Abstract
Human papillomavirus (HPV) infection is the most common sexually transmitted infection (STI) in the United States, and persistent HPV infection has been established as playing a major role in the development of cervical cancer. Providing HPV vaccination and regular screening tests have reduced the risk of developing cervical cancer or helped to detect the cancer at an early stage. Despite the above measures, cervical cancer still remains a major public health problem worldwide. Infection with HPV, and consequently cervical cancer, affects all people with an intact cervix, so not only heterosexual women, but also women from sexual minorities (SMW) together with people assigned female at birth (AFAB). These populations may be even more likely to develop cervical cancer, mainly because they are less likely to be aware of HPV transmission and prevention of cervical cancer. In our review, we summarized the current state of HPV knowledge, collected data assessing the orientation of this issue among SMW and AFAB, and indicated the causes of possible negligence in the prevention of cervical cancer.
1. Introduction
Human papillomavirus (HPV) infection is the most common sexually transmitted infection (STI) in the United States [1]. It is newly diagnosed among 7 million American women each year, especially between 16 and 25 years old [2]. HPV can be transmitted from sexual partners of any sex or gender as well as through skin-to-skin sexual contact and sharing fomites, i.e., sex toys during vaginal, anal and oral sex. Studies indicate specific genetic and behavioral factors increase the risk of HPV infection such as early age of sexual initiation, numerous sexual partners and an immunocompromised state among people after transplant therapy and with HIV infection. Persistent HPV infection is playing a major role in the development of lower genital tract precancerous and cancerous diseases. Each year, around 23,300 American women are diagnosed with HPV-related malignancy such as cervical cancer [1]. Providing HPV vaccination and regular screening tests has reduced the risk of developing cervical cancer or helped to detect the cancer at an early stage. Despite the above measures, cervical cancer still remains a major public health problem worldwide.
HPV infection and, consequently, cervical cancer is indicated among all people with intact cervixes regardless sexual or gender identity The disease affects not only heterosexual women, but also people such as sexual minority women (SMW, e.g., lesbians, bisexual, queer women and women with same-sex sexual attractions or partners, regardless of their sexual identity) and people assigned female at birth (AFAB, e.g., nonbinary and trans-men) [3]. Evidence of the frequency of HPV infection in the AFAB population together with the incidence and prevalence of cervical cancer among SMW and AFAB is limited, because sexual and gender identity data are not collected in most cancer surveillance programs [4,5]. Analysis of available research has shown that HPV infection was confirmed among 36–53% of women with same-sex partners and 41–43.9% women with no same-sex partner [6].
Despite the similar prevalence of HPV infection among both sexual minority and heterosexual women, recent studies imply that SMW perceive themselves as less likely to acquire the HPV infection compared to all women. Moreover, these women may also receive limited cervical cancer prevention and screening. In this review, we try to gather and summarize the HPV-related knowledge and risk perceptions along with attitudes and behaviors among SMW and AFAB. Organized cervical cancer prevention and screening programs should consider the needs of these populations.
2. SMW and AFAB
2.1. HPV Knowledge
The United States includes at least four million SMW and around 150,000 youth (aged 13–17 years) along with 1.4 million adults (aged 18 years and older) identifying themselves as transgender [7]. Another study has shown that self-identified transgender and gender nonconforming individuals represented 0.1–2% of the global population [8]. The analysis of the data received from adolescent and adult residents of the Netherlands using an Internet-based survey showed that 0.2% respondents were AFAB [9]. In a similar study conducted among residents of the Flanders region in Belgium, AFAB constituted 0.6% [10]. A recent population-based study showed that among 50,157 Swedish adults, 2.3% of participants expressed feeling like someone of a different sex, and 2.8% wanted to live or be treated as a person of another sex [11]. Interestingly, interviews conducted among Taiwanese university students revealed that 7% of them are AFAB [12]. The literature describing the proportion of AFAB youth (younger than 19 years of age) in the general population is limited. A total of 0.65% (n = 96) of New Zealand school students described themselves as AFAB, and 1.5% (n = 121) reported that they were not sure [13]. Two surveys conducted among American youth showed that 0.56% (n = 17) from Boston and 1.8% (n = 1465) from Minnesota described themselves as AFAB [14,15]. Another analysis of data collected from public schools in San Francisco revealed that 1.3% of respondents considered themselves transgender, but the AMAB (assigned male at birth)/AFAB status was not provided [16]. Understanding the size of SMW and AFAB populations is a critical step in drawing attention to the needs of these societies along with establishing knowledge about cervical cancer prevention and screening.
2.1.1. HPV Infection
There are over 150 genotypes of HPV. The 40 of them that infect the anogenital tract are divided into high-risk and low-risk groups (hrHPV and lrHPV, respectively), based on their oncogenic potential [17]. HPV16 and HPV18 are high-risk genotypes and are responsible for nearly 70% of high-grade cervical cancers in the US [18]. HPV infection is usually asymptomatic. Nearly 90% of all HPV viruses are either eliminated by immune systems or become inactive up to two years after infection [19]. Current data confirms that in most women, cervical cancer development was observed at least 3 to 5 years after hrHPV infection [20]. Persistent HPV infection is responsible for more than 5% of all cancers worldwide and more than 50% of all malignancies related to infection [21,22,23]. Therefore, early detection of both HPV infection and HPV-induced lesions are key to preventing the development of cervical cancer.
The prevalence of HPV in sexual and gender minority people with intact cervixes varies between 15 and 51%, and the prevalence is similar among heterosexual women [4]. Current studies have confirmed HPV infection among 58% of bisexual women, 36% of lesbians and 41% of heterosexual women [3]. Moreover, the national health and nutrition examination survey (NHANES) data confirmed the prevalence of HPV among women with same-sex partners as 51–53%, in contrast to 43.9% in women with no same-sex partners [4,5]. Moreover, the HPV-positive rate among lesbians in Western countries is estimated between 13–21%. Another study confirmed 25% of HPV-positive results among 300 investigated Chinese lesbians, and the hrHPV rate was 21.33%. Data describing the prevalence of HPV among AFAB are limited. We found only one study describing HPV prevalence among transgender men, showing 16% of hrHPV positive results in self-collected vaginal swabs [24].
2.1.2. Cervical Cancer
The number of deaths caused by cervical cancer has decreased in the US from 2.8 per 1000 women in 2000 to 2.3 per 1000 in 2015 [25,26]. Around 99.7% cases of cervical cancer are associated with HPV infection. Cervical cancer is preventable through HPV vaccination, and regular cervical screening can detect the cancer at an early stage. However, most cases of cervical cancer deaths occur among inadequately screened women [25]. The incidence and prevalence of cervical cancer among SMW and AFAB populations are both limited since gender and sexual identity data are not collected in most cancer surveillance programs [6]. Only one study showed that that sexual minority women had increased rates of cervical cancer diagnosis compared to heterosexual women [4].
2.1.3. HPV Vaccine
The Centers for Disease Control and Prevention (CDC) recommends routine HPV vaccination at ages 11–12 for all sexes, however it can be started as early as age 9 [27]. In public health programs, HPV vaccine is recommended for people under age 25 at the initiation of vaccinations. However, some scientific societies (i.e., Centers for Disease Control and Prevention, American Cancer Society) allow HPV vaccination in older patients if the benefits outweigh the risks related to the vaccine. HPV vaccines can be administered in two different regiments—two doses and three-doses, depending on the age at initiation of vaccination and immune status [28,29] (Table 1). Since 2016, only 9-valent HPV vaccine (against HPV types 6, 11, 16, 18, 31, 33, 45, 52 and 58) has been available for use in the US [29]. Moreover, the HPV vaccine may be given at the same time as other vaccines. Recent studies have confirmed HPV vaccine uptake of 10–18.2% among SMW women [30,31,32]. These low rates of vaccination were similar across all subsamples: lesbian (20.8%, n = 40) and bisexual women (30.8%, n = 95) [33]. It might be speculated that perceptions of low HPV risk are responsible for lower HPV vaccine rates among lesbians when compared with heterosexual women, as well as among women with only female sexual partners in contrast to women with male sexual partners [1]. There are currently no data available on the number of AFAB who have received the HPV vaccine.

Table 1.
HPV vaccine regiments (for ninevalent vaccine) [28].
2.1.4. Cervical Screening and HPV Screening
The Papanicolaou (Pap) test is an important cancer screening procedure for people with intact cervixes. It allows health care providers to detect precancerous and cancerous lesions caused by HPV infection. Current data indicate the lack of clinically important differences between conventional and liquid-based cytology (LBC) [34]. According to the American College of Obstetricians and Gynecologists (ACOG) recommendations, cis-gender women (women whose gender identity aligns with the sex assigned to them at birth). along with AFAB individuals with intact cervixes, should follow the same screening guidelines as heterosexual women [7,35]. Cervical cancer screening should be performed per age-related guidelines for all people with intact uteruses [26,36] (Table 2). However, certain risk factors such as a compromised immune system or previous treatment of a high-grade precancerous stage may increase cervical cancer risk. Therefore, people with these factors should receive individualized follow-up. Additionally, it is worth paying attention to the particular circumstances relating to the AFAB population, such as future plans for cervix removal. Permanent sterilization, defined as the definitive removal of the reproductive organs, used to be listed as an obligatory step to change legal gender, and it is still mandatory in Japan and in some American states. Growing acceptance of transgender people may lead to less restrictive laws and two important conditions. First, as legal requirements for hysterectomies are not necessary, we can expect a growing population of transgender men with intact cervixes will need cervical screening. Second, while some transgender men who will retain their cervix remain registered legally as women, others will be registered as men and may not be able to take part in the national cervical cancer screening program. The US transgender survey reports that 71% of transgender men (n = 7950) have received androgen therapy and 14% have had hysterectomies [37]. Another problem with the AFAB population, especially transgender men, is cervical and vaginal atrophy secondary to testosterone, which may hamper the cervical cancer screening. Trans-male individuals have been confirmed to have a 10-times higher rate of unsatisfactory cytological results, in contrary to cis-genders [38]. However, the recent study on 84 transgender men showed that they were not at a higher or lower risk of abnormal Pap test results compared with women [39]. Nevertheless, larger studies are needed to support above findings.

Table 2.
Cervical screening recommendations [26,36].
A HPV test is performed in the same way as a Pap test to determine the risk of cervical infection by hrHPV genotypes. The American Cancer Society recommends it in individuals with cervixes at ages 25 to 65. The ACOG states that self-collected HPV specimens may be appropriate for those patients who do not have access to screening or for whom speculum insertion is physically difficult or may be emotionally traumatic. Nevertheless, there is no self-collected HPV test approved by the U.S. Food and Drug Administration (FDA). AHPV test should be performed individually every 3 years or together with a Pap test (called a co-test) every 5 years.
2.2. Risk Perceptions
Risk perceptions play a key role in creating health behaviors. Based on the available literature, it is slightly different in the discussed populations in terms of both HPV transmission and vaccine.
HPV Transmission
Several studies have confirmed that lesbians and women with only AFAB partners tend to perceive themselves as less likely to acquire HPV than bisexual women and (AMAB) [4,40,41,42]. Moreover, studies have shown that SMW who have sex with bisexual women and AFAB individuals who have AMAB partners have a notable HPV risk factor [43]. Interestingly, participants’ HPV risk perceptions do not vary by race or ethnicity [32]. Marazzo et al. reported that young adult lesbian and bisexual women believed that female-to-female HPV transmission was decreased when compared to heterosexual intercourse [44]. Other studies showed that only 59.6% of Italian [2] and 44.8% of Australian [45] participants, including lesbian, gay men and bisexuals, have heard about HPV infection. In contrast, 79% of gay and 93% of bisexual Americans reported hearing about HPV [46,47]. Data on the perception of HPV transmission among AFAB is scarce. One of the studies showed that the majority of sexually active AFAB individuals was aware of being at some risk of acquiring HPV, regardless of gender and sexual identity. However, based on survey data, a hierarchy of perceived HPV risk emerged based on sexual behavior and sexual identity. Precisely, most of the participants believed that the highest risk of HPV was among those who have penile-vaginal sex. The risk of acquiring HPV from AFAB sexual partners was perceived incorrectly as low or negligible [39]. Several studies confirmed that most participants erroneously attributed the risk of HPV transmission in the context of sex among AFAB individuals to the exchange of genital fluids rather than to skin-to-skin sexual contact [39,47,48]. Some researchers have suggested that the misconceptions about the risk of transmitting HPV may be due to comparing the acquisition of this infection to sexually transmitted infections via body fluids (e.g., HIV) [48,49].
Consequently, low HPV risk perceptions are also responsible for less safe -sex practices that include not limiting the number of sexual partners and reduced use of barrier methods among SMW and AFAB. Nevertheless, the widespread occurrence of HPV carries the risk of infection even after one intercourse. Moreover, the use of condoms reduces but does not eliminate, the risk of HPV infection because the virus can be transmitted from skin not covered by the condom that must be used from the beginning to the end of intercourse [2]. These misperceptions may undermine individuals’ engagement in HPV prevention strategies, including HPV vaccination and cervical screening.
2.3. Attitudes and Behavior
2.3.1. HPV Vaccine
It has been suggested that perceived HPV risk was positively associated with HPV vaccination among American young adults [1]. With regard to the level of HPV vaccine knowledge from recent studies, only 42.1% of Italian participants reported that they were aware of available protection against HPV. Higher awareness of HPV vaccine has been observed among Australian lesbians and bisexual women (50% and 54.6%, respectively). Currently, there are no data reporting knowledge about both HPV infection and vaccine among AFAB.
Current data on the initiation and completion of HPV vaccination in different sexual orientation groups are inconsistent. Several studies suggest that lesbians are less likely to vaccinate against HPV than heterosexual women. However, there were no difference in initiation of HPV vaccination between bisexual and heterosexual women [1,31,39]. In contrast, other researchers have shown that both lesbians and heterosexual women are equally likely to initiate and complete HPV vaccination, whereas bisexual women are more likely to receive the complete course of HPV vaccination than other women are [50,51]. Another study suggested that women experience similar protection against HPV infections, regardless of sexual orientation [52]. This finding might be explained by the fact that the HPV vaccine is normally administered prior to the development of sexual orientation. Nevertheless, there are no HPV vaccine recommendations to sexual and gender minority people or parents of SMW and AFAB adolescents defining a vaccination regimen determined by the age of the patient.
2.3.2. HPV/Cervical Screening
Even in the relatively extensive literature on sexual identity and cytology tests, studies assessing the frequency of cervical screening in people from sexual minorities are limited. According to current data, only 10–12% SMW continue regular cervical screenings [53]. Another study has shown that during the three years before the study, 48–81% of American lesbians reported having a Pap test, around 57% in the United Kingdom and 78% in Australia [54]. The study also confirms that cervical screening among lesbians is 5–18% lower than in heterosexual women. Moreover, there is no data determining the age at which these individuals have their first Pap tests or HPV test. Taking the Pap test too late together with not performing screening for hrHPV may put both SMW and AFAB at higher risk of HPV complications such as late diagnosis and poorer cancer outcomes [55]. Nevertheless, most of the guidelines still do not specify cervical cancer screening recommendations for sexual and gender minorities, which is crucial to reduce the cervical cancer risk. Moreover, to the best of our knowledge, the progress of dysplasia and cancer genesis among SMW and AFAB has not yet been described in the literature.
3. Cervical Cancer Screening Barriers for Sexual Minority People (SMW and AFAB)
We have described the barriers that SMW and AFAB face in accessing cervical screening (Figure 1). Reduced cervical cancer screening may have a multifactorial background.
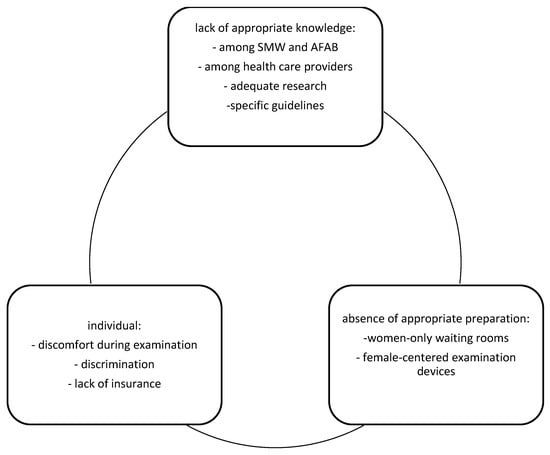
Figure 1.
Cervical cancer screening barriers for SMW and AFAB.
First, lack of adequate knowledge indicates several levels—interested individuals, health care providers, appropriate research and guidelines. Several studies showed that sexual minority women believed that they do not need screening if they did not have sex with men [56,57]. On the other hand, further data reported that most participants believed that HPV and cervical cancer risk did not differ by gender or sexual identity, but they could not answer how often cervical cancer screening should be performed [58]. In contrast, some believed that testosterone use increased their risk of cervical cancer [59]. Moreover, beliefs in low HPV risk led to reduced use of barrier methods such as condoms [58,60]. Taken together, the data confirm a lack of consensus among participants about how their gender minority status influenced both cervical cancer risk and therefore screening [59]. Furthermore, access to health care providers may also shape patterns of cervical cancer screening behavior [61,62]. Receiving adequate information about HPV transmission, together with cervical cancer prevention and screening, may lead to the opposite situation. Reduced need for contraception limits necessity of medical visits [63,64]. Moreover, restricted knowledge about HPV transmission and risky sexual behavior may arise from misinformation and lack of proper training among health care providers [54,60]. According to US data, only 20% of 141 gynecology trainees received postgraduate training on the care of gender minority patients [61]. When asked particularly about care among gender minorities, 89% admitted that cervical cancer screening should be performed, but only 29% felt confident performing the screening [54,62]. Possible explanations may be either the lack of proper education in how to perform the examination or psychological barriers to including ‘female’ designated procedures if the patient has already changed their legal gender [54,63]. Lack of knowledge about the examination technique was confirmed by several studies [1,63], and in addition, some care providers reported that if the patient had lower sexual risk and was waiting for a total hysterectomy, screening could be postponed or avoided [40]. Moreover, lack of knowledge among health care providers arises from the limited relevant research concerning the course of HPV infection and cervical cancer development among the abovementioned populations. The lack of scientific outcomes and therefore of suitable guidelines lead to the neglect of the different conditions and needs of SMW and AFAB in both cancer screening and prevention programs.
Second, is the lack of preparation for the cervical cancer screening. Starting from women-only waiting rooms and female-concentrated tools for cervical cancer screening, such as specula, most of the health care centers are not adequately prepared to provide services for these people.
Additionally, one of the studies showed that many AFAB do not identify as women. Therefore, the speculum is the most off-putting aspect of a cervical examination, and consequently, application of the conventional Pap smear is restricted [65]. That is why one of the studies emphasized the need to provide alternative screening options for sexual minorities [24] that were also considered in other studies [51,65,66]. Interestingly, another research with 131 gender minority participants compared a conventional Pap test with hrHPV DNA hybridization assays from both self-and provider-collected vaginal swabs [24]. In contrast to Pap tests, self-collected vaginal specimen assays for hrHPV had a sensitivity of 71% and a specificity of 98%, with substantial concordance between techniques (κ = 0.75; p < 0.0001), whereas in the provider-collected vaginal swab, stated to be the gold standard, the DNA assay conducted on the self-collected vaginal specimens had 86% sensitivity and 98% specificity, with near perfect concordance (κ = 0.84; p < 0.0001). Moreover, self-collected HPV swabs were considered by participants less inconvenient than the conventional Pap test [67]. Another study confirmed that among cisgender women (nontransgender), self-swab HPV-DNA testing as a primary cervical cancer screening method and self-swab specimen collection for other STIs have sufficient level of acceptance [68]. Therefore, it might be speculated that use of cotesting, including Pap smear and HPV DNA testing, every 5 years as per heterosexual women, might be both the most effective and the most acceptable method of cervical cancer screening among sexual and gender minority people, including trans and nonbinary individuals experiencing gender dysphoria (GD).
Finally, individual barriers are tied to discomfort resulting from the examination, discrimination and lack of insurance. Sexual and gender minority people such as AFAB are more likely to experience discrimination in health care, which may explain sexual and gender orientation-related health disparities [50]. Therefore, the social and economic consequences of living with a stigmatized sexual and gender identity are thought to explain sexual and gender orientation-related health disparities. Moreover, in many countries, lack of insurance covering gender-affirming surgical procedures or participation in free cervical cancer screening program for female members may also lead to the lack of appropriate cervical screening.
4. Conclusions
In conclusion, general knowledge and risk perceptions in HPV transmission among SMW and even more with AFAB is insufficient. In our review, we summarized the current state of HPV knowledge, collected data assessing knowledge of this issue among SMW and AFAB, and indicated the causes of possible negligence in the prevention of cervical cancer. Taken together, our results should draw the attention of many populations, not only medical ones, to the problems related to HPV infection among the discussed populations. This knowledge might also lead to the implementation of targeted intervention programs for the mentioned populations. Moreover, they can also provide parents and caregivers with the knowledge and communication skills they need to deliver accurate information about HPV risk and prevention to their adolescent and young adult family members from sexual and gender minorities.
Author Contributions
Conceptualization, M.P., B.G. and K.S.-W.; methodology, M.P.; software, A.Z.; validation, M.P., A.Z., R.J. and M.D.-W.; formal analysis, A.W. and R.J.; investigation, M.P.; resources, M.P., M.D.-W. and K.S.-W.; data curation, K.S.-W.; writing—original draft preparation, M.P. and B.G.; writing—review and editing, K.S.-W.; visualization, M.P. and B.G.; supervision, K.S.-W. and R.J.; project administration, K.S.-W.; funding acquisition, K.S.-W. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by “Foundation for the development of gynecological endocrinology, oncological gynecology and fertility treatment” and by the Dean of Jagiellonian University Medical College.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Agénor, M.; Jahn, J.L.; Kay, E.; Bishop, R.A.; Peitzmeier, S.M.; Potter, J.; Austin, S.B. Human Papillomavirus Risk Perceptions among Young Adult Sexual Minority Cisgender Women and Nonbinary Individuals Assigned Female at Birth. Perspect. Sex. Reprod. Health 2019, 51, 27–34. [Google Scholar] [CrossRef] [PubMed]
- Pelullo, C.P.; Di Giuseppe, G.; Angelillo, I.F. Human papillomavirus infection: Knowledge, attitudes, and behaviors among lesbian, gay men, and bisexual in Italy. PLoS ONE 2012, 7, e42856. [Google Scholar] [CrossRef] [PubMed]
- Reiter, P.L.; McRee, A.L. HPV infection among a population-based sample of sexual minority women from USA. Sex. Transm. Infect. 2017, 93, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Branstetter, A.J.; McRee, A.L.; Reiter, P.L. Correlates of Human Papillomavirus Infection among a National Sample of Sexual Minority Women. J. Womens Health 2017, 26, 1004–1011. [Google Scholar] [CrossRef]
- Hariri, S.; Unger, E.R.; Sternberg, M.; Dunne, E.F.; Swan, D.; Patel, S.; Markowitz, L.E. Prevalence of genital human papillomavirus among females in the United States, the National Health and Nutrition Examination Survey, 2003–2006. J. Infect. Dis. 2011, 204, 566–573. [Google Scholar] [CrossRef] [PubMed]
- Boehmer, U.; Miao, X.; Ozonoff, A. Cancer survivorship and sexual orientation. Cancer 2011, 117, 3796–3804. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Committee Opinion no. 512: Health care for transgender individuals. Obstet. Gynecol. 2011, 118, 1454–1458. [Google Scholar] [CrossRef]
- Goodman, M.; Adams, N.; Corneil, T.; Kreukels, B.; Motmans, J.; Coleman, E. Size and Distribution of Transgender and Gender Nonconforming Populations: A Narrative Review. Endocrinol. Metab. Clin. N. Am. 2019, 48, 303–321. [Google Scholar] [CrossRef]
- Kuyper, L.; Wijsen, C. Gender identities and gender dysphoria in the Netherlands. Arch. Sex. Behav. 2014, 43, 377–385. [Google Scholar] [CrossRef]
- Van Caenegem, E.; Wierckx, K.; Elaut, E.; Buysse, A.; Dewaele, A.; Van Nieuwerburgh, F.; De Cuypere, G.; T’Sjoen, G. Prevalence of Gender Nonconformity in Flanders, Belgium. Arch. Sex. Behav. 2015, 44, 1281–1287. [Google Scholar] [CrossRef]
- Åhs, J.W.; Dhejne, C.; Magnusson, C.; Dal, H.; Lundin, A.; Arver, S.; Dalman, C.; Kosidou, K. Proportion of adults in the general population of Stockholm County who want gender-affirming medical treatment. PLoS ONE 2018, 13, e0204606. [Google Scholar] [CrossRef] [PubMed]
- Lai, M.C.; Chiu, Y.N.; Gadow, K.D.; Gau, S.S.; Hwu, H.G. Correlates of gender dysphoria in Taiwanese university students. Arch. Sex. Behav. 2010, 39, 1415–1428. [Google Scholar] [CrossRef] [PubMed]
- Clark, T.C.; Lucassen, M.F.; Bullen, P.; Denny, S.J.; Fleming, T.; Robinson, E.M.; Rossen, F.V. The health and well-being of transgender high school students: Results from the New Zealand adolescent health survey (Youth’12). J. Adolesc. Health 2014, 55, 93–99. [Google Scholar] [CrossRef]
- Almeida, J.; Johnson, R.M.; Corliss, H.L.; Molnar, B.E.; Azrael, D. Emotional distress among LGBT youth: The influence of perceived discrimination based on sexual orientation. J. Youth Adolesc. 2009, 38, 1001–1014. [Google Scholar] [CrossRef]
- Eisenberg, M.E.; Gower, A.L.; McMorris, B.J.; Rider, G.N.; Shea, G.; Coleman, E. Risk and Protective Factors in the Lives of Transgender/Gender Nonconforming Adolescents. J. Adolesc. Health 2017, 61, 521–526. [Google Scholar] [CrossRef] [PubMed]
- Shields, J.P.; Cohen, R.; Glassman, J.R.; Whitaker, K.; Franks, H.; Bertolini, I. Estimating population size and demographic characteristics of lesbian, gay, bisexual, and transgender youth in middle school. J. Adolesc. Health 2013, 52, 248–250. [Google Scholar] [CrossRef]
- Egawa, N.; Doorbar, J. The low-risk papillomaviruses. Virus Res. 2017, 231, 119–127. [Google Scholar] [CrossRef]
- Aranda-Rivera, A.K.; Cruz-Gregorio, A.; Briones-Herrera, A.; Pedraza-Chaverri, J. Regulation of autophagy by high- and low-risk human papillomaviruses. Rev. Med. Virol. 2021, 31, e2169. [Google Scholar] [CrossRef]
- Soheili, M.; Keyvani, H.; Nasseri, S. Human papilloma virus: A review study of epidemiology, carcinogenesis, diagnostic methods, and treatment of all HPV-related cancers. Med. J. Islam. Repub. Iran. 2021, 35, 65. [Google Scholar] [CrossRef]
- Nasioutziki, M.; Chatzistamatiou, K.; Loufopoulos, P.D.; Vavoulidis, E.; Tsampazis, N.; Pratilas, G.-C.; Liberis, A.; Karpa, V.; Parcharidis, E.; Daniilidis, A.; et al. Cervical, anal and oral HPV detection and HPV type concordance among women referred for colposcopy. Infect. Agents Cancer 2020, 15, 22. [Google Scholar] [CrossRef]
- Forman, D.; de Martel, C.; Lacey, C.J.; Soerjomataram, I.; Lortet-Tieulent, J.; Bruni, L.; Vignat, J.; Ferlay, J.; Bray, F.; Plummer, M.; et al. Global burden of human papillomavirus and related diseases. Vaccine 2012, 30 (Suppl. 5), F12–F23. [Google Scholar] [CrossRef] [PubMed]
- de Martel, C.; Ferlay, J.; Franceschi, S.; Vignat, J.; Bray, F.; Forman, D.; Plummer, M. Global burden of cancers attributable to infections in 2008: A review and synthetic analysis. Lancet Oncol. 2012, 13, 607–615. [Google Scholar] [CrossRef]
- Plummer, M.; de Martel, C.; Vignat, J.; Ferlay, J.; Bray, F.; Franceschi, S. Global burden of cancers attributable to infections in 2012: A synthetic analysis. Lancet Glob. Health 2016, 4, e609–e616. [Google Scholar] [CrossRef]
- Reisner, S.L.; Deutsch, M.B.; Peitzmeier, S.M.; Hughto, J.M.W.; Cavanaugh, T.P.; Pardee, D.J.; McLean, S.A.; Panther, L.A.; Gelman, M.; Mimiaga, M.J.; et al. Test performance and acceptability of self- versus provider-collected swabs for high-risk HPV DNA testing in female-to-male trans masculine patients. PLoS ONE 2018, 13, e0190172. [Google Scholar] [CrossRef] [PubMed]
- Melnikow, J.; Henderson, J.T.; Burda, B.U.; Senger, C.A.; Durbin, S.; Weyrich, M.S. Screening for Cervical Cancer with High-Risk Human Papillomavirus Testing: Updated Evidence Report and Systematic Review for the US Preventive Services Task Force. JAMA 2018, 320, 687–705. [Google Scholar] [CrossRef] [PubMed]
- Curry, S.J.; Krist, A.H.; Owens, D.K.; Owens, D.K.; Barry, M.J.; Caughey, A.B.; Davidson, K.; Doubeni, C.A.; Epling, J.W.; Kemper, A.R.; et al. Screening for Cervical Cancer: US Preventive Services Task Force Recommendation Statement. JAMA 2018, 320, 674–686. [Google Scholar] [CrossRef]
- Petrosky, E.; Bocchini, J.A.; Hariri, S.; Chesson, H.; Curtis, C.R.; Saraiya, M.; Unger, E.R.; Markowitz, L.E. Use of 9-valent human papillomavirus (HPV) vaccine: Updated HPV vaccination recommendations of the advisory committee on immunization practices. MMWR Morb. Mortal. Wkly. Rep. 2015, 64, 300–304. [Google Scholar]
- Oshman, L.D.; Davis, A.M. Human Papillomavirus Vaccination for Adults: Updated Recommendations of the Advisory Committee on Immunization Practices (ACIP). JAMA 2020, 323, 468–469. [Google Scholar] [CrossRef]
- Seyferth, E.R.; Bratic, J.S.; Bocchini, J.A. Human papillomavirus epidemiology and vaccine recommendations: Selected review of the recent literature. Curr. Opin. Pediatr. 2016, 28, 400–406. [Google Scholar] [CrossRef]
- Porsch, L.M.; Zhang, H.; Dayananda, I.; Dean, G. Comparing Receipt of Cervical Cancer Screening and Completion of Human Papillomavirus Vaccination Using a New Construct of Sexual Orientation: A Serial Cross-Sectional Study. LGBT Health 2019, 6, 184–191. [Google Scholar] [CrossRef]
- Agénor, M.; Krieger, N.; Austin, S.B.; Haneuse, S.; Gottlieb, B.R. Sexual orientation disparities in Papanicolaou test use among US women: The role of sexual and reproductive health services. Am. J. Public Health 2014, 104, e68–e73. [Google Scholar] [CrossRef] [PubMed]
- LoSchiavo, C.; Greene, R.E.; Halkitis, P.N. Human Papillomavirus Prevalence, Genotype Diversity, and Risk Factors among Transgender Women and Nonbinary Participants in the P18 Cohort Study. AIDS Patient Care STDs 2020, 34, 502–505. [Google Scholar] [CrossRef] [PubMed]
- Griffin, M.; Jaiswal, J.; Stults, C.B. Human Papillomavirus Vaccination Rates by Gender Identity and Sexual Orientation among 18-44-Year-Olds in the U.S. Arch. Sex. Behav. 2021, 50, 3079–3092. [Google Scholar] [CrossRef] [PubMed]
- Rozemeijer, K.; Naber, S.K.; Penning, C.; Overbeek, L.I.H.; Looman, C.W.N.; De Kok, I.M.C.M.; Matthijsse, S.M.; Rebolj, M.; Van Kemenade, F.J.; Van Ballegooijen, M. Cervical cancer incidence after normal cytological sample in routine screening using SurePath, ThinPrep, and conventional cytology: Population based study. BMJ 2017, 356, j504. [Google Scholar] [CrossRef]
- American College of Obstetricians and Gynecologists. Health Care for Transgender and Gender Diverse Individuals: ACOG Committee Opinion, Number 823. Obstet. Gynecol. 2021, 137, e75–e88. [Google Scholar] [CrossRef]
- Fontham, E.T.H.; Wolf, A.M.D.; Church, T.R.; Etzioni, R.; Flowers, C.R.; Herzig, A.; Guerra, C.E.; Oeffinger, K.C.; Shih, Y.T.; Walter, L.C.; et al. Cervical cancer screening for individuals at average risk: 2020 guideline update from the American Cancer Society. CA Cancer J. Clin. 2020, 70, 321–346. [Google Scholar] [CrossRef]
- Kachen, A.; Pharr, J.R. Health Care Access and Utilization by Transgender Populations: A United States Transgender Survey Study. Transgend. Health 2020, 5, 141–148. [Google Scholar] [CrossRef]
- Peitzmeier, S.M.; Reisner, S.L.; Harigopal, P.; Potter, J. Female-to-male patients have high prevalence of unsatisfactory Paps compared to non-transgender females: Implications for cervical cancer screening. J. Gen. Intern. Med. 2014, 29, 778–784. [Google Scholar] [CrossRef]
- Cacis, K.; Kwon, R.; Graham, A.; White, M.; Maleki, Z.; Rodriguez, E. Comparison of cervical cancer screen results on female-to-male transgender patients with female patients. Am. J. Clin. Pathol. 2021, 7, aqab158. [Google Scholar]
- Agénor, M.; Peitzmeier, S.M.; Bernstein, I.M.; McDowell, M.; Alizaga, N.M.; Reisner, S.L.; Pardee, D.J.; Potter, J. Perceptions of cervical cancer risk and screening among transmasculine individuals: Patient and provider perspectives. Cult. Health Sex. 2016, 18, 1192–1206. [Google Scholar] [CrossRef]
- Kim, S.; Lee, S.Y.; Choi-Kwon, S. Cervical Cancer Screening and Human Papillomavirus Vaccination among Korean Sexual Minority Women by Sex of Their Sexual Partners. Int. J. Environ. Res. Public Health 2020, 17, 8924. [Google Scholar] [CrossRef] [PubMed]
- Paschen-Wolff, M.M.; Greene, M.Z.; Hughes, T.L. Sexual Minority Women’s Sexual and Reproductive Health Literacy: A Qualitative Descriptive Study. Health Educ. Behav. 2020, 47, 728–739. [Google Scholar] [CrossRef] [PubMed]
- Colledge, L.; Hickson, F.; Reid, D.; Weatherburn, P. Poorer mental health in UK bisexual women than lesbians: Evidence from the UK 2007 Stonewall Women’s Health Survey. J. Public Health 2015, 37, 427–437. [Google Scholar] [CrossRef] [PubMed]
- Marrazzo, J.M.; Coffey, P.; Bingham, A. Sexual practices, risk perception and knowledge of sexually transmitted disease risk among lesbian and bisexual women. Perspect. Sex. Reprod. Health 2005, 37, 6–12. [Google Scholar] [CrossRef]
- Pitts, M.K.; Fox, C.; Willis, J.; Anderson, J. What do gay men know about human papillomavirus? Australian gay men’s knowledge and experience of anal cancer screening and human papillomavirus. Sex. Transm. Dis. 2007, 34, 170–173. [Google Scholar] [CrossRef]
- Brewer, N.T.; Ng, T.W.; McRee, A.L.; Reiter, P.L. Men’s beliefs about HPV-related disease. J. Behav. Med. 2010, 33, 274–281. [Google Scholar] [CrossRef]
- Reiter, P.L.; Brewer, N.T.; McRee, A.L.; Gilbert, P.; Smith, J.S. Acceptability of HPV vaccine among a national sample of gay and bisexual men. Sex. Transm. Dis. 2010, 37, 197–203. [Google Scholar] [CrossRef]
- McNair, R. Risks and prevention of sexually transmissible infections among women who have sex with women. Sex. Health 2005, 2, 209–217. [Google Scholar] [CrossRef]
- Balán, I.C.; Lopez-Rios, J.; Dolezal, C.; Rael, C.T.; Lentz, C. Low sexually transmissible infection knowledge, risk perception and concern about infection among men who have sex with men and transgender women at high risk of infection. Sex. Health 2019, 16, 580–586. [Google Scholar] [CrossRef]
- Reyna, V.F.; Adam, M.B. Fuzzy-trace theory, risk communication, and product labeling in sexually transmitted diseases. Risk Anal. 2003, 23, 325–342. [Google Scholar] [CrossRef]
- Macapagal, K.; Bhatia, R.; Greene, G.J. Differences in Healthcare Access, Use, and Experiences within a Community Sample of Racially Diverse Lesbian, Gay, Bisexual, Transgender, and Questioning Emerging Adults. LGBT Health 2016, 3, 434–442. [Google Scholar] [CrossRef] [PubMed]
- Solazzo, A.L.; Agénor, M.; Austin, S.B.; Chavarro, J.E.; Charlton, B.M. Sexual Orientation Differences in Cervical Cancer Prevention among a Cohort of U.S. Women. Womens Health Issues 2020, 30, 306–312. [Google Scholar] [CrossRef] [PubMed]
- Charlton, B.M.; Reisner, S.L.; Agénor, M.; Gordon, A.R.; Sarda, V.; Austin, S.B. Sexual Orientation Disparities in Human Papillomavirus Vaccination in a Longitudinal Cohort of U.S. Males and Females. LGBT Health 2017, 4, 202–209. [Google Scholar] [CrossRef] [PubMed]
- Peitzmeier, S.M.; Agénor, M.; Bernstein, I.M.; McDowell, M.; Alizaga, N.M.; Reisner, S.L.; Pardee, D.J.; Potter, J. “It Can Promote an Existential Crisis”: Factors Influencing Pap Test Acceptability and Utilization Among Transmasculine Individuals. Qual. Health Res. 2017, 27, 2138–2149. [Google Scholar] [CrossRef]
- Waterman, L.; Voss, J. HPV, cervical cancer risks, and barriers to care for lesbian women. Nurse Pract. 2015, 40, 46–53. [Google Scholar] [CrossRef] [PubMed]
- Dobbs, S.P.; Asmussen, T.; Nunns, D.; Hollingworth, J.; Brown, L.J.; Ireland, D. Does histological incomplete excision of cervical intraepithelial neoplasia following large loop excision of transformation zone increase recurrence rates? A six year cytological follow up. BJOG 2000, 107, 1298–1301. [Google Scholar] [CrossRef]
- Gonzales, G.; Henning-Smith, C. Barriers to Care among Transgender and Gender Nonconforming Adults. Milbank Q. 2017, 95, 726–748. [Google Scholar] [CrossRef]
- Shires, D.A.; Stroumsa, D.; Jaffee, K.D.; Woodford, M.R. Primary Care Clinicians’ Willingness to Care for Transgender Patients. Ann. Fam. Med. 2018, 16, 555–558. [Google Scholar] [CrossRef]
- Youatt, E.J.; Harris, L.H.; Harper, G.W.; Janz, N.K.; Bauermeister, J.A. Sexual Health Care Services among Young Adult Sexual Minority Women. Sex. Res. Soc. Policy 2017, 14, 345–357. [Google Scholar] [CrossRef]
- Mohr, S.; Gygax, L.N.; Imboden, S.; Mueller, M.D.; Kuhn, A. Screening for HPV and dysplasia in transgender patients: Do we need it? Eur. J. Obstet. Gynecol. Reprod. Biol. 2021, 260, 177–182. [Google Scholar] [CrossRef]
- Johnson, M.J.; Nemeth, L.S.; Mueller, M.; Eliason, M.J.; Stuart, G.W. Qualitative Study of Cervical Cancer Screening Among Lesbian and Bisexual Women and Transgender Men. Cancer Nurs. 2016, 39, 455–463. [Google Scholar] [CrossRef] [PubMed]
- Unger, C.A. Care of the transgender patient: A survey of gynecologists’ current knowledge and practice. J. Womens Health 2015, 24, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Connolly, D.; Hughes, X.; Berner, A. Barriers and facilitators to cervical cancer screening among transgender men and non-binary people with a cervix: A systematic narrative review. Prev. Med. 2020, 135, 106071. [Google Scholar] [CrossRef] [PubMed]
- Greene, M.Z.; Meghani, S.H.; Sommers, M.S.; Hughes, T.L. Health Care-Related Correlates of Cervical Cancer Screening among Sexual Minority Women: An Integrative Review. J. Midwifery Womens Health 2018, 63, 550–577. [Google Scholar] [CrossRef]
- Charlton, B.M.; Corliss, H.L.; Missmer, S.A.; Frazier, A.L.; Rosario, M.; Kahn, J.A.; Austin, S.B. Influence of hormonal contraceptive use and health beliefs on sexual orientation disparities in Papanicolaou test use. Am. J. Public Health 2014, 104, 319–325. [Google Scholar] [CrossRef]
- McDowell, M.; Pardee, D.J.; Peitzmeier, S.; Reisner, S.L.; Agénor, M.; Alizaga, N.; Bernstein, I.; Potter, J. Cervical Cancer Screening Preferences among Trans-Masculine Individuals: Patient-Collected Human Papillomavirus Vaginal Swabs versus Provider-Administered Pap Tests. LGBT Health 2017, 4, 252–259. [Google Scholar] [CrossRef]
- Seay, J.; Ranck, A.; Weiss, R.; Salgado, C.; Fein, L.; Kobetz, E. Understanding Transgender Men’s Experiences with and Preferences for Cervical Cancer Screening: A Rapid Assessment Survey. LGBT Health 2017, 4, 304–309. [Google Scholar] [CrossRef]
- Reisner, S.L.; Deutsch, M.B.; Peitzmeier, S.M.; Hughto, J.M.W.; Cavanaugh, T.; Pardee, D.J.; McLean, S.; Marrow, E.J.; Mimiaga, M.J.; Panther, L.; et al. Comparing self- and provider-collected swabbing for HPV DNA testing in female-to-male transgender adult patients: A mixed-methods biobehavioral study protocol. BMC Infect. Dis. 2017, 17, 444. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).