intensity modulated radiotherapy (IMRT) in benign giant cell tumors--a single institution case series and a short review of the literature | Radiat Oncol | Retrospective Case Series | 3 |
| Journal Article | [44] | P. Ruggieri; A. F. Mavrogenis; G. Ussia; A. Angelini; P. J. Papagelopoulos; M. Mercuri | 2010 | Recurrence after and complications associated with adjuvant treatments for sacral giant cell tumor | Clin Orthop Relat Res | Retrospective Case Series | 3 |
| Journal Article | [20] | B. K. Sanjay; F. H. Sim; K. K. Unni; R. A. McLeod; R. A. Klassen | 1993 | Giant-cell tumours of the spine | J Bone Joint Surg Br | Retrospective Case Series | 3 |
| Journal Article | [51] | D. R. Santiago-Dieppa; L. S. Hwang; A. Bydon; Z. L. Gokaslan; E. F. McCarthy; T. F. Witham | 2014 | L4 and L5 spondylectomy for en bloc resection of giant cell tumor and review of the literature | Evidence-based spine-care journal | Case Report | 4 |
| Journal Article | [42] | R. R. Sharma; A. K. Mahapatra; S. J. Pawar; J. Sousa; E. J. Dev | 2002 | Craniospinal giant cell tumors: clinicoradiological analysis in a series of 11 cases | J Clin Neurosci | Retrospective Case Series | 3 |
| Journal Article | [27] | L. S. Shi; Y. Q. Li; W. J. Wu; Z. K. Zhang; F. Gao; M. Latif | 2015 | Imaging appearance of giant cell tumour of the spine above the sacrum | The British journal of radiology | Retrospective Case Series | 3 |
| Journal Article | [52] | Y. Shimada; M. Hongo; N. Miyakoshi; Y. Kasukawa; S. Ando; E. Itoi; E. Abe | 2007 | Giant cell tumor of fifth lumbar vertebrae: two case reports and review of the literature | Spine J | Case Report | 4 |
| Journal Article | [25] | M.-J. Si; C.-G. Wang; C.-S. Wang; L.-J. Du; X.-Y. Ding; W.-B. Zhang; Y. Lu; J.-Y. Zu | 2014 | Giant cell tumours of the mobile spine: characteristic imaging features and differential diagnosis | La radiologia medica | Retrospective Case Series | 3 |
| Journal Article | [35] | K. A. Siebenrock; K. K. Unni; M. G. Rock | 1998 | Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up | J Bone Joint Surg Br | Retrospective Case Series | 3 |
| Journal Article | [12] | D. Thomas; R. Henshaw; K. Skubitz; S. Chawla; A. Staddon; J.-Y. Blay; M. Roudier; J. Smith; Z. Ye; W. Sohn; R. Dansey; S. Jun | 2010 | Denosumab in patients with giant-cell tumour of bone: an open-label, phase 2 study | The Lancet Oncology | Prospective Cohort | 2 |
| Journal Article | [17] | A. J. Verschoor; J. Bovée; M. J. L. Mastboom; P. D. Sander Dijkstra; M. A. J. Van De Sande; H. Gelderblom | 2018 | Incidence and demographics of giant cell tumor of bone in The Netherlands: First nationwide Pathology Registry Study | Acta Orthop | Database Study | 4 |
| Journal Article | [21] | S. Wilartratsami; S. Muangsomboon; S. Benjarassameroj; R. Phimolsarnti; C. Chavasiri; P. Luksanapruksa | 2014 | Prevalence of primary spinal tumors: 15-year data from Siriraj Hospital | J Med Assoc Thai | Retrospective Case Series | 3 |
| Journal Article | [7] | W. Xu; X. Li; W. Huang; Y. Wang; S. Han; S. Chen; L. Xu; X. Yang; T. Liu; J. Xiao | 2013 | Factors Affecting Prognosis of Patients with Giant Cell Tumors of the Mobile Spine: Retrospective Analysis of 102 Patients in a Single Center | Annals of Surgical Oncology | Retrospective Case Series | 3 |
| Journal Article | [14] | T. Yayama; K. Mori; A. Nakamura; T. Mimura; S. Imai | 2020 | Denosumab Therapy for Giant-cell Tumor of the Lumbar Spine: A Case Report and Immunohistochemical Examination | Journal of orthopaedic case reports | Case Report | 4 |
| Journal Article | [31] | H. Yin; M. Cheng; B. Li; B. Li; P. Wang; T. Meng; J. Wang; W. Zhou; W. Yan; J. Xiao | 2015 | Treatment and outcome of malignant giant cell tumor in the spine | Journal of Neuro-Oncology | Retrospective Case Series | 3 |
| Journal Article | [8] | N. Yokogawa; H. Murakami; S. Demura; S. Kato; K. Yoshioka; T. Shimizu; N. Oku; R. Kitagawa; H. Tsuchiya | 2018 | Total spondylectomy for Enneking stage III giant cell tumor of the mobile spine | European Spine Journal | Retrospective Case Series | 3 |
| Journal Article | [15] | N. Yonezawa; H. Murakami; S. Kato; A. Takeuchi; H. Tsuchiya | 2017 | Giant cell tumor of the thoracic spine completely removed by total spondylectomy after neoadjuvant denosumab therapy | Eur Spine J | Case Report | 4 |
| Journal Article | [9] | H. Zhou; L. Jiang; F. Wei; M. Yu; F. l. Wu; X. g. Liu; Z. j. Liu | 2018 | Surgical approach selection for total spondylectomy for the treatment of giant cell tumors in the lumbar spine: A retrospective analysis of 12 patients from a single center | Asia-Pacific journal of clinical oncology | Retrospective Case Series | 3 |
| Journal Article | [10] | M. Zhou; H. Yang; K. Chen; G. Wang; J. Lu; Y. Ji; C. Wu; C. Chen; H. Hu | 2013 | Surgical treatment of giant cell tumors of the sacrum and spine combined with pre-operative transarterial embolization | Oncol Lett | Retrospective Case Series | 3 |
References
- Anract, P.; De Pinieux, G.; Cottias, P.; Pouillart, P.; Forest, M.; Tomeno, B. Malignant giant-cell tumours of bone. Int. Orthop. 1998, 22, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Kwon, J.W.; Chung, H.W.; Cho, E.Y.; Hong, S.H.; Choi, S.H.; Yoon, Y.C.; Yi, S.K. MRI findings of giant cell tumors of the spine. AJR Am. J. Roentgenol. 2007, 189, 246–250. [Google Scholar] [CrossRef] [PubMed]
- Hoy, D.; Brooks, P.; Blyth, F.; Buchbinder, R. The Epidemiology of low back pain. Best Pract. Res. Clin. Rheumatol. 2010, 24, 769–781. [Google Scholar] [CrossRef] [PubMed]
- Boriani, S.; Weinstein, J.N.; Biagini, R. Primary bone tumors of the spine. Terminology and surgical staging. Spine 1997, 22, 1036–1044. [Google Scholar] [CrossRef] [PubMed]
- Boriani, S.; Bandiera, S.; Casadei, R.; Boriani, L.; Donthineni, R.; Gasbarrini, A.; Pignotti, E.; Biagini, R.; Schwab, J.H. Giant cell tumor of the mobile spine: A review of 49 cases. Spine 2012, 37, E37–E45. [Google Scholar] [CrossRef] [PubMed]
- Hart, R.A.; Boriani, S.; Biagini, R.; Currier, B.; Weinstein, J.N. A system for surgical staging and management of spine tumors. A clinical outcome study of giant cell tumors of the spine. Spine 1997, 22, 1773–1782; discussion 1783. [Google Scholar] [CrossRef]
- Xu, W.; Li, X.; Huang, W.; Wang, Y.; Han, S.; Chen, S.; Xu, L.; Yang, X.; Liu, T.; Xiao, J. Factors Affecting Prognosis of Patients with Giant Cell Tumors of the Mobile Spine: Retrospective Analysis of 102 Patients in a Single Center. Ann. Surg. Oncol. 2013, 20, 804–810. [Google Scholar] [CrossRef]
- Yokogawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Shimizu, T.; Oku, N.; Kitagawa, R.; Tsuchiya, H. Total spondylectomy for Enneking stage III giant cell tumor of the mobile spine. Eur. Spine J. 2018, 27, 3084–3091. [Google Scholar] [CrossRef]
- Zhou, H.; Jiang, L.; Wei, F.; Yu, M.; Wu, F.l.; Liu, X.g.; Liu, Z.j. Surgical approach selection for total spondylectomy for the treatment of giant cell tumors in the lumbar spine: A retrospective analysis of 12 patients from a single center. Asia-Pac. J. Clin. Oncol. 2018, 14, e103–e108. [Google Scholar] [CrossRef]
- Zhou, M.; Yang, H.; Chen, K.; Wang, G.; Lu, J.; Ji, Y.; Wu, C.; Chen, C.; Hu, H. Surgical treatment of giant cell tumors of the sacrum and spine combined with pre-operative transarterial embolization. Oncol. Lett. 2013, 6, 185–190. [Google Scholar] [CrossRef]
- Boriani, S.; Cecchinato, R.; Cuzzocrea, F.; Bandiera, S.; Gambarotti, M.; Gasbarrini, A. Denosumab in the treatment of giant cell tumor of the spine. Preliminary report, review of the literature and protocol proposal. Eur. Spine J. 2020, 29, 257–271. [Google Scholar] [CrossRef] [PubMed]
- Thomas, D.; Henshaw, R.; Skubitz, K.; Chawla, S.; Staddon, A.; Blay, J.-Y.; Roudier, M.; Smith, J.; Ye, Z.; Sohn, W.; et al. Denosumab in patients with giant-cell tumour of bone: An open-label, phase 2 study. Lancet Oncol. 2010, 11, 275–280. [Google Scholar] [CrossRef]
- Xu, S.F.; Adams, B.; Yu, X.C.; Xu, M. Denosumab and Giant Cell Tumour of Bone—A Review and Future Management Considerations. Curr. Oncol. 2013, 20, 442–447. [Google Scholar] [CrossRef] [PubMed]
- Yayama, T.; Mori, K.; Nakamura, A.; Mimura, T.; Imai, S. Denosumab Therapy for Giant-cell Tumor of the Lumbar Spine: A Case Report and Immunohistochemical Examination. J. Orthop. Case Rep. 2020, 10, 76–79. [Google Scholar] [CrossRef]
- Yonezawa, N.; Murakami, H.; Demura, S.; Kato, S.; Yoshioka, K.; Takeuchi, A.; Shinmura, K.; Yokogawa, N.; Shimizu, T.; Oku, N.; et al. Morphologic Changes After Denosumab Therapy in Patients with Giant Cell Tumor of the Spine: Report of Four Cases and a Review of the Literature. World Neurosurg. 2019, 127, 38–46. [Google Scholar] [CrossRef]
- Murphey, M.D.; Andrews, C.L.; Flemming, D.J.; Temple, H.T.; Smith, W.S.; Smirniotopoulos, J.G. From the archives of the AFIP. Primary tumors of the spine: Radiologic pathologic correlation. Radiographics 1996, 16, 1131–1158. [Google Scholar] [CrossRef]
- Verschoor, A.J.; Bovée, J.; Mastboom, M.J.L.; Sander Dijkstra, P.D.; Van De Sande, M.A.J.; Gelderblom, H. Incidence and demographics of giant cell tumor of bone in The Netherlands: First nationwide Pathology Registry Study. Acta Orthop. 2018, 89, 570–574. [Google Scholar] [CrossRef]
- Mendenhall, W.M.; Zlotecki, R.A.; Scarborough, M.T.; Gibbs, C.P.; Mendenhall, N.P. Giant cell tumor of bone. Am. J. Clin. Oncol. 2006, 29, 96–99. [Google Scholar] [CrossRef]
- Orguc, S.; Arkun, R. Primary tumors of the spine. Semin. Musculoskelet. Radiol. 2014, 18, 280–299. [Google Scholar] [CrossRef]
- Sanjay, B.K.; Sim, F.H.; Unni, K.K.; McLeod, R.A.; Klassen, R.A. Giant-cell tumours of the spine. J. Bone Joint Surg. Br. 1993, 75, 148–154. [Google Scholar] [CrossRef]
- Wilartratsami, S.; Muangsomboon, S.; Benjarassameroj, S.; Phimolsarnti, R.; Chavasiri, C.; Luksanapruksa, P. Prevalence of primary spinal tumors: 15-year data from Siriraj Hospital. J. Med. Assoc. Thai. 2014, 97 (Suppl. 9), S83–S87. [Google Scholar] [PubMed]
- Jia, Q.; Chen, G.; Cao, J.; Yang, X.; Zhou, Z.; Wei, H.; Liu, T.; Xiao, J. Clinical features and prognostic factors of pediatric spine giant cell tumors: Report of 31 clinical cases in a single center. Spine J. 2019, 19, 1232–1241. [Google Scholar] [CrossRef] [PubMed]
- Campanacci, M.; Baldini, N.; Boriani, S.; Sudanese, A. Giant-cell tumor of bone. JBJS 1987, 69, 106–114. [Google Scholar] [CrossRef]
- Donthineni, R.; Boriani, L.; Ofluoglu, O.; Bandiera, S. Metastatic behaviour of giant cell tumour of the spine. Int. Orthop. 2009, 33, 497–501. [Google Scholar] [CrossRef] [PubMed]
- Si, M.-J.; Wang, C.-G.; Wang, C.-S.; Du, L.-J.; Ding, X.-Y.; Zhang, W.-B.; Lu, Y.; Zu, J.-Y. Giant cell tumours of the mobile spine: Characteristic imaging features and differential diagnosis. Radiol. Med. 2014, 119, 681–693. [Google Scholar] [CrossRef] [PubMed]
- Randhawa, S.S.; Kwan, A.K.N.; Chiu, C.K.; Chan, C.Y.W.; Kwan, M.K. Neurological Recovery in Two Patients with Cauda Equina Syndrome Secondary to L5 Lumbar Spine Giant Cell Tumour after Treatment with Denosumab without Surgery. Asian Spine J. 2016, 10, 945–949. [Google Scholar] [CrossRef] [PubMed]
- Shi, L.S.; Li, Y.Q.; Wu, W.J.; Zhang, Z.K.; Gao, F.; Latif, M. Imaging appearance of giant cell tumour of the spine above the sacrum. Br. J. Radiol. 2015, 88, 20140566. [Google Scholar] [CrossRef]
- Murphey, M.D.; Nomikos, G.C.; Flemming, D.J.; Gannon, F.H.; Temple, H.T.; Kransdorf, M.J. From the archives of AFIP. Imaging of giant cell tumor and giant cell reparative granuloma of bone: Radiologic-pathologic correlation. Radiographics 2001, 21, 1283–1309. [Google Scholar] [CrossRef]
- Aoki, J.; Tanikawa, H.; Ishii, K.; Seo, G.S.; Karakida, O.; Sone, S.; Ichikawa, T.; Kachi, K. MR findings indicative of hemosiderin in giant-cell tumor of bone: Frequency, cause, and diagnostic significance. AJR Am. J. Roentgenol. 1996, 166, 145–148. [Google Scholar] [CrossRef]
- Rimondi, E.; Staals, E.L.; Errani, C.; Bianchi, G.; Casadei, R.; Alberghini, M.; Malaguti, M.C.; Rossi, G.; Durante, S.; Mercuri, M. Percutaneous CT-guided biopsy of the spine: Results of 430 biopsies. Eur. Spine J. 2008, 17, 975–981. [Google Scholar] [CrossRef]
- Yin, H.; Cheng, M.; Li, B.; Li, B.; Wang, P.; Meng, T.; Wang, J.; Zhou, W.; Yan, W.; Xiao, J. Treatment and outcome of malignant giant cell tumor in the spine. J. Neuro-Oncol. 2015, 124, 275–281. [Google Scholar] [CrossRef] [PubMed]
- Dominkus, M.; Ruggieri, P.; Bertoni, F.; Briccoli, A.; Picci, P.; Rocca, M.; Mercuri, M. Histologically verified lung metastases in benign giant cell tumours--14 cases from a single institution. Int. Orthop. 2006, 30, 499–504. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Seethalakshmi, V.; Jambhekar, N.A.; Prabhudesai, S.; Merchant, N.; Puri, A.; Agarwal, M. Clinicopathologic profile of 470 giant cell tumors of bone from a cancer hospital in western India. Ann. Diagn. Pathol. 2008, 12, 239–248. [Google Scholar] [CrossRef] [PubMed]
- Rock, M.G.; Pritchard, D.J.; Unni, K.K. Metastases from histologically benign giant-cell tumor of bone. J. Bone Joint Surg. Am. 1984, 66, 269–274. [Google Scholar] [CrossRef]
- Siebenrock, K.A.; Unni, K.K.; Rock, M.G. Giant-cell tumour of bone metastasising to the lungs. A long-term follow-up. J. Bone Joint Surg. Br. 1998, 80, 43–47. [Google Scholar] [CrossRef]
- Rock, M. Curettage of giant cell tumor of bone. Factors influencing local recurrences and metastasis. Chir Organi Mov 1990, 75, 204–205. [Google Scholar]
- Patil, S.; Shah, K.C.; Bhojraj, S.Y.; Nene, A.M. Recurrent Spinal Giant Cell Tumors: A Study of Risk Factors and Recurrence Patterns. Asian Spine J. 2016, 10, 129–135. [Google Scholar] [CrossRef][Green Version]
- Goldschlager, T.; Dea, N.; Boyd, M.; Reynolds, J.; Patel, S.; Rhines, L.D.; Mendel, E.; Pacheco, M.; Ramos, E.; Mattei, T.A.; et al. Giant cell tumors of the spine: Has denosumab changed the treatment paradigm? J. Neurosurg. Spine SPI 2015, 22, 526–533. [Google Scholar] [CrossRef]
- Mattei, T.A.; Ramos, E.; Rehman, A.A.; Shaw, A.; Patel, S.R.; Mendel, E. Sustained long-term complete regression of a giant cell tumor of the spine after treatment with denosumab. Spine J. 2014, 14, e15–e21. [Google Scholar] [CrossRef]
- Aghaloo, T.L.; Felsenfeld, A.L.; Tetradis, S. Osteonecrosis of the Jaw in a Patient on Denosumab. J. Oral Maxillofac. Surg. 2010, 68, 959–963. [Google Scholar] [CrossRef]
- Khan, D.C.; Malhotra, S.; Stevens, R.E.; Steinfeld, A.D. Radiotherapy for the treatment of giant cell tumor of the spine: A report of six cases and review of the literature. Cancer Invest. 1999, 17, 110–113. [Google Scholar] [CrossRef] [PubMed]
- Sharma, R.R.; Mahapatra, A.K.; Pawar, S.J.; Sousa, J.; Dev, E.J. Craniospinal giant cell tumors: Clinicoradiological analysis in a series of 11 cases. J. Clin. Neurosci. 2002, 9, 41–50. [Google Scholar] [CrossRef] [PubMed]
- Chakravarti, A.; Spiro, I.J.; Hug, E.B.; Mankin, H.J.; Efird, J.T.; Suit, H.D. Megavoltage radiation therapy for axial and inoperable giant-cell tumor of bone. J. Bone Joint Surg. Am. 1999, 81, 1566–1573. [Google Scholar] [CrossRef] [PubMed]
- Ruggieri, P.; Mavrogenis, A.F.; Ussia, G.; Angelini, A.; Papagelopoulos, P.J.; Mercuri, M. Recurrence after and complications associated with adjuvant treatments for sacral giant cell tumor. Clin. Orthop. Relat. Res. 2010, 468, 2954–2961. [Google Scholar] [CrossRef]
- Leggon, R.E.; Zlotecki, R.; Reith, J.; Scarborough, M.T. Giant cell tumor of the pelvis and sacrum: 17 cases and analysis of the literature. Clin. Orthop. Relat. Res. 2004, 423, 196–207. [Google Scholar] [CrossRef]
- Roeder, F.; Timke, C.; Zwicker, F.; Thieke, C.; Bischof, M.; Debus, J.; Huber, P.E. Intensity modulated radiotherapy (IMRT) in benign giant cell tumors--a single institution case series and a short review of the literature. Radiat. Oncol. 2010, 5, 18. [Google Scholar] [CrossRef]
- Chin, B.Z.; Ji, T.; Tang, X.; Yang, R.; Guo, W. Three-Level Lumbar En Bloc Spondylectomy with Three-Dimensional−Printed Vertebrae Reconstruction for Recurrent Giant Cell Tumor. World Neurosurg. 2019, 129, 531–537.e1. [Google Scholar] [CrossRef]
- De Carvalho Cavalcante, R.A.; Silva Marques, R.A.; dos Santos, V.G.; Sabino, E.; Fraga, J.A.C.; Zaccariotti, V.A.; Arruda, J.B.; Fernandes, Y.B. Spondylectomy for Giant Cell Tumor After Denosumab Therapy. Spine 2016, 41, E178–E182. [Google Scholar] [CrossRef]
- Kinoshita, H.; Orita, S.; Yonemoto, T.; Ishii, T.; Iwata, S.; Kamoda, H.; Tsukanishi, T.; Inage, K.; Abe, K.; Inoue, M.; et al. Successful total en bloc spondylectomy of the L3 vertebra with a paravertebral giant cell tumor following preoperative treatment with denosumab: A case report. J. Med. Case Rep. 2019, 13, 116. [Google Scholar] [CrossRef]
- Minato, K.; Hirano, T.; Kawashima, H.; Yamagishi, T.; Watanabe, K.; Ohashi, M.; Ogose, A.; Endo, N. Minimally Invasive Spinal Stabilization with Denosumab before Total Spondylectomy for a Collapsing Lower Lumbar Spinal Giant Cell Tumor. Acta Med. Okayama 2021, 75, 95–101. [Google Scholar] [CrossRef]
- Santiago-Dieppa, D.R.; Hwang, L.S.; Bydon, A.; Gokaslan, Z.L.; McCarthy, E.F.; Witham, T.F. L4 and L5 spondylectomy for en bloc resection of giant cell tumor and review of the literature. Evid. Based Spine Care J. 2014, 5, 151–157. [Google Scholar] [CrossRef] [PubMed]
- Shimada, Y.; Hongo, M.; Miyakoshi, N.; Kasukawa, Y.; Ando, S.; Itoi, E.; Abe, E. Giant cell tumor of fifth lumbar vertebrae: Two case reports and review of the literature. Spine J. 2007, 7, 499–505. [Google Scholar] [CrossRef]
- Palmerini, E.; Staals, E.L.; Jones, L.B.; Donati, D.M.; Longhi, A.; Randall, R.L. Role of (Neo)adjuvant Denosumab for Giant Cell Tumor of Bone. Curr. Treat. Options Oncol. 2020, 21, 68. [Google Scholar] [CrossRef] [PubMed]
- Lador, R.; Regev, G.; Salame, K.; Khashan, M.; Lidar, Z. Use of 3-Dimensional Printing Technology in Complex Spine Surgeries. World Neurosurg. 2020, 133, e327–e341. [Google Scholar] [CrossRef] [PubMed]
- Boriani, S.; De Iure, F.; Campanacci, L.; Gasbarrini, A.; Bandiera, S.; Biagini, R.; Bertoni, F.; Picci, P. Aneurysmal bone cyst of the mobile spine: Report on 41 cases. Spine 2001, 26, 27–35. [Google Scholar] [CrossRef]
- Leithner, A.; Windhager, R.; Lang, S.; Haas, O.A.; Kainberger, F.; Kotz, R. Aneurysmal bone cyst. A population based epidemiologic study and literature review. Clin. Orthop. Relat. Res. 1999, 363, 176–179. [Google Scholar]
- Wu, Z.; Yang, X.; Xiao, J.; Feng, D.; Huang, Q.; Zheng, W.; Huang, W.; Zhou, Z. Aneurysmal Bone Cyst Secondary to Giant Cell Tumor of the Mobile Spine: A Report of 11 Cases. Spine 2011, 36, E1385–E1390. [Google Scholar] [CrossRef]
- Alhumaid, I.; Abu-Zaid, A. Denosumab Therapy in the Management of Aneurysmal Bone Cysts: A Comprehensive Literature Review. Cureus 2019, 11, e3989. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Leggett, A.R.; Berg, A.R.; Hullinger, H.; Benevenia, J.B. Diagnosis and Treatment of Lumbar Giant Cell Tumor of the Spine: Update on Current Management Strategies. Diagnostics 2022, 12, 857. https://doi.org/10.3390/diagnostics12040857
Leggett AR, Berg AR, Hullinger H, Benevenia JB. Diagnosis and Treatment of Lumbar Giant Cell Tumor of the Spine: Update on Current Management Strategies. Diagnostics. 2022; 12(4):857. https://doi.org/10.3390/diagnostics12040857
Chicago/Turabian StyleLeggett, Andrew R., Ari R. Berg, Heidi Hullinger, and Joseph B. Benevenia. 2022. "Diagnosis and Treatment of Lumbar Giant Cell Tumor of the Spine: Update on Current Management Strategies" Diagnostics 12, no. 4: 857. https://doi.org/10.3390/diagnostics12040857
APA StyleLeggett, A. R., Berg, A. R., Hullinger, H., & Benevenia, J. B. (2022). Diagnosis and Treatment of Lumbar Giant Cell Tumor of the Spine: Update on Current Management Strategies. Diagnostics, 12(4), 857. https://doi.org/10.3390/diagnostics12040857





