Clinical Spectrum and CSF Findings in Patients with West-Nile Virus Infection, a Retrospective Cohort Review
Abstract
1. Introduction
2. Materials and Methods
2.1. Serological Testing
2.2. Molecular Biological Analysis
2.3. Statistical Analysis
3. Results
3.1. Demographics and Comorbidities
3.2. Presenting Symptoms
3.3. CSF Analysis and Serology
3.4. Treatment
3.5. Complications
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Smithburn, K.C.; Hughes, T.P.; Burke, A.W.; Paul, J.H. A Neurotropic Virus Isolated from the Blood of a Native of Uganda1. Am. J. Trop. Med. Hyg. 1940, 20, 471–492. [Google Scholar] [CrossRef]
- Murgue, B.; Murri, S.; Triki, H.; Deubel, V.; Zeller, H.G. West Nile in the Mediterranean basin: 1950–2000. Ann. N. Y. Acad. Sci. 2001, 951, 117–126. [Google Scholar] [CrossRef] [PubMed]
- Goldblum, N.; Sterk, V.V.; Paderski, B. West Nile fever; the clinical features of the disease and the isolation of West Nile virus from the blood of nine human cases. Am. J. Hyg. 1954, 59, 89–103. [Google Scholar] [PubMed]
- Bernkopf, H.; Levine, S.; Nerson, R. Isolation of West Nile virus in Israel. J. Infect. Dis. 1953, 93, 207–218. [Google Scholar] [CrossRef]
- Goldblum, N.; Jasinska-Klingberg, W.; Klingberg, M.A.; Marberg, K.; Sterk, V.V. The natural history of West Nile Fever. I. Clinical observations during an epidemic in Israel. Am. J. Hyg. 1956, 64, 259–269. [Google Scholar]
- Chancey, C.; Grinev, A.; Volkova, E.; Rios, M. The global ecology and epidemiology of West Nile virus. Biomed. Res. Int. 2015, 2015, 376230. [Google Scholar] [CrossRef]
- van der Meulen, K.M.; Pensaert, M.B.; Nauwynck, H.J. West Nile virus in the vertebrate world. Arch. Virol. 2005, 150, 637–657. [Google Scholar] [CrossRef]
- De Madrid, A.T.; Porterfield, J.S. The flaviviruses (group B arboviruses): A cross-neutralization study. J. Gen. Virol. 1974, 23, 91–96. [Google Scholar] [CrossRef]
- Pealer, L.N.; Marfin, A.A.; Petersen, L.R.; Lanciotti, R.S.; Page, P.L.; Stramer, S.L.; Stobierski, M.G.; Signs, K.; Newman, B.; Kapoor, H.; et al. Transmission of West Nile virus through blood transfusion in the United States in 2002. N. Engl. J. Med. 2003, 349, 1236–1245. [Google Scholar] [CrossRef]
- Ognjan, A.; Boulton, M.L.; Somsel, P.; Stobierski, M.G.; Stoltman, G.; Downes, F.; Smith, K.; Chapman, L.; Petersen, L.; Marfin, A.; et al. Possible West Nile Virus transmission to an infant through breast-feeding—Michigan, 2002—(Reprinted from MMWR, vol 51, pg 877–878, 2002). JAMA 2002, 288, 1976–1977. [Google Scholar]
- Meny, G.M.; Santos-Zabala, L.; Szallasi, A.; Stramer, S.L. West Nile virus infection transmitted by granulocyte transfusion. Blood 2011, 117, 5778–5779. [Google Scholar] [CrossRef]
- Petersen, L.R.; Marfin, A.A. West Nile virus: A primer for the clinician. Ann. Intern. Med. 2002, 137, 173–179. [Google Scholar] [CrossRef]
- Granwehr, B.P.; Lillibridge, K.M.; Higgs, S.; Mason, P.W.; Aronson, J.F.; Campbell, G.A.; Barrett, A.D. West Nile virus: Where are we now? Lancet Infect. Dis. 2004, 4, 547–556. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Weekly Updates: 2018 West Nile Fever Transmission Season; ECDC: Stockholm, Sweden, 2018. [Google Scholar]
- KÖZPONT NN. Heti tájékoztató a hazai járványügyi helyzetről. 43. Hét. 2018. Available online: https://www.google.com/url?sa=t&rct=j&q=&esrc=s&source=web&cd=&cad=rja&uact=8&ved=2ahUKEwjTwIPeoOD2AhUTsFYBHYraA0gQFnoECBAQAQ&url=https%3A%2F%2Fwww.antsz.hu%2Fdata%2Fcms88419%2F2018_34_honlapheti.pdf&usg=AOvVaw2OwSjXV9UVxDJpN_RrbaSj (accessed on 16 November 2018).
- European Union. Commission Implementing Decision (EU) 2018/945 of 22 June 2018 on the Communicable Diseases and Related Special Health Issues to Be Covered by Epidemiological Surveillance as Well as Relevant Case Definitions. Off. J. Eur. Union 2018, 61, 1–74. [Google Scholar]
- Szomor, K.N.; Rigo, Z.; Ban, E.; Nagy, L.; Szalkai, T.; Balogh, Z.; Ferenczi, E.; Takács, M. Serologic evidence of West Nile virus infection in patients with exanthema in Hungary. Acta Microbiol. Immunol. Hung. 2011, 58, 157–167. [Google Scholar] [CrossRef]
- Linke, S.; Ellerbrok, H.; Niedrig, M.; Nitsche, A.; Pauli, G. Detection of West Nile virus lineages 1 and 2 by real-time PCR. J. Virol. Methods 2007, 146, 355–358. [Google Scholar] [CrossRef]
- Chaskopoulou, A.; Dovas, C.; Chaintoutis, S.; Bouzalas, I.; Ara, G.; Papanastassopoulou, M. Evidence of enzootic circulation of West Nile virus (Nea Santa-Greece-2010, lineage 2), Greece, May to July 2011. Eurosurveillance 2011, 16, 19933. [Google Scholar] [CrossRef]
- Nikolay, B.; Weidmann, M.; Dupressoir, A.; Faye, O.; Boye, C.S.; Diallo, M.; Sall, A.A. Development of a Usutu virus specific real-time reverse transcription PCR assay based on sequenced strains from Africa and Europe. J. Virol. Methods 2014, 197, 51–54. [Google Scholar] [CrossRef]
- European Centre for Disease Prevention and Control (ECDC). Transmission of West Nile Fever, May to November 2017—Table of Cases. 2017. Available online: https://www.ecdc.europa.eu/en/publications-data/table-transmission-west-nile-fever-may-november-2017-table-cases-2017 (accessed on 1 July 2019).
- Hershberger, V.S.; Augsburger, J.J.; Hutchins, R.K.; Miller, S.A.; Horwitz, J.A.; Bergmann, M. Chorioretinal lesions in nonfatal cases of West Nile virus infection. Ophthalmology 2003, 110, 1732–1736. [Google Scholar] [CrossRef]
- Bains, H.S.; Jampol, L.M.; Caughron, M.C.; Parnell, J.R. Vitritis and chorioretinitis in a patient with West Nile virus infection. Arch. Ophthalmol. 2003, 121, 205–207. [Google Scholar]
- Garg, S.; Jampol, L.M. Systemic and intraocular manifestations of West Nile virus infection. Surv. Ophthalmol. 2005, 50, 3–13. [Google Scholar] [CrossRef]
- Kuchtey, R.W.; Kosmorsky, G.S.; Martin, D.; Lee, M.S. Uveitis associated with West Nile virus infection. Arch. Ophthalmol. 2003, 121, 1648–1649. [Google Scholar] [CrossRef][Green Version]
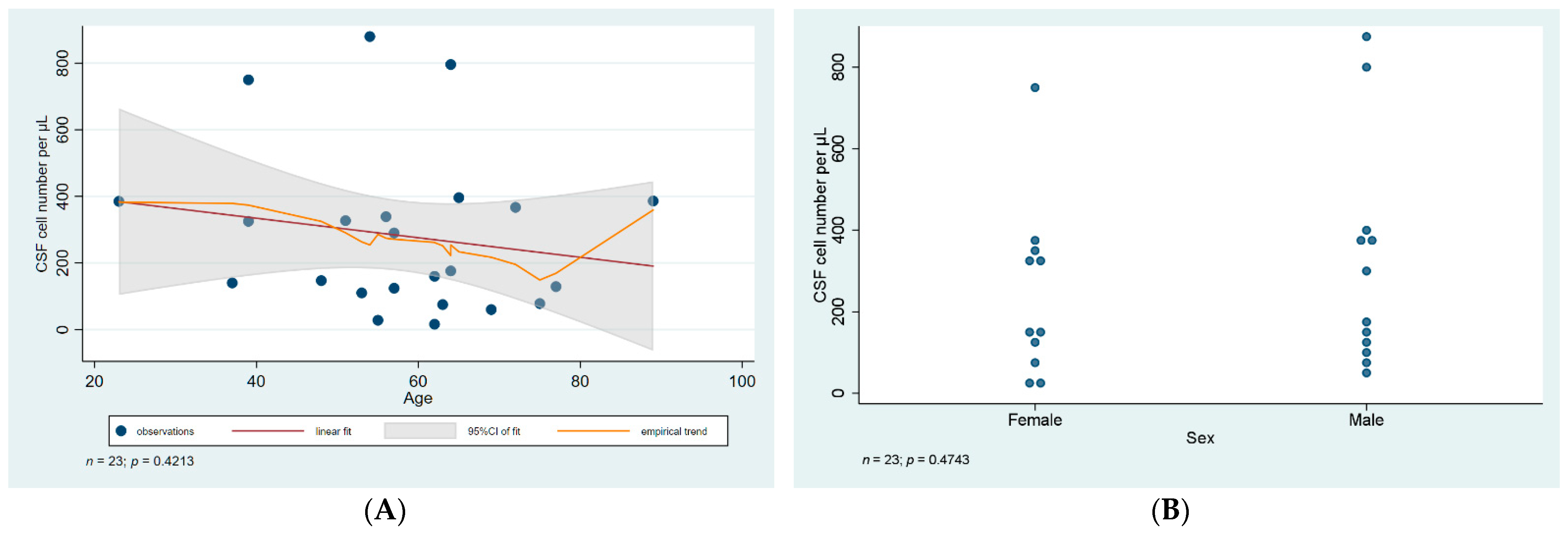
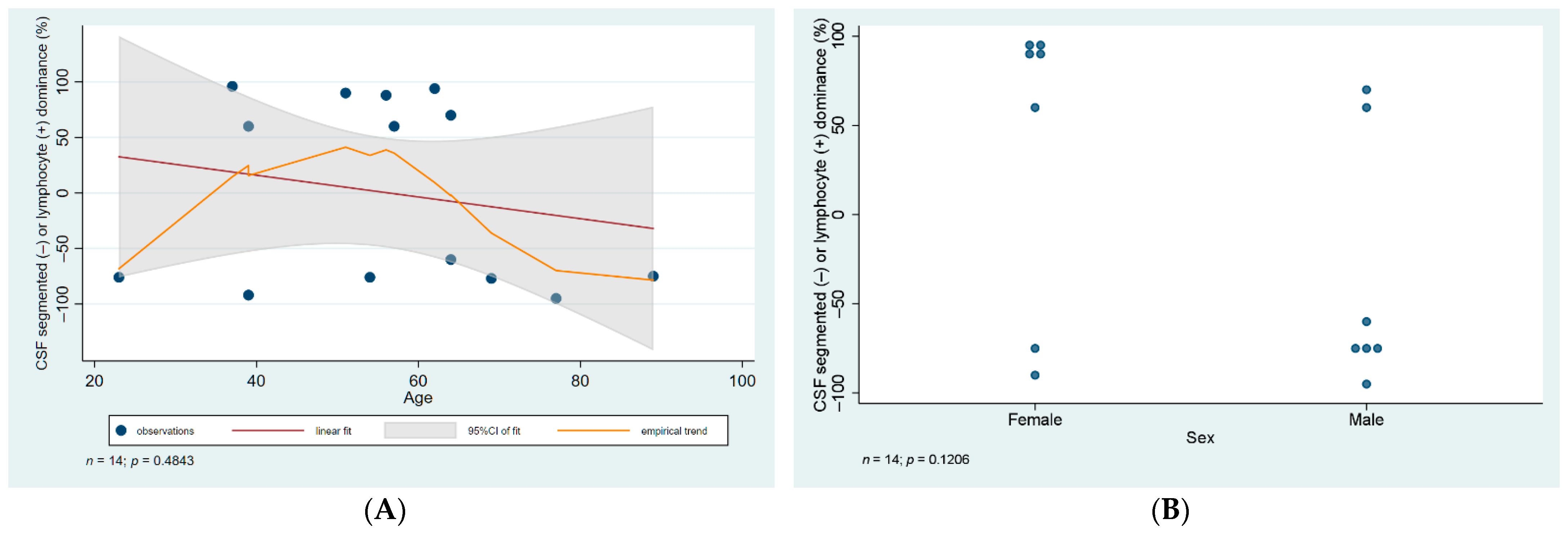

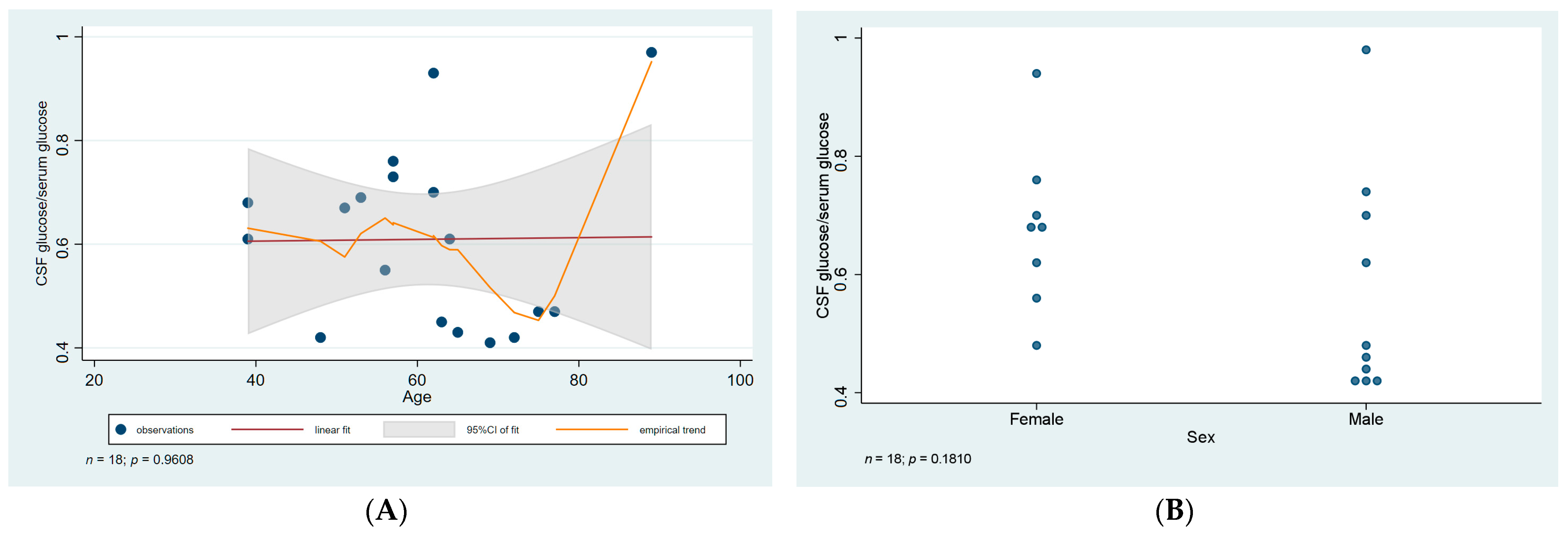
| ID | Age | Sex | CSF WNV IgM IFA | Serum WNV IgM ELISA | WNV RT-PCR Blood | WNV RT-PCR Urine | CSF Cell Number Per µL | CSF Cell Type | CSF Total Protein (mg/L) | Ratio of CSF to Serum Glucose | Treatment Duration (Days) | Presentation |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| X01 | 56 | F | Positive | Positive | Negative | Negative | 339 | 88% lymphocytes | 864 | 0.55 | 9 | mild |
| X04 * | 51 | F | Negative | Positive | Negative | Negative | 327 | 90% lymphocytes | 607 | 0.67 | 6 | moderate |
| X03 | 62 | F | Negative | Positive | Positive | Positive | 16 | ND | 771 | 0.7 | 4 | moderate |
| X02 * | 39 | F | Negative | Positive | Negative | Negative | 750 | 92% segmented | 657 | 0.61 | 7 | mild |
| X08 | 62 | F | Positive | Positive | Negative | Negative | 160 | 94% lymphocytes | 1497 | 0.93 | 10 | severe |
| X06 | 23 | F | Positive | Positive | Positive | Positive | 385 | 76% segmented | 955 | ND | 4 | moderate |
| X05 ** | 75 | F | NA | Positive | Negative | Negative | 78 | ND | 1800 | 0.47 | 6 | moderate |
| X07 | 55 | F | Positive | Positive | Negative | Negative | 28 | ND | 548 | ND | 7 | mild |
| X09 | 37 | F | Positive | Positive | NA | Negative | 140 | 96% lymphocytes | 633 | ND | 6 | moderate |
| X13 * | 39 | F | Indeterminate | Positive | Indeterminate | Negative | 325 | 60% lymphocytes | 649 | 0.68 | 6 | moderate |
| X11 | 57 | F | Positive | Positive | Indeterminate | Negative | 124 | ND | 635 | 0.76 | 4 | severe |
| Y01 | 69 | M | NA | Positive | Positive | Negative | 60 | 77% segmented | 1008 | 0.41 | 6 | severe |
| Y02 | 89 | M | Indeterminate | Positive | Positive | Positive | 386 | 75% segmented | 1647 | 0.97 | 9 | severe/fatal |
| Y03 ** | 77 | M | NA | Positive | Negative | Positive | 129 | 95% segmented | 1701 | 0.47 | 13 | severe |
| Y04 | 65 | M | Positive | Positive | Positive | Positive | 396 | ND | 1510 | 0.43 | 5 | moderate |
| Y06 | 53 | M | Positive | Positive | Positive | Indeterminate | 110 | ND | 830 | 0.69 | 8 | moderate |
| Y07 | 72 | M | NA | Positive | Positive | Indeterminate | 367 | ND | 1008 | 0.42 | 7 | moderate |
| Y05 * | 48 | M | Negative | Positive | Negative | Positive | 147 | ND | 890 | 0.42 | 7 | moderate |
| Y08 | 54 | M | Positive | Positive | Positive | Positive | 880 | 76% segmented | 1445 | ND | 8 | severe |
| Y09 ** | 64 | M | Negative | Positive | Negative | Negative | 796 | 60% segmented | 1086 | 0.61 | 8 | severe |
| Y10 ** | 64 | M | NA | Positive | Negative | NA | 176 | 70% lymphocytes | 1246 | ND | 5 | severe |
| Y11 ** | 63 | M | NA | Positive | Indeterminate | Negative | 75 | ND | 870 | 0.45 | 11 | mild |
| Y12 | 57 | M | Positive | Positive | Negative | Negative | 290 | 60% lymphocytes | 737 | 0.73 | 7 | mild |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bakos, I.; Mahdi, M.; Kardos, L.; Nagy, A.; Várkonyi, I. Clinical Spectrum and CSF Findings in Patients with West-Nile Virus Infection, a Retrospective Cohort Review. Diagnostics 2022, 12, 805. https://doi.org/10.3390/diagnostics12040805
Bakos I, Mahdi M, Kardos L, Nagy A, Várkonyi I. Clinical Spectrum and CSF Findings in Patients with West-Nile Virus Infection, a Retrospective Cohort Review. Diagnostics. 2022; 12(4):805. https://doi.org/10.3390/diagnostics12040805
Chicago/Turabian StyleBakos, Imre, Mohamed Mahdi, László Kardos, Anna Nagy, and István Várkonyi. 2022. "Clinical Spectrum and CSF Findings in Patients with West-Nile Virus Infection, a Retrospective Cohort Review" Diagnostics 12, no. 4: 805. https://doi.org/10.3390/diagnostics12040805
APA StyleBakos, I., Mahdi, M., Kardos, L., Nagy, A., & Várkonyi, I. (2022). Clinical Spectrum and CSF Findings in Patients with West-Nile Virus Infection, a Retrospective Cohort Review. Diagnostics, 12(4), 805. https://doi.org/10.3390/diagnostics12040805






