Fluid Biomarkers in Alzheimer’s Disease and Other Neurodegenerative Disorders: Toward Integrative Diagnostic Frameworks and Tailored Treatments
Abstract
:1. Introduction
2. Fluid Biomarkers for NDDs
3. Candidate Biomarkers
3.1. Blood Biomarkers
3.1.1. Aβ Peptides
3.1.2. t-tau
3.1.3. p-tau181
3.1.4. p-tau Alternative Isoforms
3.1.5. Nfl
3.2. Novel CSF Biomarkers
3.2.1. CSF Ng and Other Synaptic Biomarkers
| Reference | Protein | Study Population | Diagnostic Value | Prognostic Value | Application |
|---|---|---|---|---|---|
| Blood Matrix | |||||
| Tau Pathology | |||||
| Janelidze S. et al., 2020 [35] | p-tau181 | Cohort 1: n = 182 (MCI n = 28, AD n = 38, non-AD dementia n = 52, CU n = 64) Cohort 2: n = 344 (MCI n = 125, CU n = 219) Cohort 3 (neuropathology cohort): n = 63 (AD n = 16, non-AD dementia n = 47) | p-tau181 differentiating: 1. Cohort 1: - tau-positive vs. negative AuROC = 0.87 with tau-PET as reference; - AD vs. non-AD dementia: AuROC = 0.94 with clinical diagnosis 2. Cohort 1 and cohort 2 together: - Aβ + vs. Aβ-participants: AuROC = 0.80 with Aβ PET as reference 3. Cohort 3: - AD vs. non-AD dementia: AuROC = 0.85 neuropathological reference | Cohort 2: higher p-tau levels are associated with progression to AD for both CU (HR = 2.48) and MCI (HR = 3.07) | Feasible for screening and early diagnosis of AD, with p-tau231 displaying the earliest increase during the disease course |
| Thijssen E. et al., 2020 [36] | Cohort 1: n = 362 (MCI n = 47, AD n = 56, CBS n = 39, PSP n = 48, bvFTD n = 50, nfvPPA n = 27, svPPA n = 26, CU n = 69) Cohort 2: n = 42 (MCI, AD) | p-tau181 differentiating: 1. Cohort 1 - AD vs. FTLD (n = 190): AuROC = 0.89 with clinical diagnosis as reference; - Aβ-PET + vs.-: AuROC = 0.86 with amyloid-PET as reference; - autopsy-confirmed AD (n = 15) vs. FTLD-tau (n = 52) AuROC = 0.86 with neuropathology as reference | NA | ||
| Karikari T. et al., 2020 [37] | n = 1331 Discovery cohort: n = 37 (AD n = 19, CU n = 18) Validation cohort 1: n = 763 (MCI n = 45, AD n = 33, FTD n = 8, CU n = 140) Validation cohort 2: n = 763 (MCI n = 191, AD n = 126, bvFTD/PPA n = 18, PD/MSA n = 36, VD n = 12, PSP/CBS n = 21, CU n = 337) Primary care cohort: n = 105 (MCI n = 12, AD n = 10, CU n = 83) | p-tau181 differentiating: 1. Across cohorts AD vs. CU: AuROCs = 0.90–0.98 2. Validation cohort 1 AD vs. FTD: AuROCs = 0.76–0.82 with clinical diagnosis as reference ● Across cohorts tau-PET + vs. tau-PET-: AuROCs = 0.83–0.93 with tau-PET as reference; AD vs. Aβ-: AuROC = 0.99 with amyloid-PET as reference ● Validation cohort 2 - AD vs. VD: AuROC = 0.92 - AD vs. PSP/CBS: AuROC = 0.89 - AD vs. PD/MSA: AuROC = 0.82 with clinical diagnosis as reference ● Primary care cohort - AD vs. CU young adults: AuROC = 1.0 - AD vs. CU older adults: AuROC = 0.84 with clinical diagnosis as reference | NA | NA | |
| Palmqvist S. et al., 2020 [41] | p-tau217 | Cohort 1 (neuropathology cohort): n = 81 (AD n = 34, non-AD n = 47) Cohort 2: n = 699 (MCI n = 178, AD dementia n = 121, non-AD dementia: PD/PDD/MSA, PSP/CBS, bvFTD/PPA, VD n = 99, CU n = 301) - Cohort 3: n = 622 PSEN1 mutation carriers n = 365; age- and sex-matched noncarriers: n = 257 | p-tau181 differentiating: 1. Cohort 1 AD vs. non-AD: AuROC = 0.89 with neuropathology as reference 2. Cohort 2 - AD vs. non-AD dementia: AuROC = 0.96 with clinical diagnosis as reference - tau + vs. tau-: AuROC = 0.93 with tau-PET as reference- amyloid + vs. amyloid -: AuROC = 0.87 with amyloid-PET as reference | NA | NA |
| Barthélemy NR. et al., 2020 [40] | Discovery cohort: n = 36 (non-AD MCI n = 2, preclinical AD n = 5, AD-MCI n = 8, moderate AD n = 4, CU n = 17) Validation cohort: n = 92 (non-AD MCI n = 11, preclinical AD n = 20, AD-MCI n = 24, moderate AD n = 6, CU n = 31) | p-tau181 differentiating amyloid + vs. amyloid- - discovery cohort: AuROC = 0.98 - validation cohort: AuROC = 0.75 with amyloid-PET or CSF Aβ1-42/Aβ1-40 as reference; p-tau217 differentiating amyloid + vs. amyloid- - discovery cohort: AuROC = 0.99; - validation cohort: AuROC = 0.92 with amyloid-PET or CSF Aβ1-42/Aβ1-40 as reference | NA | NA | |
| Ashton NJ. et al., 2021 [43] | p-tau231 | Cohort 1 n = 48 (AD n = 20, CU n = 18) Cohort 2 n = 313 (AD n = 42, MCI n = 54, non-AD dementia n = 26, CU n = 191) Cohort 3 n = 190 (AD n = 34, MCI n = 17, CU n = 139) Cohort 4 (neuropathology cohort) n = 47 (AD n = 36, non-AD n = 11) | p-tau231 differentiating: - AD vs. amyloid-PET- CU: AuROCs = 0.92–0.94 - AD vs. non-AD NDDs (FTD, PSP, CBS, VD, HS, CAA) AuROC = 0.93 - AD vs. amyloid-PET-MCI: AuROC = 0.89 with clinical diagnosis as reference - AD vs. non-AD pathology: AuROC = 0.99 with neuropathology as reference | NA | NA |
| Mattsson N. et al., 2017 [50] | NfL | n = 570 (AD n = 180, MCI n = 197, CU n = 193) | NfL discriminating AD vs. CU participants: AuROC = 0.87 with clinical diagnosis as reference | NA | Potentially suitable to rule out neurodegeneration across different NDDs in primary care settings, eligible for diagnostic and prognostic purposes in AD and ALS |
| Lewczuk P. et al., 2018 [45] | n = 99 (AD n = 33, MCI n = 25, CU n = 41) | NfL differentiating → - diseased participants vs. CU: AuROC = 0.85 - AD vs. CU: AuROC = 0.92 with clinical diagnosis as reference | NA | ||
| Gille B. et al., 2019 [54] | n = 250 (ALS n = 149, ALS mimics n = 19, disease controls n = 82) | NfL discriminating ALS vs. ALS mimics: AuROC = 0.85 with clinical diagnosis as reference | Nfl levels in the prediction of mortality among ALS patients: -upper tertile: HR = 5.34 -middle tertile: HR = 4.47 | ||
| Verde F. et al., 2019 [57] | n = 283 (ALS n = 124, FTD n = 20, AD n = 20, PD n = 19, CJD n = 6, disease controls n = 44, non-NDDs controls n = 50) | Nfl differentiating ALS vs. - non-NDDs controls: AuROC = 0.97; - disease controls: AuROC = 0.87; - all other categories together: AuROC = 0.88 with clinical diagnosis as reference | In the prediction of mortality: Nfl levels above the median (125 pg/mL): HR = 2.39 | ||
| Thouvenot E. et al., 2020 [58] | n = 219 (ALS n = 198, CU n = 21) | Nfl differentiating ALS vs. CU: AuROC = 0.99 with clinical diagnosis as reference | In the prediction of mortality: Nfl levels ≥ 71,2 pg/mL HR = 4.7 | ||
| Mattsson N. et al., 2016 [73] | Ng | n = 389 (AD n = 93, MCI n = 187, CU n = 109) | Ng differentiating AD vs. CU: AuROC = 0.71 with clinical diagnosis as reference | NA | Candidate supportive biomarker in the diagnostic/prognostic work-up of AD |
| Tarawneh R. et al., 2016 [72] | n = 302 (AD n = 95, CU n = 207) | In the discrimination of AD from CU → - Ng: AuROC = 0.71 - Ng + Aβ1-42: AuROC = 0.81 with clinical diagnosis as reference | In predicting the conversion from HC to AD → -Ng: adjusted HR = 1.89 - Ng/Aβ1-42: adjusted HR = 27.9 | ||
| Blennow K. et al., 2019 [71] | n = 191 (AD n = 46, CJD n = 81, CU n = 64) | Ng differentiating → - CJD vs. CU: AuROC = 0.96 - AD vs. CJD: AuROC = 0.85 - AD vs. CU: AuROC = 0.73 with clinical diagnosis as reference | NA | ||
3.2.2. CSF YKL-40
4. Alternative Matrices
4.1. Saliva
4.2. Olfactory Mucosa
4.3. Skin
4.4. Urine
4.5. Retina
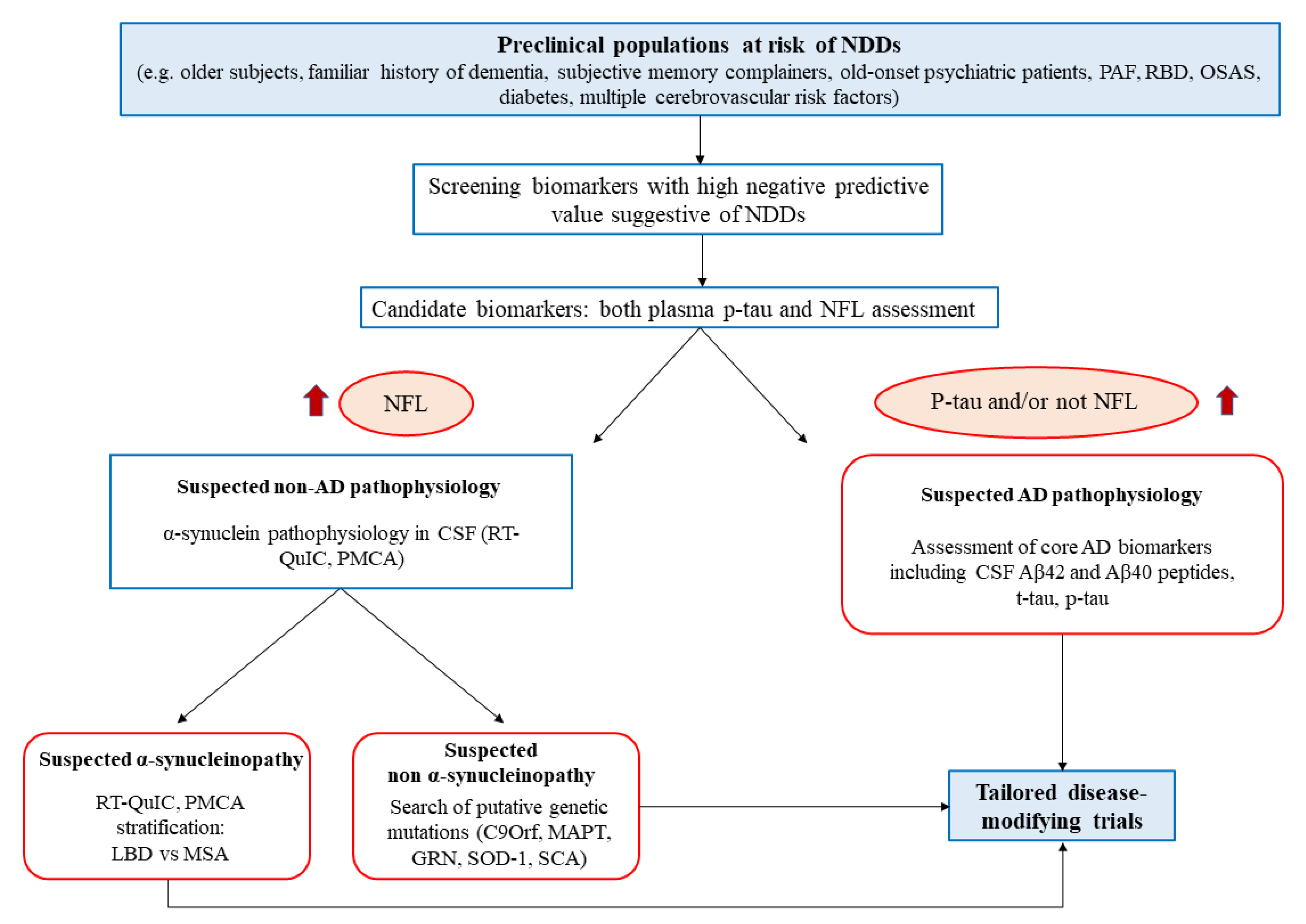
5. Protein Misfolding Amplification Assays for the In Vivo Dissection of NDDs and for the Stratification of at High-Risk Populations
5.1. Protein Misfolding Amplification Assays: An Overview
5.2. Protein Misfolding Amplification Assays in NDDs: Promising Tools for Disease Stratification
6. Biomarkers in Clinical Trials for NDDs
6.1. AD
6.1.1. Anti-Amyloid Strategies
6.1.2. Anti-tau Strategies
6.1.3. Neuroinflammation
6.2. Genetic Forms of NDDs
6.2.1. FTD
6.2.2. ALS
7. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Numbers of People with Dementia Worldwide: An Update to the Estimates in the World Alzheimer Report. 2015. Available online: https://www.alzint.org/resource/numbers-of-people-with-dementia-worldwide/ (accessed on 22 June 2021).
- Mehta, D.; Jackson, R.; Paul, G.; Shi, J.; Sabbagh, M. Why do trials for Alzheimer’s disease drugs keep failing? A discontinued drug perspective for 2010–2015. Expert Opin. Investig. Drugs 2017, 26, 735–739. [Google Scholar] [CrossRef] [PubMed]
- Jack, C.R.; Knopman, D.S.; Jagust, W.J.; Shaw, L.M.; Aisen, P.S.; Weiner, M.W.; Petersen, R.C.; Trojanowski, J.Q. Hypothetical model of dynamic biomarkers of the Alzheimer’s pathological cascade. Lancet Neurol. 2010, 9, 119–128. [Google Scholar] [CrossRef] [Green Version]
- Hawkes, C.H. The prodromal phase of sporadic Parkinson’s disease: Does it exist and if so how long is it? Mov. Disord. 2008, 23, 1799–1807. [Google Scholar] [CrossRef] [PubMed]
- Savica, R.; Bradley, B.F.; Mielke, M.M. When do a-Synucleinopathies start? An epidemiological timeline a review. JAMA Neurol. 2018, 75, 503–509. [Google Scholar] [CrossRef]
- Van Zundert, B.; Izaurieta, P.; Fritz, E.; Alvarez, F.J. Early pathogenesis in the adult-onset neurodegenerative disease amyotrophic lateral sclerosis. J. Cell. Biochem. 2012, 113, 3301–3312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Beach, T.G.; Monsell, S.E.; Phillips, L.E.; Kukull, W. Accuracy of the clinical diagnosis of Alzheimer disease at National Institute on Aging Alzheimer Disease Centers, 2005–2010. J. Neuropathol. Exp. Neurol. 2012, 71, 266–273. [Google Scholar] [CrossRef] [PubMed]
- Rizzo, G.; Arcuti, S.; Copetti, M.; Alessandria, M.; Savica, R.; Fontana, A.; Liguori, R.; Logroscino, G. Accuracy of clinical diagnosis of dementia with Lewy bodies: A systematic review and meta-analysis. J. Neurol. Neurosurg. Psychiatry 2018, 89, 358–366. [Google Scholar] [CrossRef]
- Jack, C.R.; Bennett, D.A.; Blennow, K.; Carrillo, M.C.; Dunn, B.; Haeberlein, S.B.; Holtzman, D.M.; Jagust, W.; Jessen, F.; Karlawish, J.; et al. NIA-AA Research Framework: Toward a biological definition of Alzheimer’s disease. Alzheimer’s Dement. 2018, 14, 535–562. [Google Scholar] [CrossRef]
- Albert, M.S.; DeKosky, S.T.; Dickson, D.; Dubois, B.; Feldman, H.H.; Fox, N.C.; Gamst, A.; Holtzman, D.M.; Jagust, W.J.; Petersen, R.C. The diagnosis of mild cognitive impairment due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 270–279. [Google Scholar] [CrossRef] [Green Version]
- Sperling, R.A.; Aisen, P.S.; Beckett, L.A.; Bennett, D.A.; Craft, S.; Fagan, A.M.; Iwatsubo, T.; Jack, C.R.; Kaye, J.; Montine, T.J.; et al. Toward defining the preclinical stages of Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 280–292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Heinzel, S.; Berg, D.; Gasser, T.; Chen, H.; Yao, C.; Postuma, R.B. Update of the MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2019, 34, 1464–1470. [Google Scholar] [CrossRef]
- Berg, D.; Postuma, R.B.; Adler, C.H.; Bloem, B.R.; Chan, P.; Dubois, B.; Gasser, T.; Goetz, C.G.; Halliday, G.; Joseph, L.; et al. MDS research criteria for prodromal Parkinson’s disease. Mov. Disord. 2015, 30, 1600–1611. [Google Scholar] [CrossRef]
- McKeith, I.G.; Ferman, T.J.; Thomas, A.J.; Blanc, F.; Boeve, B.F.; Fujishiro, H.; Kantarci, K.; Muscio, C.; O’Brien, J.T.; Postuma, R.B.; et al. Research criteria for the diagnosis of prodromal dementia with Lewy bodies. Neurology 2020, 94, 743–755. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Mazzucchi, S.; Della Vecchia, A.; Giampietri, L.; Giannini, N.; Koronyo-Hamaoui, M.; Ceravolo, R.; Siciliano, G.; Bonuccelli, U.; Elahi, F.M.; et al. The path to biomarker-based diagnostic criteria for the spectrum of neurodegenerative diseases. Expert Rev. Mol. Diagn. 2020, 20, 421–441. [Google Scholar] [CrossRef] [PubMed]
- Kovacs, G.G.; Milenkovic, I.; Wöhrer, A.; Höftberger, R.; Gelpi, E.; Haberler, C.; Hönigschnabl, S.; Reiner-Concin, A.; Heinzl, H.; Jungwirth, S.; et al. Non-Alzheimer neurodegenerative pathologies and their combinations are more frequent than commonly believed in the elderly brain: A community-based autopsy series. Acta Neuropathol. 2013, 126, 365–384. [Google Scholar] [CrossRef] [PubMed]
- Campese, N.; Beatino, M.F.; Del Gamba, C.; Belli, E.; Giampietri, L.; Del Prete, E.; Galgani, A.; Vergallo, A.; Siciliano, G.; Ceravolo, R.; et al. Ultrasensitive techniques and protein misfolding amplification assays for biomarker-guided reconceptualization of Alzheimer’s and other neurodegenerative diseases. Expert Rev. Neurother. 2021, 21, 949–967. [Google Scholar] [CrossRef]
- Robinson, J.L.; Lee, E.B.; Xie, S.X.; Rennert, L.; Suh, E.; Bredenberg, C.; Caswell, C.; Van Deerlin, V.M.; Yan, N.; Yousef, A.; et al. Neurodegenerative disease concomitant proteinopathies are prevalent, age-related and APOE4-associated. Brain 2018, 141, 2181–2193. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thomas, D.X.; Bajaj, S.; McRae-McKee, K.; Hadjichrysanthou, C.; Anderson, R.M.; Collinge, J. Association of TDP-43 proteinopathy, cerebral amyloid angiopathy, and Lewy bodies with cognitive impairment in individuals with or without Alzheimer’s disease neuropathology. Sci. Rep. 2020, 10, 14579. [Google Scholar] [CrossRef] [PubMed]
- McKhann, G.M.; Knopman, D.S.; Chertkow, H.; Hyman, B.T.; Jack, C.R.; Kawas, C.H.; Klunk, W.E.; Koroshetz, W.J.; Manly, J.J.; Mayeux, R. The diagnosis of dementia due to Alzheimer’s disease: Recommendations from the National Institute on Aging-Alzheimer’s Association workgroups on diagnostic guidelines for Alzheimer’s disease. Alzheimer’s Dement. 2011, 7, 263–269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dubois, B.; Feldman, H.H.; Jacova, C.; Hampel, H.; Molinuevo, J.L.; Blennow, K.; DeKosky, S.T.; Gauthier, S.; Selkoe, D.; Bateman, R.; et al. Advancing research diagnostic criteria for Alzheimer’s disease: The IWG-2 criteria. Lancet Neurol. 2014, 13, 614–629. [Google Scholar] [CrossRef]
- Hermann, P.; Appleby, B.; Brandel, J.P.; Caughey, B.; Collins, S.; Geschwind, M.D.; Green, A.; Haïk, S.; Kovacs, G.G.; Ladogana, A.; et al. Biomarkers and diagnostic guidelines for sporadic Creutzfeldt-Jakob disease. Lancet Neurol. 2021, 20, 235–246. [Google Scholar] [CrossRef]
- Verberk, I.M.W.; Slot, R.E.; Verfaillie, S.C.J.; Heijst, H.; Prins, N.D.; van Berckel, B.N.M.; Scheltens, P.; Teunissen, C.E.; van der Flier, W.M. Plasma Amyloid as Prescreener for the Earliest Alzheimer Pathological Changes. Ann. Neurol. 2018, 84, 648–658. [Google Scholar] [CrossRef] [PubMed]
- Vergallo, A.; Mégret, L.; Lista, S.; Cavedo, E.; Zetterberg, H.; Blennow, K.; Vanmechelen, E.; De Vos, A.; Habert, M.-O.O.; Potier, M.-C.C.; et al. Plasma amyloid β 40/42 ratio predicts cerebral amyloidosis in cognitively normal individuals at risk for Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 764–775. [Google Scholar] [CrossRef]
- Chatterjee, P.; Elmi, M.; Goozee, K.; Shah, T.; Sohrabi, H.R.; Dias, C.B.; Pedrini, S.; Shen, K.; Asih, P.R.; Dave, P.; et al. Ultrasensitive Detection of Plasma Amyloid-β as a Biomarker for Cognitively Normal Elderly Individuals at Risk of Alzheimer’s Disease. J. Alzheimer’s Dis. 2019, 71, 775–783. [Google Scholar] [CrossRef] [Green Version]
- de Wolf, F.; Ghanbari, M.; Licher, S.; McRae-McKee, K.; Gras, L.; Weverling, G.J.; Wermeling, P.; Sedaghat, S.; Ikram, M.K.; Waziry, R.; et al. Plasma tau, neurofilament light chain and amyloid-β levels and risk of dementia; a population-based cohort study. Brain 2020, 143, 1220–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ovod, V.; Ramsey, K.N.; Mawuenyega, K.G.; Bollinger, J.G.; Hicks, T.; Schneider, T.; Sullivan, M.; Paumier, K.; Holtzman, D.M.; Morris, J.C.; et al. Amyloid β concentrations and stable isotope labeling kinetics of human plasma specific to central nervous system amyloidosis. Alzheimer’s Dement. 2017, 13, 841–849. [Google Scholar] [CrossRef]
- Nakamura, A.; Kaneko, N.; Villemagne, V.L.; Kato, T.; Doecke, J.; Doré, V.; Fowler, C.; Li, Q.-X.; Martins, R.; Rowe, C.; et al. High performance plasma amyloid-β biomarkers for Alzheimer’s disease. Nature 2018, 554, 249–254. [Google Scholar] [CrossRef] [PubMed]
- Nabers, A.; Perna, L.; Lange, J.; Mons, U.; Schartner, J.; Güldenhaupt, J.; Saum, K.; Janelidze, S.; Holleczek, B.; Rujescu, D.; et al. Amyloid blood biomarker detects Alzheimer’s disease. EMBO Mol. Med. 2018, 10, e8763. [Google Scholar] [CrossRef] [PubMed]
- Stockmann, J.; Verberk, I.M.W.; Timmesfeld, N.; Denz, R.; Budde, B.; Lange-Leifhelm, J.; Scheltens, P.; van der Flier, W.M.; Nabers, A.; Teunissen, C.E.; et al. Amyloid-β misfolding as a plasma biomarker indicates risk for future clinical Alzheimer’s disease in individuals with subjective cognitive decline. Alzheimer’s Res. Ther. 2020, 12, 169. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Hagen, C.E.; Wennberg, A.M.V.; Airey, D.C.; Savica, R.; Knopman, D.S.; Machulda, M.M.; Roberts, R.O.; Jack, C.R.; Petersen, R.C.; et al. Association of Plasma Total Tau Level With Cognitive Decline and Risk of Mild Cognitive Impairment or Dementia in the Mayo Clinic Study on Aging. JAMA Neurol. 2017, 74, 1073. [Google Scholar] [CrossRef]
- Park, J.-C.; Han, S.-H.; Yi, D.; Byun, M.S.; Lee, J.H.; Jang, S.; Ko, K.; Jeon, S.Y.; Lee, Y.-S.; Kim, Y.K.; et al. Plasma tau/amyloid-β1–42 ratio predicts brain tau deposition and neurodegeneration in Alzheimer’s disease. Brain 2019, 142, 771–786. [Google Scholar] [CrossRef] [PubMed]
- Mielke, M.M.; Hagen, C.E.; Xu, J.; Chai, X.; Vemuri, P.; Lowe, V.J.; Airey, D.C.; Knopman, D.S.; Roberts, R.O.; Machulda, M.M.; et al. Plasma phospho-tau181 increases with Alzheimer’s disease clinical severity and is associated with tau- and amyloid-positron emission tomography. Alzheimer’s Dement. 2018, 14, 989–997. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.; Mengel, D.; Keshavan, A.; Rissman, R.A.; Billinton, A.; Perkinton, M.; Percival-Alwyn, J.; Schultz, A.; Properzi, M.; Johnson, K.; et al. Learnings about the complexity of extracellular tau aid development of a blood-based screen for Alzheimer’s disease. Alzheimer’s Dement. 2019, 15, 487–496. [Google Scholar] [CrossRef]
- Janelidze, S.; Mattsson, N.; Palmqvist, S.; Smith, R.; Beach, T.G.; Serrano, G.E.; Chai, X.; Proctor, N.K.; Eichenlaub, U.; Zetterberg, H.; et al. Plasma P-tau181 in Alzheimer’s disease: Relationship to other biomarkers, differential diagnosis, neuropathology and longitudinal progression to Alzheimer’s dementia. Nat. Med. 2020, 26, 379–386. [Google Scholar] [CrossRef] [PubMed]
- Thijssen, E.H.; La Joie, R.; Wolf, A.; Strom, A.; Wang, P.; Iaccarino, L.; Bourakova, V.; Cobigo, Y.; Heuer, H.; Spina, S.; et al. Diagnostic value of plasma phosphorylated tau181 in Alzheimer’s disease and frontotemporal lobar degeneration. Nat. Med. 2020, 26, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Karikari, T.K.; Pascoal, T.A.; Ashton, N.J.; Janelidze, S.; Benedet, A.L.; Rodriguez, J.L.; Chamoun, M.; Savard, M.; Kang, M.S.; Therriault, J.; et al. Blood phosphorylated tau 181 as a biomarker for Alzheimer’s disease: A diagnostic performance and prediction modelling study using data from four prospective cohorts. Lancet Neurol. 2020, 19, 422–433. [Google Scholar] [CrossRef]
- Janelidze, S.; Stomrud, E.; Smith, R.; Palmqvist, S.; Mattsson, N.; Airey, D.C.; Proctor, N.K.; Chai, X.; Shcherbinin, S.; Sims, J.R.; et al. Cerebrospinal fluid p-tau217 performs better than p-tau181 as a biomarker of Alzheimer’s disease. Nat. Commun. 2020, 11, 1683. [Google Scholar] [CrossRef] [Green Version]
- Karikari, T.K.; Emeršič, A.; Vrillon, A.; Lantero-Rodriguez, J.; Ashton, N.J.; Kramberger, M.G.; Dumurgier, J.; Hourregue, C.; Čučnik, S.; Brinkmalm, G.; et al. Head-to-head comparison of clinical performance of CSF phospho-tau T181 and T217 biomarkers for Alzheimer’s disease diagnosis. Alzheimer’s Dement. 2021, 17, 755–767. [Google Scholar] [CrossRef]
- Barthélemy, N.R.; Horie, K.; Sato, C.; Bateman, R.J. Blood plasma phosphorylated-tau isoforms track CNS change in Alzheimer’s disease. J. Exp. Med. 2020, 217, e20200861. [Google Scholar] [CrossRef]
- Palmqvist, S.; Janelidze, S.; Quiroz, Y.T.; Zetterberg, H.; Lopera, F.; Stomrud, E.; Su, Y.; Chen, Y.; Serrano, G.E.; Leuzy, A.; et al. Discriminative Accuracy of Plasma Phospho-tau217 for Alzheimer Disease vs Other Neurodegenerative Disorders. JAMA 2020, 324, 772. [Google Scholar] [CrossRef]
- Spiegel, J.; Pirraglia, E.; Osorio, R.S.; Glodzik, L.; Li, Y.; Tsui, W.; Saint Louis, L.A.; Randall, C.; Butler, T.; Xu, J.; et al. Greater Specificity for Cerebrospinal Fluid P-tau231 over P-tau181 in the Differentiation of Healthy Controls from Alzheimer’s Disease. J. Alzheimer’s Dis. 2015, 49, 93–100. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ashton, N.J.; Pascoal, T.A.; Karikari, T.K.; Benedet, A.L.; Lantero-Rodriguez, J.; Brinkmalm, G.; Snellman, A.; Schöll, M.; Troakes, C.; Hye, A.; et al. Plasma p-tau231: A new biomarker for incipient Alzheimer’s disease pathology. Acta Neuropathol. 2021, 141, 709–724. [Google Scholar] [CrossRef] [PubMed]
- Palermo, G.; Mazzucchi, S.; Della Vecchia, A.; Siciliano, G.; Bonuccelli, U.; Azuar, C.; Ceravolo, R.; Lista, S.; Hampel, H.; Baldacci, F. Different Clinical Contexts of Use of Blood Neurofilament Light Chain Protein in the Spectrum of Neurodegenerative Diseases. Mol. Neurobiol. 2020, 57, 4667–4691. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, P.; Ermann, N.; Andreasson, U.; Schultheis, C.; Podhorna, J.; Spitzer, P.; Maler, J.M.; Kornhuber, J.; Blennow, K.; Zetterberg, H. Plasma neurofilament light as a potential biomarker of neurodegeneration in Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 71. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Cullen, N.C.; Andreasson, U.; Zetterberg, H.; Blennow, K. Association between Longitudinal Plasma Neurofilament Light and Neurodegeneration in Patients with Alzheimer Disease. JAMA Neurol. 2019, 76, 791–799. [Google Scholar] [CrossRef] [PubMed]
- Pereira, J.B.; Westman, E.; Hansson, O. Association between cerebrospinal fluid and plasma neurodegeneration biomarkers with brain atrophy in Alzheimer’s disease. Neurobiol. Aging 2017, 58, 14–29. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.-Y.; Chen, W.; Xu, W.; Li, J.-Q.; Hou, X.-H.; Ou, Y.-N.; Yu, J.-T.; Tan, L. Neurofilament Light Chain in Cerebrospinal Fluid and Blood as a Biomarker for Neurodegenerative Diseases: A Systematic Review and Meta-Analysis. J. Alzheimer’s Dis. 2019, 72, 1353–1361. [Google Scholar] [CrossRef] [PubMed]
- Ashton, N.J.; Leuzy, A.; Lim, Y.M.; Troakes, C.; Hortobágyi, T.; Höglund, K.; Aarsland, D.; Lovestone, S.; Schöll, M.; Blennow, K.; et al. Increased plasma neurofilament light chain concentration correlates with severity of post-mortem neurofibrillary tangle pathology and neurodegeneration. Acta Neuropathol. Commun. 2019, 7, 5. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Andreasson, U.; Zetterberg, H.; Blennow, K.; Alzheimer’s Disease Neuroimaging Initiative. Association of Plasma Neurofilament Light With Neurodegeneration in Patients with Alzheimer Disease. JAMA Neurol. 2017, 74, 557. [Google Scholar] [CrossRef] [PubMed]
- Hu, H.; Chen, K.-L.; Ou, Y.-N.; Cao, X.-P.; Chen, S.-D.; Cui, M.; Dong, Q.; Tan, L.; Yu, J.-T. Neurofilament light chain plasma concentration predicts neurodegeneration and clinical progression in nondemented elderly adults. Aging 2019, 11, 6904–6914. [Google Scholar] [CrossRef]
- Preische, O.; Schultz, S.A.; Apel, A.; Kuhle, J.; Kaeser, S.A.; Barro, C.; Gräber, S.; Kuder-Buletta, E.; LaFougere, C.; Laske, C.; et al. Serum neurofilament dynamics predicts neurodegeneration and clinical progression in presymptomatic Alzheimer’s disease. Nat. Med. 2019, 25, 277–283. [Google Scholar] [CrossRef] [PubMed]
- Weston, P.S.J.; Poole, T.; Ryan, N.S.; Nair, A.; Liang, Y.; Macpherson, K.; Druyeh, R.; Malone, I.B.; Ahsan, R.L.; Pemberton, H.; et al. Serum neurofilament light in familial Alzheimer disease. Neurology 2017, 89, 2167–2175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gille, B.; De Schaepdryver, M.; Goossens, J.; Dedeene, L.; De Vocht, J.; Oldoni, E.; Goris, A.; Van Den Bosch, L.; Depreitere, B.; Claeys, K.G.; et al. Serum neurofilament light chain levels as a marker of upper motor neuron degeneration in patients with Amyotrophic Lateral Sclerosis. Neuropathol. Appl. Neurobiol. 2019, 45, 291–304. [Google Scholar] [CrossRef] [PubMed]
- Steinacker, P.; Semler, E.; Anderl-Straub, S.; Diehl-Schmid, J.; Schroeter, M.L.; Uttner, I.; Foerstl, H.; Landwehrmeyer, B.; von Arnim, C.A.F.F.; Kassubek, J.; et al. Neurofilament as a blood marker for diagnosis and monitoring of primary progressive aphasias. Neurology 2017, 88, 961–969. [Google Scholar] [CrossRef]
- Gaiani, A.; Martinelli, I.; Bello, L.; Querin, G.; Puthenparampil, M.; Ruggero, S.; Toffanin, E.; Cagnin, A.; Briani, C.; Pegoraro, E.; et al. Diagnostic and Prognostic Biomarkers in Amyotrophic Lateral Sclerosis. JAMA Neurol. 2017, 74, 525. [Google Scholar] [CrossRef] [PubMed]
- Verde, F.; Steinacker, P.; Weishaupt, J.H.; Kassubek, J.; Oeckl, P.; Halbgebauer, S.; Tumani, H.; von Arnim, C.A.F.; Dorst, J.; Feneberg, E.; et al. Neurofilament light chain in serum for the diagnosis of amyotrophic lateral sclerosis. J. Neurol. Neurosurg. Psychiatry 2019, 90, 157–164. [Google Scholar] [CrossRef]
- Thouvenot, E.; Demattei, C.; Lehmann, S.; Maceski-Maleska, A.; Hirtz, C.; Juntas-Morales, R.; Pageot, N.; Esselin, F.; Alphandéry, S.; Vincent, T.; et al. Serum neurofilament light chain at time of diagnosis is an independent prognostic factor of survival in amyotrophic lateral sclerosis. Eur. J. Neurol. 2020, 27, 251–257. [Google Scholar] [CrossRef]
- Poesen, K.; De Schaepdryver, M.; Stubendorff, B.; Gille, B.; Muckova, P.; Wendler, S.; Prell, T.; Ringer, T.M.; Rhode, H.; Stevens, O.; et al. Neurofilament markers for ALS correlate with extent of upper and lower motor neuron disease. Neurology 2017, 88, 2302–2309. [Google Scholar] [CrossRef]
- Weydt, P.; Oeckl, P.; Huss, A.; Müller, K.; Volk, A.E.; Kuhle, J.; Knehr, A.; Andersen, P.M.; Prudlo, J.; Steinacker, P.; et al. Neurofilament levels as biomarkers in asymptomatic and symptomatic familial amyotrophic lateral sclerosis. Ann. Neurol. 2016, 79, 152–158. [Google Scholar] [CrossRef]
- Al Shweiki, M.R.; Steinacker, P.; Oeckl, P.; Hengerer, B.; Danek, A.; Fassbender, K.; Diehl-Schmid, J.; Jahn, H.; Anderl-Straub, S.; Ludolph, A.C.; et al. Neurofilament light chain as a blood biomarker to differentiate psychiatric disorders from behavioural variant frontotemporal dementia. J. Psychiatr. Res. 2019, 113, 137–140. [Google Scholar] [CrossRef]
- Katisko, K.; Cajanus, A.; Jääskeläinen, O.; Kontkanen, A.; Hartikainen, P.; Korhonen, V.E.; Helisalmi, S.; Haapasalo, A.; Koivumaa-Honkanen, H.; Herukka, S.-K.; et al. Serum neurofilament light chain is a discriminative biomarker between frontotemporal lobar degeneration and primary psychiatric disorders. J. Neurol. 2020, 267, 162–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hansson, O.; Janelidze, S.; Hall, S.; Magdalinou, N.; Lees, A.J.; Andreasson, U.; Norgren, N.; Linder, J.; Forsgren, L.; Constantinescu, R.; et al. Blood-based NfL. Neurology 2017, 88, 930–937. [Google Scholar] [CrossRef]
- Parnetti, L.; Gaetani, L.; Eusebi, P.; Paciotti, S.; Hansson, O.; El-Agnaf, O.; Mollenhauer, B.; Blennow, K.; Calabresi, P. CSF and blood biomarkers for Parkinson’s disease. Lancet Neurol. 2019, 18, 573–586. [Google Scholar] [CrossRef]
- Marques, T.M.; van Rumund, A.; Oeckl, P.; Kuiperij, H.B.; Esselink, R.A.J.; Bloem, B.R.; Otto, M.; Verbeek, M.M. Serum NFL discriminates Parkinson disease from atypical parkinsonisms. Neurology 2019, 92, e1479–e1486. [Google Scholar] [CrossRef] [PubMed]
- Singer, W.; Schmeichel, A.M.; Shahnawaz, M.; Schmelzer, J.D.; Sletten, D.M.; Gehrking, T.L.; Gehrking, J.A.; Olson, A.D.; Suarez, M.D.; Misra, P.P.; et al. Alpha-Synuclein Oligomers and Neurofilament Light Chain Predict Phenoconversion of Pure Autonomic Failure. Ann. Neurol. 2021, 89, 1212–1220. [Google Scholar] [CrossRef]
- Molinuevo, J.L.; Ayton, S.; Batrla, R.; Bednar, M.M.; Bittner, T.; Cummings, J.; Fagan, A.M.; Hampel, H.; Mielke, M.M.; Mikulskis, A.; et al. Current state of Alzheimer’s fluid biomarkers. Acta Neuropathol. 2018, 136, 821–853. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mazzucchi, S.; Palermo, G.; Campese, N.; Galgani, A.; Della Vecchia, A.; Vergallo, A.; Siciliano, G.; Ceravolo, R.; Hampel, H.; Baldacci, F. The role of synaptic biomarkers in the spectrum of neurodegenerative diseases. Expert Rev. Proteom. 2020, 17, 543–559. [Google Scholar] [CrossRef] [PubMed]
- Kvartsberg, H.; Duits, F.H.; Ingelsson, M.; Andreasen, N.; Öhrfelt, A.; Andersson, K.; Brinkmalm, G.; Lannfelt, L.; Minthon, L.; Hansson, O.; et al. Cerebrospinal fluid levels of the synaptic protein neurogranin correlates with cognitive decline in prodromal Alzheimer’s disease. Alzheimer’s Dement. 2015, 11, 1180–1190. [Google Scholar] [CrossRef] [PubMed]
- Kester, M.I.; Teunissen, C.E.; Crimmins, D.L.; Herries, E.M.; Ladenson, J.H.; Scheltens, P.; van der Flier, W.M.; Morris, J.C.; Holtzman, D.M.; Fagan, A.M. Neurogranin as a Cerebrospinal Fluid Biomarker for Synaptic Loss in Symptomatic Alzheimer Disease. JAMA Neurol. 2015, 72, 1275. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blennow, K.; Diaz-Lucena, D.; Zetterberg, H.; Villar-Pique, A.; Karch, A.; Vidal, E.; Hermann, P.; Schmitz, M.; Ferrer Abizanda, I.; Zerr, I.; et al. CSF neurogranin as a neuronal damage marker in CJD: A comparative study with AD. J. Neurol. Neurosurg. Psychiatry 2019, 90, 846–853. [Google Scholar] [CrossRef]
- Tarawneh, R.; D’Angelo, G.; Crimmins, D.; Herries, E.; Griest, T.; Fagan, A.M.; Zipfel, G.J.; Ladenson, J.H.; Morris, J.C.; Holtzman, D.M. Diagnostic and Prognostic Utility of the Synaptic Marker Neurogranin in Alzheimer Disease. JAMA Neurol. 2016, 73, 561. [Google Scholar] [CrossRef] [PubMed]
- Mattsson, N.; Insel, P.S.; Palmqvist, S.; Portelius, E.; Zetterberg, H.; Weiner, M.; Blennow, K.; Hansson, O. Cerebrospinal fluid tau, neurogranin, and neurofilament light in Alzheimer’s disease. EMBO Mol. Med. 2016, 8, 1184–1196. [Google Scholar] [CrossRef] [PubMed]
- Lista, S.; Toschi, N.; Baldacci, F.; Zetterberg, H.; Blennow, K.; Kilimann, I.; Teipel, S.J.; Cavedo, E.; dos Santos, A.M.; Epelbaum, S.; et al. Cerebrospinal Fluid Neurogranin as a Biomarker of Neurodegenerative Diseases: A Cross-Sectional Study. J. Alzheimer’s Dis. 2017, 59, 1327–1334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wellington, H.; Paterson, R.W.; Suárez-González, A.; Poole, T.; Frost, C.; Sjöbom, U.; Slattery, C.F.; Magdalinou, N.K.; Lehmann, M.; Portelius, E.; et al. CSF neurogranin or tau distinguish typical and atypical Alzheimer disease. Ann. Clin. Transl. Neurol. 2018, 5, 162–171. [Google Scholar] [CrossRef] [PubMed]
- Portelius, E.; Zetterberg, H.; Skillbäck, T.; Törnqvist, U.; Andreasson, U.; Trojanowski, J.Q.; Weiner, M.W.; Shaw, L.M.; Mattsson, N. Cerebrospinal fluid neurogranin: Relation to cognition and neurodegeneration in Alzheimer’s disease. Brain 2015, 138, 3373–3385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Headley, A.; De Leon-Benedetti, A.; Dong, C.; Levin, B.; Loewenstein, D.; Camargo, C.; Rundek, T.; Zetterberg, H.; Blennow, K.; Wright, C.B.; et al. Neurogranin as a predictor of memory and executive function decline in MCI patients. Neurology 2018, 90, e887–e895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bos, I.; Vos, S.; Verhey, F.; Scheltens, P.; Teunissen, C.; Engelborghs, S.; Sleegers, K.; Frisoni, G.; Blin, O.; Richardson, J.C.; et al. Cerebrospinal fluid biomarkers of neurodegeneration, synaptic integrity, and astroglial activation across the clinical Alzheimer’s disease spectrum. Alzheimer’s Dement. 2019, 15, 644–654. [Google Scholar] [CrossRef] [PubMed]
- Tible, M.; Sandelius, Å.; Höglund, K.; Brinkmalm, A.; Cognat, E.; Dumurgier, J.; Zetterberg, H.; Hugon, J.; Paquet, C.; Blennow, K. Dissection of synaptic pathways through the CSF biomarkers for predicting Alzheimer disease. Neurology 2020, 95, e953–e961. [Google Scholar] [CrossRef] [PubMed]
- Brinkmalm, A.; Brinkmalm, G.; Honer, W.G.; Frölich, L.; Hausner, L.; Minthon, L.; Hansson, O.; Wallin, A.; Zetterberg, H.; Blennow, K.; et al. SNAP-25 is a promising novel cerebrospinal fluid biomarker for synapse degeneration in Alzheimer’s disease. Mol. Neurodegener. 2014, 9, 53. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Therriault, J.; Kang, M.S.; Ng, K.P.; Pascoal, T.A.; Rosa-Neto, P.; Gauthier, S. Cerebrospinal fluid synaptosomal-associated protein 25 is a key player in synaptic degeneration in mild cognitive impairment and Alzheimer’s disease. Alzheimer’s Res. Ther. 2018, 10, 80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Öhrfelt, A.; Brinkmalm, A.; Dumurgier, J.; Zetterberg, H.; Bouaziz-Amar, E.; Hugon, J.; Paquet, C.; Blennow, K. A Novel ELISA for the Measurement of Cerebrospinal Fluid SNAP-25 in Patients with Alzheimer’s Disease. Neuroscience 2019, 420, 136–144. [Google Scholar] [CrossRef] [PubMed]
- Öhrfelt, A.; Brinkmalm, A.; Dumurgier, J.; Brinkmalm, G.; Hansson, O.; Zetterberg, H.; Bouaziz-Amar, E.; Hugon, J.; Paquet, C.; Blennow, K. The pre-synaptic vesicle protein synaptotagmin is a novel biomarker for Alzheimer’s disease. Alzheimer’s Res. Ther. 2016, 8, 41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Del Prete, E.; Beatino, M.F.; Campese, N.; Giampietri, L.; Siciliano, G.; Ceravolo, R.; Baldacci, F. Fluid Candidate Biomarkers for Alzheimer’s Disease: A Precision Medicine Approach. J. Pers. Med. 2020, 10, 221. [Google Scholar] [CrossRef]
- Baldacci, F.; Lista, S.; Palermo, G.; Giorgi, F.S.; Vergallo, A.; Hampel, H. The neuroinflammatory biomarker YKL-40 for neurodegenerative diseases: Advances in development. Expert Rev. Proteom. 2019, 16, 593–600. [Google Scholar] [CrossRef] [PubMed]
- Baldacci, F.; Lista, S.; Cavedo, E.; Bonuccelli, U.; Hampel, H. Diagnostic function of the neuroinflammatory biomarker YKL-40 in Alzheimer’s disease and other neurodegenerative diseases. Expert Rev. Proteom. 2017, 14, 285–299. [Google Scholar] [CrossRef] [PubMed]
- Querol-Vilaseca, M.; Colom-Cadena, M.; Pegueroles, J.; San Martín-Paniello, C.; Clarimon, J.; Belbin, O.; Fortea, J.; Lleó, A. YKL-40 (Chitinase 3-like I) is expressed in a subset of astrocytes in Alzheimer’s disease and other tauopathies. J. Neuroinflam. 2017, 14, 118. [Google Scholar] [CrossRef]
- Alcolea, D.; Vilaplana, E.; Suárez-Calvet, M.; Illán-Gala, I.; Blesa, R.; Clarimón, J.; Lladó, A.; Sánchez-Valle, R.; Molinuevo, J.L.; García-Ribas, G.; et al. CSF sAPPβ, YKL-40, and neurofilament light in frontotemporal lobar degeneration. Neurology 2017, 89, 178–188. [Google Scholar] [CrossRef] [PubMed]
- Oeckl, P.; Weydt, P.; Steinacker, P.; Anderl-Straub, S.; Nordin, F.; Volk, A.E.; Diehl-Schmid, J.; Andersen, P.M.; Kornhuber, J.; Danek, A.; et al. Different neuroinflammatory profile in amyotrophic lateral sclerosis and frontotemporal dementia is linked to the clinical phase. J. Neurol. Neurosurg. Psychiatry 2019, 90, 4–10. [Google Scholar] [CrossRef] [PubMed]
- Thompson, A.G.B.; Luk, C.; Heslegrave, A.J.; Zetterberg, H.; Mead, S.H.; Collinge, J.; Jackson, G.S. Neurofilament light chain and tau concentrations are markedly increased in the serum of patients with sporadic Creutzfeldt-Jakob disease, and tau correlates with rate of disease progression. J. Neurol. Neurosurg. Psychiatry 2018, 89, 955–961. [Google Scholar] [CrossRef] [Green Version]
- Pekeles, H.; Qureshi, H.Y.; Paudel, H.K.; Schipper, H.M.; Gornistky, M.; Chertkow, H. Development and validation of a salivary tau biomarker in Alzheimer’s disease. Alzheimer’s Dement. Diagn. Assess. Dis. Monit. 2018, 10, 53–60. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, Y.; Liu, G.; Jiang, Y.; Wang, X.; Wang, Z.; Feng, T. α-Synuclein in salivary extracellular vesicles as a potential biomarker of Parkinson’s disease. Neurosci. Lett. 2019, 696, 114–120. [Google Scholar] [CrossRef] [PubMed]
- Song, W.; Kothari, V.; Velly, A.M.; Cressatti, M.; Liberman, A.; Gornitsky, M.; Schipper, H.M. Evaluation of salivary heme oxygenase-1 as a potential biomarker of early Parkinson’s disease. Mov. Disord. 2018, 33, 583–591. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Lee, E.B.; Moberg, P.J.; Stutzbach, L.; Kazi, H.; Han, L.-Y.; Lee, V.M.Y.; Trojanowski, J.Q. Olfactory epithelium amyloid-β and paired helical filament-tau pathology in Alzheimer disease. Ann. Neurol. 2010, 67, 462–469. [Google Scholar] [CrossRef] [PubMed]
- Wu, N.; Rao, X.; Gao, Y.; Wang, J.; Xu, F. Amyloid-β Deposition and Olfactory Dysfunction in an Alzheimer’s Disease Model. J. Alzheimer’s Dis. 2013, 37, 699–712. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pellkofer, H.; Ihler, F.; Weiss, B.G.; Trothe, J.; Kadavath, H.; Chongtham, M.; Kunadt, M.; Riedel, D.; Lornsen, F.; Wilken, P.; et al. Evaluation of the methoxy-X04 derivative BSC4090 for diagnosis of prodromal and early Alzheimer’s disease from bioptic olfactory mucosa. Eur. Arch. Psychiatry Clin. Neurosci. 2019, 269, 973–984. [Google Scholar] [CrossRef] [Green Version]
- De Luca, C.M.G.; Elia, A.E.; Portaleone, S.M.; Cazzaniga, F.A.; Rossi, M.; Bistaffa, E.; De Cecco, E.; Narkiewicz, J.; Salzano, G.; Carletta, O.; et al. Efficient RT-QuIC seeding activity for α-synuclein in olfactory mucosa samples of patients with Parkinson’s disease and multiple system atrophy. Transl. Neurodegener. 2019, 8, 24. [Google Scholar] [CrossRef] [PubMed]
- Perra, D.; Bongianni, M.; Novi, G.; Janes, F.; Bessi, V.; Capaldi, S.; Sacchetto, L.; Tagliapietra, M.; Schenone, G.; Morbelli, S.; et al. Alpha-synuclein seeds in olfactory mucosa and cerebrospinal fluid of patients with dementia with Lewy bodies. Brain Commun. 2021, 3, fcab045. [Google Scholar] [CrossRef] [PubMed]
- Stefani, A.; Iranzo, A.; Holzknecht, E.; Perra, D.; Bongianni, M.; Gaig, C.; Heim, B.; Serradell, M.; Sacchetto, L.; Garrido, A.; et al. Alpha-synuclein seeds in olfactory mucosa of patients with isolated REM sleep behaviour disorder. Brain 2021, 144, 1118–1126. [Google Scholar] [CrossRef] [PubMed]
- Al-Qassabi, A.; Tsao, T.-S.; Racolta, A.; Kremer, T.; Cañamero, M.; Belousov, A.; Santana, M.A.; Beck, R.C.; Zhang, H.; Meridew, J.; et al. Immunohistochemical Detection of Synuclein Pathology in Skin in Idiopathic Rapid Eye Movement Sleep Behavior Disorder and Parkinsonism. Mov. Disord. 2021, 36, 895–904. [Google Scholar] [CrossRef] [PubMed]
- Doppler, K.; Jentschke, H.-M.; Schulmeyer, L.; Vadasz, D.; Janzen, A.; Luster, M.; Höffken, H.; Mayer, G.; Brumberg, J.; Booij, J.; et al. Dermal phospho-alpha-synuclein deposits confirm REM sleep behaviour disorder as prodromal Parkinson’s disease. Acta Neuropathol. 2017, 133, 535–545. [Google Scholar] [CrossRef] [Green Version]
- Wang, Z.; Becker, K.; Donadio, V.; Siedlak, S.; Yuan, J.; Rezaee, M.; Incensi, A.; Kuzkina, A.; Orrú, C.D.; Tatsuoka, C.; et al. Skin α-Synuclein Aggregation Seeding Activity as a Novel Biomarker for Parkinson Disease. JAMA Neurol. 2021, 78, 30. [Google Scholar] [CrossRef] [PubMed]
- Sun, R.; Wang, H.; Shi, Y.; Gao, D.; Sun, Z.; Chen, Z.; Jiang, H.; Zhang, J. A Pilot Study of Urinary Exosomes in Alzheimer’s Disease. Neurodegener. Dis. 2019, 19, 184–191. [Google Scholar] [CrossRef] [PubMed]
- Yilmaz, A.; Ugur, Z.; Bisgin, H.; Akyol, S.; Bahado-Singh, R.; Wilson, G.; Imam, K.; Maddens, M.E.; Graham, S.F. Targeted Metabolic Profiling of Urine Highlights a Potential Biomarker Panel for the Diagnosis of Alzheimer’s Disease and Mild Cognitive Impairment: A Pilot Study. Metabolites 2020, 10, 357. [Google Scholar] [CrossRef]
- Nam, D.; Kim, A.; Han, S.J.; Lee, S.-I.; Park, S.-H.; Seol, W.; Son, I.; Ho, D.H. Analysis of α-synuclein levels related to LRRK2 kinase activity: From substantia nigra to urine of patients with Parkinson’s disease. Animal Cells Syst. 2021, 25, 28–36. [Google Scholar] [CrossRef]
- Lunetta, C.; Lizio, A.; Gerardi, F.; Tarlarini, C.; Filippi, M.; Riva, N.; Tremolizzo, L.; Diamanti, S.; Dellanoce, C.C.; Mosca, L.; et al. Urinary neopterin, a new marker of the neuroinflammatory status in amyotrophic lateral sclerosis. J. Neurol. 2020, 267, 3609–3616. [Google Scholar] [CrossRef]
- Ong, Y.-T.; Hilal, S.; Cheung, C.Y.; Venketasubramanian, N.; Niessen, W.J.; Vrooman, H.; Anuar, A.R.; Chew, M.; Chen, C.; Wong, T.Y.; et al. Retinal neurodegeneration on optical coherence tomography and cerebral atrophy. Neurosci. Lett. 2015, 584, 12–16. [Google Scholar] [CrossRef]
- Hart, N.J.; Koronyo, Y.; Black, K.L.; Koronyo-Hamaoui, M. Ocular indicators of Alzheimer’s: Exploring disease in the retina. Acta Neuropathol. 2016, 132, 767–787. [Google Scholar] [CrossRef] [Green Version]
- Mirzaei, N.; Shi, H.; Oviatt, M.; Doustar, J.; Rentsendorj, A.; Fuchs, D.-T.; Sheyn, J.; Black, K.L.; Koronyo, Y.; Koronyo-Hamaoui, M. Alzheimer’s Retinopathy: Seeing Disease in the Eyes. Front. Neurosci. 2020, 14, 921. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Shi, C.; Shen, M.; Lu, F. Advances in retina imaging as potential biomarkers for early diagnosis of Alzheimer’s disease. Transl. Neurodegener. 2021, 10, 6. [Google Scholar] [CrossRef]
- Robbins, C.B.; Thompson, A.C.; Bhullar, P.K.; Koo, H.Y.; Agrawal, R.; Soundararajan, S.; Yoon, S.P.; Polascik, B.W.; Scott, B.L.; Grewal, D.S.; et al. Characterization of Retinal Microvascular and Choroidal Structural Changes in Parkinson Disease. JAMA Ophthalmol. 2021, 139, 182. [Google Scholar] [CrossRef]
- Zanusso, G.; Monaco, S.; Pocchiari, M.; Caughey, B. Advanced tests for early and accurate diagnosis of Creutzfeldt–Jakob disease. Nat. Rev. Neurol. 2016, 12, 325–333. [Google Scholar] [CrossRef]
- Paciotti, S.; Bellomo, G.; Gatticchi, L.; Parnetti, L. Are We Ready for Detecting α-Synuclein Prone to Aggregation in Patients? The Case of “Protein-Misfolding Cyclic Amplification” and “Real-Time Quaking-Induced Conversion” as Diagnostic Tools. Front. Neurol. 2018, 9, 415. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Candelise, N.; Baiardi, S.; Franceschini, A.; Rossi, M.; Parchi, P. Towards an improved early diagnosis of neurodegenerative diseases: The emerging role of in vitro conversion assays for protein amyloids. Acta Neuropathol. Commun. 2020, 8, 117. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, N.D.C.; Caughey, B. Proteopathic Seed Amplification Assays for Neurodegenerative Disorders. Clin. Lab. Med. 2020, 40, 257–270. [Google Scholar] [CrossRef] [PubMed]
- Green, A.J.E.E.; Zanusso, G. Prion protein amplification techniques. Handb. Clin. Neurol. 2018, 153, 357–370. [Google Scholar] [PubMed]
- Cazzaniga, F.A.; De Luca, C.M.G.; Bistaffa, E.; Consonni, A.; Legname, G.; Giaccone, G.; Moda, F. Cell-free amplification of prions: Where do we stand? Prog. Mol. Biol. Transl. Sci. 2020, 175, 325–358. [Google Scholar] [PubMed]
- van Rumund, A.; Green, A.J.E.; Fairfoul, G.; Esselink, R.A.J.; Bloem, B.R.; Verbeek, M.M. α-Synuclein real-time quaking-induced conversion in the cerebrospinal fluid of uncertain cases of parkinsonism. Ann. Neurol. 2019, 85, 777–781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bongianni, M.; Ladogana, A.; Capaldi, S.; Klotz, S.; Baiardi, S.; Cagnin, A.; Perra, D.; Fiorini, M.; Poleggi, A.; Legname, G.; et al. α-Synuclein RT-QuIC assay in cerebrospinal fluid of patients with dementia with Lewy bodies. Ann. Clin. Transl. Neurol. 2019, 6, 2120–2126. [Google Scholar] [CrossRef] [PubMed]
- Groveman, B.R.; Orrù, C.D.; Hughson, A.G.; Raymond, L.D.; Zanusso, G.; Ghetti, B.; Campbell, K.J.; Safar, J.; Galasko, D.; Caughey, B. Rapid and ultra-sensitive quantitation of disease-associated α-synuclein seeds in brain and cerebrospinal fluid by αSyn RT-QuIC. Acta Neuropathol. Commun. 2018, 6, 7. [Google Scholar] [CrossRef] [PubMed]
- Rossi, M.; Candelise, N.; Baiardi, S.; Capellari, S.; Giannini, G.; Orrù, C.D.; Antelmi, E.; Mammana, A.; Hughson, A.G.; Calandra-Buonaura, G.; et al. Ultrasensitive RT-QuIC assay with high sensitivity and specificity for Lewy body-associated synucleinopathies. Acta Neuropathol. 2020, 140, 49–62. [Google Scholar] [CrossRef]
- Shahnawaz, M.; Mukherjee, A.; Pritzkow, S.; Mendez, N.; Rabadia, P.; Liu, X.; Hu, B.; Schmeichel, A.; Singer, W.; Wu, G.; et al. Discriminating α-synuclein strains in Parkinson’s disease and multiple system atrophy. Nature 2020, 578, 273–277. [Google Scholar] [CrossRef] [PubMed]
- Shahnawaz, M.; Tokuda, T.; Waragai, M.; Mendez, N.; Ishii, R.; Trenkwalder, C.; Mollenhauer, B.; Soto, C. Development of a Biochemical Diagnosis of Parkinson Disease by Detection of α-Synuclein Misfolded Aggregates in Cerebrospinal Fluid. JAMA Neurol. 2017, 74, 163. [Google Scholar] [CrossRef]
- Singer, W.; Schmeichel, A.M.; Shahnawaz, M.; Schmelzer, J.D.; Boeve, B.F.; Sletten, D.M.; Gehrking, T.L.; Gehrking, J.A.; Olson, A.D.; Savica, R.; et al. Alpha-Synuclein Oligomers and Neurofilament Light Chain in Spinal Fluid Differentiate Multiple System Atrophy from Lewy Body Synucleinopathies. Ann. Neurol. 2020, 88, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Peng, C.; Gathagan, R.J.; Lee, V.M.-Y. Distinct α-Synuclein strains and implications for heterogeneity among α-Synucleinopathies. Neurobiol. Dis. 2018, 109, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Fairfoul, G.; McGuire, L.I.; Pal, S.; Ironside, J.W.; Neumann, J.; Christie, S.; Joachim, C.; Esiri, M.; Evetts, S.G.; Rolinski, M.; et al. Alpha-synuclein RT-QuIC in the CSF of patients with alpha-synucleinopathies. Ann. Clin. Transl. Neurol. 2016, 3, 812–818. [Google Scholar] [CrossRef] [PubMed]
- Iranzo, A.; Fairfoul, G.; Ayudhaya, A.C.N.; Serradell, M.; Gelpi, E.; Vilaseca, I.; Sanchez-Valle, R.; Gaig, C.; Santamaria, J.; Tolosa, E.; et al. Detection of α-synuclein in CSF by RT-QuIC in patients with isolated rapid-eye-movement sleep behaviour disorder: A longitudinal observational study. Lancet Neurol. 2021, 20, 203–212. [Google Scholar] [CrossRef]
- Garrido, A.; Fairfoul, G.; Tolosa, E.S.; Martí, M.J.; Green, A. Alpha-synuclein RT-QuIC in cerebrospinal fluid of LRRK 2-linked Parkinson’s disease. Ann. Clin. Transl. Neurol. 2019, 6, 1024–1032. [Google Scholar] [CrossRef] [Green Version]
- Saijo, E.; Ghetti, B.; Zanusso, G.; Oblak, A.; Furman, J.L.; Diamond, M.I.; Kraus, A.; Caughey, B. Ultrasensitive and selective detection of 3-repeat tau seeding activity in Pick disease brain and cerebrospinal fluid. Acta Neuropathol. 2017, 133, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Saijo, E.; Metrick, M.A.; Koga, S.; Parchi, P.; Litvan, I.; Spina, S.; Boxer, A.; Rojas, J.C.; Galasko, D.; Kraus, A.; et al. 4-Repeat tau seeds and templating subtypes as brain and CSF biomarkers of frontotemporal lobar degeneration. Acta Neuropathol. 2020, 139, 63–77. [Google Scholar] [CrossRef] [PubMed]
- Scialò, C.; Tran, T.H.; Salzano, G.; Novi, G.; Caponnetto, C.; Chiò, A.; Calvo, A.; Canosa, A.; Moda, F.; Caroppo, P.; et al. TDP-43 real-time quaking induced conversion reaction optimization and detection of seeding activity in CSF of amyotrophic lateral sclerosis and frontotemporal dementia patients. Brain Commun. 2020, 2, fcaa142. [Google Scholar] [CrossRef] [PubMed]
- Salvadores, N.; Shahnawaz, M.; Scarpini, E.; Tagliavini, F.; Soto, C. Detection of Misfolded Aβ Oligomers for Sensitive Biochemical Diagnosis of Alzheimer’s Disease. Cell Rep. 2014, 7, 261–268. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Canevelli, M.; Remoli, G.; Bacigalupo, I.; Valletta, M.; Blasi, M.T.; Sciancalepore, F.; Bruno, G.; Cesari, M.; Vanacore, N. Use of biomarkers in ongoing research protocols on alzheimer’s disease. J. Pers. Med. 2020, 10, 68. [Google Scholar] [CrossRef]
- Panza, F.; Lozupone, M.; Logroscino, G.; Imbimbo, B.P. A critical appraisal of amyloid-β-targeting therapies for Alzheimer disease. Nat. Rev. Neurol. 2019, 15, 73–88. [Google Scholar] [CrossRef] [PubMed]
- Rombouts, F.; Kusakabe, K.-I.; Hsiao, C.C.; Gijsen, H.J.M. Small-molecule BACE1 inhibitors: A patent literature review (2011 to 2020). Expert Opin. Ther. Pat. 2021, 31, 25–52. [Google Scholar] [CrossRef] [PubMed]
- Golde, T.E.; Koo, E.H.; Felsenstein, K.M.; Osborne, B.A.; Miele, L. γ-Secretase inhibitors and modulators. Biochim. Biophys. Acta—Biomembr. 2013, 1828, 2898–2907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lacosta, A.-M.; Pascual-Lucas, M.; Pesini, P.; Casabona, D.; Pérez-Grijalba, V.; Marcos-Campos, I.; Sarasa, L.; Canudas, J.; Badi, H.; Monleón, I.; et al. Safety, Tolerability and Immunogenicity of an Active Anti-Aβ40 Vaccine (ABvac40) in Patients with Alzheimer’s Disease: A Randomised, Double-Blind, Placebo-Controlled, Phase I Trial. Alzheimer’s Res. Ther. 2018, 10, 12. [Google Scholar] [CrossRef] [PubMed]
- Molina, E.P.; Pesini, P.; Sarasa-SanJose, M.; Marcos, I.; Lacosta, A.M.; Allué, J.A.; Fandos, N.; Sarasa, M.; Boada, M. Safety, tolerability and immunogenicity of an active anti-Aβ40 vaccine (ABvac40) in patients with amnestic mild cognitive impairment (A-MCI) or very mild alzheimer’s disease (VM-AD): A randomized, double-blind, placebo-controlled, phase II trial. Alzheimer’s Dement. 2020, 16, e045720. [Google Scholar] [CrossRef]
- Safety and Immunogenicity of Repeated Doses of ABvac40 in Patients with A-MCI or Vm-AD. Available online: https://clinicaltrials.gov/show/NCT03461276 (accessed on 31 May 2021).
- Ostrowitzki, S.; Lasser, R.A.; Dorflinger, E.; Scheltens, P.; Barkhof, F.; Nikolcheva, T.; Ashford, E.; Retout, S.; Hofmann, C.; Delmar, P.; et al. A phase III randomized trial of gantenerumab in prodromal Alzheimer’s disease. Alzheimer’s Res. Ther. 2017, 9, 95. [Google Scholar] [CrossRef]
- Imbimbo, B.P.; Ottonello, S.; Frisardi, V.; Solfrizzi, V.; Greco, A.; Seripa, D.; Pilotto, A.; Panza, F. Solanezumab for the treatment of mild-to-moderate Alzheimers disease. Expert Rev. Clin. Immunol. 2012, 8, 135–149. [Google Scholar] [CrossRef] [PubMed]
- Doody, R.S.; Thomas, R.G.; Farlow, M.; Iwatsubo, T.; Vellas, B.; Joffe, S.; Kieburtz, K.; Raman, R.; Sun, X.; Aisen, P.S.; et al. Phase 3 Trials of Solanezumab for Mild-to-Moderate Alzheimer’s Disease. N. Engl. J. Med. 2014, 370, 311–321. [Google Scholar] [CrossRef]
- Honig, L.S.; Vellas, B.; Woodward, M.; Boada, M.; Bullock, R.; Borrie, M.; Hager, K.; Andreasen, N.; Scarpini, E.; Liu-Seifert, H.; et al. Trial of Solanezumab for Mild Dementia Due to Alzheimer’s Disease. N. Engl. J. Med. 2018, 378, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Clinical Trial of Solanezumab for Older Individuals Who May Be at Risk for Memory Loss. Available online: https://www.clinicaltrials.gov/ct2/show/NCT02008357 (accessed on 31 May 2021).
- Tucker, S.; Möller, C.; Tegerstedt, K.; Lord, A.; Laudon, H.; Sjödahl, J.; Söderberg, L.; Spens, E.; Sahlin, C.; Waara, E.R.; et al. The murine Version of BAN2401 (mAb158) selectively reduces amyloid-β protofibrils in brain and cerebrospinal fluid of tg-ArcSwe Mice. J. Alzheimer’s Dis. 2015, 43, 575–588. [Google Scholar] [CrossRef] [PubMed]
- Swanson, C.J.; Zhang, Y.; Dhadda, S.; Wang, J.; Kaplow, J.; Lai, R.Y.K.; Lannfelt, L.; Bradley, H.; Rabe, M.; Koyama, A.; et al. A randomized, double-blind, phase 2b proof-of-concept clinical trial in early Alzheimer’s disease with lecanemab, an anti-Aβ protofibril antibody. Alzheimer’s Res. Ther. 2021, 13, 80. [Google Scholar] [CrossRef] [PubMed]
- Arndt, J.W.; Qian, F.; Smith, B.A.; Quan, C.; Kilambi, K.P.; Bush, M.W.; Walz, T.; Pepinsky, R.B.; Bussière, T.; Hamann, S.; et al. Structural and kinetic basis for the selectivity of aducanumab for aggregated forms of amyloid-β. Sci. Rep. 2018, 8, 6412. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haeberlein, S.B.; von Hehn, C.; Tian, Y.; Chalkias, S.; Muralidharan, K.K.; Chen, T.; Wu, S.; Skordos, L.; Nisenbaum, L.; Rajagovindan, R.; et al. EMERGE and ENGAGE topline results: Phase 3 studies of aducanumab in early Alzheimer’s disease. Alzheimer’s Dement. 2020, 16, e047259. [Google Scholar] [CrossRef]
- Dominantly Inherited Alzheimer Network Trial: An Opportunity to Prevent Dementia. A Study of Potential Disease Modifying Treatments in Individuals at Risk for or with a Type of Early Onset Alzheimer’s Disease Caused by a Genetic Mutation. Master Protocol DIAN-TU001. Available online: https://clinicaltrials.gov/show/NCT01760005 (accessed on 21 June 2021).
- Farlow, M.R.; Bateman, R.J.; Aschenbrenner, A.J.; Benzinger, T.L.S.; Clifford, D.; Coalier, K.; Cruchaga, C.; Fagan, A.M.; Goate, A.M.; Gordon, B.A.; et al. Solanezumab in-depth outcomes. Alzheimer’s Dement. 2020, 16, e038028. [Google Scholar] [CrossRef]
- Salloway, S.P.; Bateman, R.J.; Aschenbrenner, A.J.; Benzinger, T.L.S.; Clifford, D.; Coalier, K.; Cruchaga, C.; Fagan, A.M.; Farlow, M.R.; Goate, A.M.; et al. Gantenerumab in-depth outcomes. Alzheimer’s Dement. 2020, 16, e038049. [Google Scholar] [CrossRef]
- Ceyzériat, K.; Zilli, T.; Millet, P.; Frisoni, G.B.; Garibotto, V.; Tournier, B.B. Learning from the Past: A Review of Clinical Trials Targeting Amyloid, Tau and Neuroinflammation in Alzheimer’s Disease. Curr. Alzheimer Res. 2020, 17, 112–125. [Google Scholar] [CrossRef] [PubMed]
- AADvac1|ALZFORUM. Available online: https://www.alzforum.org/therapeutics/aadvac1 (accessed on 14 June 2021).
- Axon Announces Positive Results from Phase II ADAMANT Trial for AADvac1 in Alzheimer’s Disease. Available online: https://www.axon-neuroscience.eu/src/pr/6.pdf (accessed on 14 June 2021).
- A Study to Evaluate the Safety, Tolerability and Immunogenicity of Tau Targeted Vaccines in Participants with Early Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT04445831 (accessed on 14 June 2021).
- N-Terminal Tau Antibodies Fade, Mid-Domain Ones Push to the Fore|ALZFORUM. Available online: https://www.alzforum.org/news/conference-coverage/n-terminal-tau-antibodies-fade-mid-domain-ones-push-fore (accessed on 15 June 2021).
- Galpern, W.R.; Mercken, M.; Van Kolen, K.; Timmers, M.; Haeverans, K.; Janssens, L.; Triana-Baltzer, G.; Kolb, H.C.; Jacobs, T.; Nandy, P.; et al. P1-052: A Single Ascending Dose Study To Evaluate The Safety, Tolerability, Pharmacokinetics, And Pharmacodynamics Of The Anti-Phospho-Tau Antibody JNJ-63733657 In Healthy Subjects. Alzheimer’s Dement. 2019, 15, P252–P253. [Google Scholar] [CrossRef]
- Aisen, P.S. The potential of anti-inflammatory drugs for the treatment of Alzheimer’s disease. Lancet Neurol. 2002, 1, 279–284. [Google Scholar] [CrossRef]
- Jordan, F.; Quinn, T.J.; McGuinness, B.; Passmore, P.; Kelly, J.P.; Tudur Smith, C.; Murphy, K.; Devane, D. Aspirin and other non-steroidal anti-inflammatory drugs for the prevention of dementia. Cochrane Database Syst. Rev. 2020, 4, CD011459. [Google Scholar] [PubMed]
- Jaturapatporn, D.; Isaac, M.G.E.K.N.; McCleery, J.; Tabet, N. Aspirin, steroidal and non-steroidal anti-inflammatory drugs for the treatment of Alzheimer’s disease. Cochrane Database Syst. Rev. 2012, 2, CD006378. [Google Scholar] [CrossRef] [PubMed]
- De Luca, C.; Colangelo, A.M.; Virtuoso, A.; Alberghina, L.; Papa, M. Neurons, glia, extracellular matrix and neurovascular unit: A systems biology approach to the complexity of synaptic plasticity in health and disease. Int. J. Mol. Sci. 2020, 21, 1539. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Mustafa, M.; Yuede, C.M.; Salazar, S.V.; Kong, P.; Long, H.; Ward, M.; Siddiqui, O.; Paul, R.; Gilfillan, S.; et al. Anti-human TREM2 induces microglia proliferation and reduces pathology in an Alzheimer’s disease model. J. Exp. Med. 2020, 217, e20200785. [Google Scholar] [CrossRef] [PubMed]
- Hampel, H.; Caraci, F.; Cuello, A.C.; Caruso, G.; Nisticò, R.; Corbo, M.; Baldacci, F.; Toschi, N.; Garaci, F.; Chiesa, P.A.; et al. A Path Toward Precision Medicine for Neuroinflammatory Mechanisms in Alzheimer’s Disease. Front. Immunol. 2020, 11, 456. [Google Scholar] [CrossRef]
- A Phase 2 Study to Evaluate Efficacy and Safety of AL002 in Participants with Early Alzheimer’s Disease. Available online: https://clinicaltrials.gov/ct2/show/NCT04592874 (accessed on 21 June 2021).
- Fisher, T.L.; Reilly, C.A.; Winter, L.A.; Pandina, T.; Jonason, A.; Scrivens, M.; Balch, L.; Bussler, H.; Torno, S.; Seils, J.; et al. Generation and preclinical characterization of an antibody specific for SEMA4D. MAbs 2016, 8, 150–162. [Google Scholar] [CrossRef]
- Evans, E.E.; Fisher, T.L.; Leonard, J.E.; Reader, A.M.; Mishra, V.; Mallow, C.L.; Balch, L.; Howell, A.; Smith, E.S.; Feigin, A.; et al. Evidence that semaphorin 4D is upregulated in neurons in Huntington’s and Alzheimer’s diseases: Effects of a SEMA4D blocking antibody on FDG-PET in a clinical trial, and treatment rationale for its use in AD. Alzheimer’s Dement. 2020, 16, e043971. [Google Scholar] [CrossRef]
- Alzforum.com. Pepinemab|ALZFORUM. Available online: https://www.alzforum.org/therapeutics/pepinemab (accessed on 20 June 2021).
- SEMA4D Blockade Safety and Brain Metabolic Activity in Alzheimer’s Disease (AD). Available online: https://clinicaltrials.gov/ct2/show/NCT04381468 (accessed on 20 June 2021).
- Greaves, C.V.; Rohrer, J.D. An update on genetic frontotemporal dementia. J. Neurol. 2019, 266, 2075–2086. [Google Scholar] [CrossRef] [Green Version]
- Broce, I.J.; Castruita, P.A.; Yokoyama, J.S. Moving toward Patient-Tailored Treatment in ALS and FTD: The Potential of Genomic Assessment as a Tool for Biological Discovery and Trial Recruitment. Front. Neurosci. 2021, 15, 150. [Google Scholar] [CrossRef]
- Gass, J.; Cannon, A.; Mackenzie, I.R.; Boeve, B.; Baker, M.; Adamson, J.; Crook, R.; Melquist, S.; Kuntz, K.; Petersen, R.; et al. Mutations in progranulin are a major cause of ubiquitin-positive frontotemporal lobar degeneration. Hum. Mol. Genet. 2006, 15, 2988–3001. [Google Scholar] [CrossRef] [Green Version]
- Murphy, N.A.; Arthur, K.C.; Tienari, P.J.; Houlden, H.; Chiò, A.; Traynor, B.J. Age-related penetrance of the C9orf72 repeat expansion. Sci. Rep. 2017, 7, 2116. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Mossevelde, S.; Engelborghs, S.; van der Zee, J.; Van Broeckhoven, C. Genotype–phenotype links in frontotemporal lobar degeneration. Nat. Rev. Neurol. 2018, 14, 363–378. [Google Scholar] [CrossRef] [PubMed]
- Winter, B.; Guenther, R.; Ludolph, A.C.; Hermann, A.; Otto, M.; Wurster, C.D. Neurofilaments and tau in CSF in an infant with SMA type 1 treated with nusinersen. J. Neurol. Neurosurg. Psychiatry 2019, 90, 1068–1069. [Google Scholar] [CrossRef] [PubMed]
- Alector Announces Data from On-Going Phase 1b Trial Demonstrating That AL001 Reverses Progranulin Deficiency in Frontotemporal Dementia Patients. Available online: https://investors.alector.com/news-releases/news-release-details/alector-announces-data-going-phase-1b-trial-demonstrating-al001 (accessed on 22 June 2021).
- A Phase 3 Study to Evaluate Efficacy and Safety of AL001 in Frontotemporal Dementia (INFRONT-3). Available online: https://clinicaltrials.gov/ct2/show/NCT04374136 (accessed on 22 June 2021).
- A Study of PBFT02 in Patients with Frontotemporal Dementia and Progranulin Mutations (FTD-GRN). Available online: https://clinicaltrials.gov/ct2/show/NCT04747431 (accessed on 21 June 2021).
- Phase 1/2 Clinical Trial of PR006 in Patients with Frontotemporal Dementia with Progranulin Mutations (FTD-GRN). Available online: https://clinicaltrials.gov/ct2/show/NCT04408625 (accessed on 22 June 2021).
- Baker, M.; Mackenzie, I.R.; Pickering-Brown, S.M.; Gass, J.; Rademakers, R.; Lindholm, C.; Snowden, J.; Adamson, J.; Sadovnick, A.D.; Rollinson, S.; et al. Mutations in progranulin cause tau-negative frontotemporal dementia linked to chromosome 17. Nature 2006, 442, 916–919. [Google Scholar] [CrossRef]
- Salado, I.G.; Redondo, M.; Bello, M.L.; Perez, C.; Liachko, N.F.; Kraemer, B.C.; Miguel, L.; Lecourtois, M.; Gil, C.; Martinez, A.; et al. Protein Kinase CK-1 Inhibitors As New Potential Drugs for Amyotrophic Lateral Sclerosis. J. Med. Chem. 2014, 57, 2755–2772. [Google Scholar] [CrossRef]
- Grad, L.I.; Rouleau, G.A.; Ravits, J.; Cashman, N.R. Clinical Spectrum of Amyotrophic Lateral Sclerosis (ALS). Cold Spring Harb. Perspect. Med. 2017, 7, a024117. [Google Scholar] [CrossRef] [Green Version]
- Renton, A.E.; Chiò, A.; Traynor, B.J. State of play in amyotrophic lateral sclerosis genetics. Nat. Neurosci. 2014, 17, 17–23. [Google Scholar] [CrossRef]
- van Es, M.A.; Hardiman, O.; Chio, A.; Al-Chalabi, A.; Pasterkamp, R.J.; Veldink, J.H.; van den Berg, L.H. Amyotrophic lateral sclerosis. Lancet 2017, 390, 2084–2098. [Google Scholar] [CrossRef]
- Kiernan, M.C.; Vucic, S.; Talbot, K.; McDermott, C.J.; Hardiman, O.; Shefner, J.M.; Al-Chalabi, A.; Huynh, W.; Cudkowicz, M.; Talman, P.; et al. Improving clinical trial outcomes in amyotrophic lateral sclerosis. Nat. Rev. Neurol. 2021, 17, 104–118. [Google Scholar] [CrossRef]
- A Study of BIIB067 When Initiated in Clinically Presymptomatic Adults with a Confirmed Superoxide Dismutase 1 Mutation. Available online: https://clinicaltrials.gov/ct2/show/NCT04856982 (accessed on 18 June 2021).
- Study to Assess the Safety, Tolerability, Pharmacokinetics, and Effect on Disease Progression of BIIB078 Administered to Previously Treated Adults C9ORF72-Associated Amyotrophic Lateral Sclerosis (ALS). Available online: https://clinicaltrials.gov/ct2/show/NCT04288856 (accessed on 19 June 2021).
- Chen, C.; Ding, X.; Akram, N.; Xue, S.; Luo, S.-Z. Fused in Sarcoma: Properties, Self-Assembly and Correlation with Neurodegenerative Diseases. Molecules 2019, 24, 1622. [Google Scholar] [CrossRef] [Green Version]
- A Study to Evaluate the Efficacy, Safety, Pharmacokinetics and Pharmacodynamics of ION363 in Amyotrophic Lateral Sclerosis Participants with Fused in Sarcoma Mutations (FUS-ALS). Available online: https://clinicaltrials.gov/ct2/show/NCT04768972 (accessed on 19 June 2021).
- Hou, Y.; Dan, X.; Babbar, M.; Wei, Y.; Hasselbalch, S.G.; Croteau, D.L.; Bohr, V.A. Ageing as a risk factor for neurodegenerative disease. Nat. Rev. Neurol. 2019, 15, 565–581. [Google Scholar] [CrossRef]
- Crous-Bou, M.; Minguillón, C.; Gramunt, N.; Molinuevo, J.L. Alzheimer’s disease prevention: From risk factors to early intervention. Alzheimer’s Res. Ther. 2017, 9, 71. [Google Scholar] [CrossRef]
- Xu, W.; Tan, L.L.; Wang, H.-F.F.; Jiang, T.; Tan, M.-S.S.; Tan, L.L.; Zhao, Q.-F.F.; Li, J.-Q.Q.; Wang, J.; Yu, J.-T.T. Meta-analysis of modifiable risk factors for Alzheimer’s disease. J. Neurol. Neurosurg. Psychiatry 2015, 86, 1299–1306. [Google Scholar] [CrossRef]
- Bubu, O.M.; Andrade, A.G.; Umasabor-Bubu, O.Q.; Hogan, M.M.; Turner, A.D.; de Leon, M.J.; Ogedegbe, G.; Ayappa, I.; Jackson, M.L.; Varga, A.W.; et al. Obstructive sleep apnea, cognition and Alzheimer’s disease: A systematic review integrating three decades of multidisciplinary research. Sleep Med. Rev. 2020, 50, 101250. [Google Scholar] [CrossRef]
- Abner, E.L.; Kryscio, R.J.; Caban-Holt, A.M.; Schmitt, F.A. Baseline subjective memory complaints associate with increased risk of incident dementia: The PREADVISE trial. J. Prev. Alzheimer’s Dis. 2015, 2, 11–16. [Google Scholar] [CrossRef]
- Dauvilliers, Y.; Schenck, C.H.; Postuma, R.B.; Iranzo, A.; Luppi, P.H.; Plazzi, G.; Montplaisir, J.; Boeve, B. REM sleep behaviour disorder. Nat. Rev. Dis. Prim. 2018, 4, 19. [Google Scholar] [CrossRef]
- Dubois, B.; Hampel, H.; Feldman, H.H.; Scheltens, P.; Aisen, P.; Andrieu, S.; Bakardjian, H.; Benali, H.; Bertram, L.; Blennow, K.; et al. Preclinical Alzheimer’s disease: Definition, natural history, and diagnostic criteria. Alzheimer’s Dement. 2016, 12, 292–323. [Google Scholar] [CrossRef]
- Ursin, F.; Timmermann, C.; Steger, F. Ethical Implications of Alzheimer’s Disease Prediction in Asymptomatic Individuals through Artificial Intelligence. Diagnostics 2021, 11, 440. [Google Scholar] [CrossRef]
- De Luca, C.; Virtuoso, A.; Maggio, N.; Izzo, S.; Papa, M.; Colangelo, A.M. Roadmap for Stroke: Challenging the Role of the Neuronal Extracellular Matrix. Int. J. Mol. Sci. 2020, 21, 7554. [Google Scholar] [CrossRef]
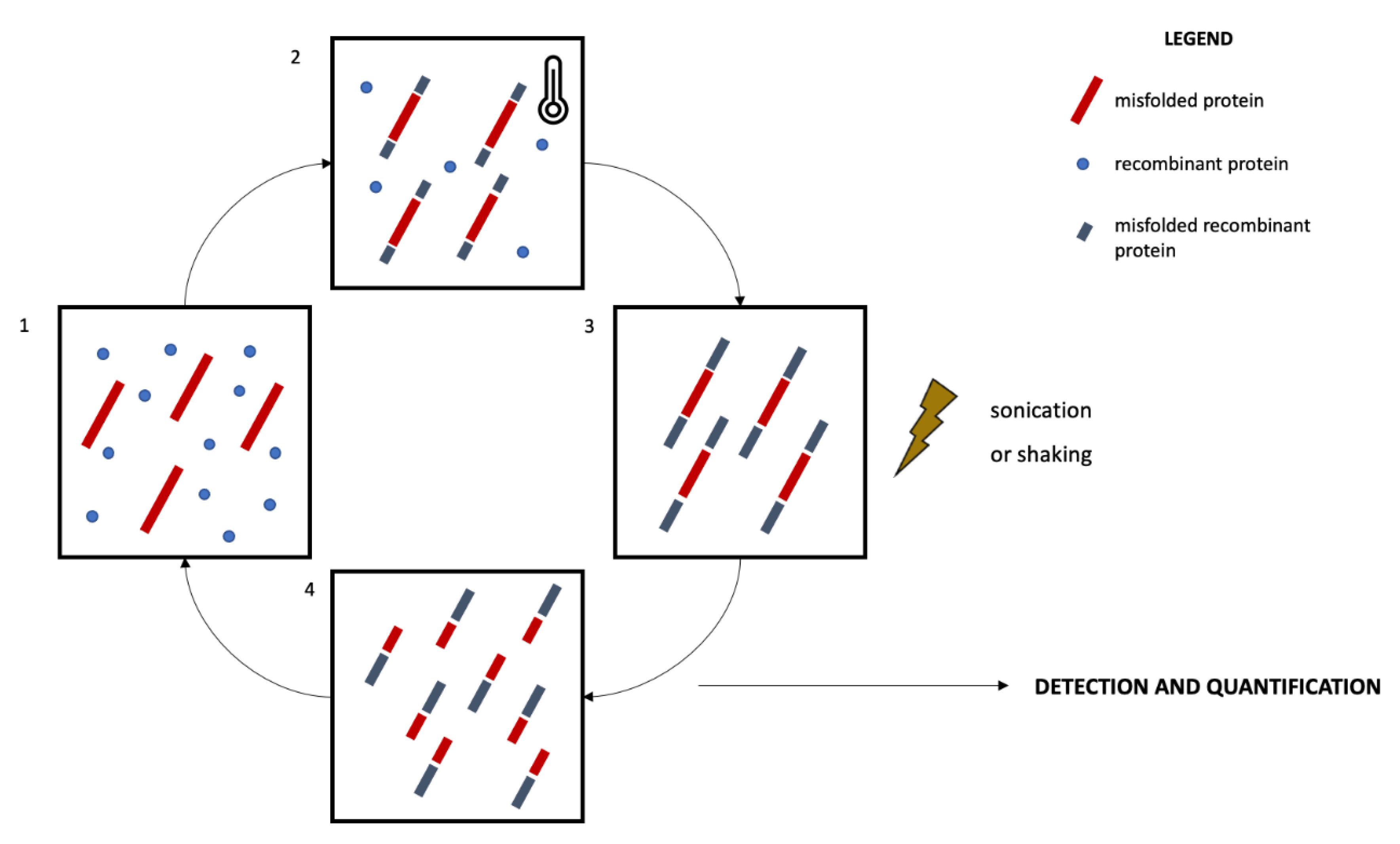
| Reference | Matrix | Protein | Study Population | Technique | Diagnostic Value | Prognostic Value |
|---|---|---|---|---|---|---|
| Pellkofer et al., 2019 [96] | Olfactory mucosa | BSC4090 (ligand for neurofibrillary tangles), t-tau, p-tau181 | n = 35 (AD n = 12, MCI n = 13, HC n = 10) | Immunohistochemistry | AD vs. HC: sensitivity 75%, specificity 80%, AUROC 0.778 MCI due to AD vs. MCI non-AD: sensitivity 100%, specificity 71.4%, AUROC 0.857 (AD + MCI due to AD) vs. (HC + MCI non-AD): sensitivity 77.8%, specificity 76.5%, AUROC 0.810 | Positive correlation between BSC4090 and p-tau181: p = 0.0466, r = 0.438846 |
| Perra et al., 2021 [98] | Olfactory mucosa | α-synuclein | n = 82 Probable DLB (n = 32), prodromal DLB (n = 5), mixed dementia (AD/LB n = 6), non-α-synucleinopathies (n = 38) | RT-QuIC | -DLB vs. non-α-synucleinopathies: sensitivity 81%, specificity 92% -good agreement between clinical diagnoses and RT-QuIC OM testing (k = 0.729) -moderate agreement between RT-QuIC assay in OM and CSF and clinical diagnoses (k = 0.498) | NA |
| Stefani et al., 2021 [99] | Olfactory mucosa | n = 163 iRBD (n = 63), PD (n = 41), HCs (n = 59) | RT-QuIC | iRBD + PD vs. HCS sensitivity 45%, specificity 90% | NA |
| Reference | Matrix | Protein | Disease | Study Population | Analytical Methods | Diagnostic Value | Prognostic Value |
|---|---|---|---|---|---|---|---|
| Wang Z. et al., 2020 [102] | Abdominal skin, scalp skin | α-syn | PD, DLB, MSA, AD, PSP, CBD | n = 130 Cohort 1: n = autopsy sample; PD n = 47, DLB n = 7, MSA n = 3, AD n = 17, PSP n = 8, CBD n = 5, HC n = 43 Cohort 2: n = 41 biopsy sample; PD n = 20, HC n = 21 | RT-QuIC, PMCA | RT-QuIC on abdominal skin (autopsy): 95% sensitivity and 100% specificity in detecting α-syn in PD vs. HCRT-QuIC on scalp skin (autopsy): 100% sensitivity and 100% specificity in detecting α-syn in PD vs. HC RT-QuIC on abdominal skin (autopsy): PD vs. NNCs AUC 0.9938 RT-QuIC on abdominal skin (autopsy): Synucleinopathies vs. non-synucleinopathies AUC 0.9696 PMCA on skin (autopsy): Synucleinopathies vs. non-synucleinopathies AUC 0.9444 RT-QuIC on skin (autopsy): PD vs. (PSP and CBD) AUC 0.975 PMCA on skin (autopsy): PD vs. (PSP and CBD) AUC 0.913 RT-QuIC on skin (living patients): 95% sensitivity and 100% specificity in detecting α-syn in PD vs. HC, AUC 0.9952 PMCA on skin (living patients): 80% sensitivity and 90% specificity in detecting α-syn in PD vs. HC, AUC 0.9250 | NA |
| Al-Qassabi et al., 2020 [100] | Skin biopsy from cervical area | α-syn | PD, RBD, PSP, vascular parkinsonism, MSA, CBD, drug-induced parkinsonism | Cohort 1 (autopsy): n = 51 (DLB n = 28, HC n = 23) Cohort 2 (living patients): n = 79 (RBD, n = 28, PD n = 20, PSP n = 4, vascular parkinsonism n = 2, MSA n = 1, CBD n = 2, drug-induced parkinsonism n = 1, HC n = 21) | Immunostaining | Skin positivity in 82.1% RBD, 70% PD, 20% atypical parkinsonism, 0% HCs. | At 3-year follow-up, 75% of RBD patients with positive biopsy phenoconverted to defined neurodegenerative disease |
| Doppler et al., 2017 [101] | Skin biopsy from cervical area, thigh, and leg | p-α-syn | PD, RBD | n = 63 (PD n = 25, RBD n = 18, HC n = 20) | Immunofluorescence | p-α-syn in dermal nerve fibres, PD vs. HC: p = 0.0001, sensitivity 80%, sensibility 100% p-α-syn in dermal nerve fibres, RBD vs. HC: p = 0.0001, sensitivity 55.6%, sensibility 100% | p-α-syn in dermal nerve fibres positively correlated with the total likelihood ratio for RBD to present prodromal PD (ρ = 0.531, p = 0.023) |
| Reference | Study Population | Analytical Method | Matrix | Diagnostic Value | Prognostic Value |
|---|---|---|---|---|---|
| Stefani A et al., 2021 [99] | iRBD n = 63 | RT-QuIC | Olfactory mucosa | iRBD + PD vs. controls: sensitivity 45.2%, specificity 89.8% | NA |
| Iranzo A et al., 2021 [127] | iRBD n = 52 | RT-QuIC | CSF | iRBD vs. controls: sensitivity 90.4%, specificity 90% | Risk of developing PD/DLB α-syn- iRBD vs. α-syn+ iRBD: HR = 0.143 |
| Rossi M et al., 2020 [121] | PAF n = 18 iRBD n = 18 | RT-QuIC | CSF | - In iRBD sensitivity 100%, specificity 100%; - In PAF: sensitivity 92.9% | NA |
| Garrido A et al., 2019 [128] | LRRK2-PD n = 15 LRRK2-NMC n = 16 | RT-QuIC | CSF | - In LRRK2-PD: sensitivity 40% - in LRRK2-NMC: sensitivity 18.8% | NA |
| Fairfoul G et al., 2016 [126] | RBD n = 3 | RT-QuIC | CSF | - In iRBD: 100% of patients had positive RT-QuIC | NA |
| Matrix | Diagnostic Value | Prognostic Value | Monitoring Treatment | |||
|---|---|---|---|---|---|---|
| Preclinical stage | Prodromal stage | Full-blown picture | ||||
| Neurodegeneration | ||||||
| Nfl | CSF, Blood | + | + | + | + | ± |
| AD pathology | ||||||
| Aβ peptides | CSF | + | + | + | ± | ± |
| p-tau | CSF, Blood | + | + | + | + | ± |
| Synucleinopathies | ||||||
| A-syn prionoids | CSF | + | + | + | ± | ± |
| Synaptic dysfunction | ||||||
| Ng | CSF | ± | + | + | + | ± |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Giampietri, L.; Belli, E.; Beatino, M.F.; Giannoni, S.; Palermo, G.; Campese, N.; Tognoni, G.; Siciliano, G.; Ceravolo, R.; De Luca, C.; et al. Fluid Biomarkers in Alzheimer’s Disease and Other Neurodegenerative Disorders: Toward Integrative Diagnostic Frameworks and Tailored Treatments. Diagnostics 2022, 12, 796. https://doi.org/10.3390/diagnostics12040796
Giampietri L, Belli E, Beatino MF, Giannoni S, Palermo G, Campese N, Tognoni G, Siciliano G, Ceravolo R, De Luca C, et al. Fluid Biomarkers in Alzheimer’s Disease and Other Neurodegenerative Disorders: Toward Integrative Diagnostic Frameworks and Tailored Treatments. Diagnostics. 2022; 12(4):796. https://doi.org/10.3390/diagnostics12040796
Chicago/Turabian StyleGiampietri, Linda, Elisabetta Belli, Maria Francesca Beatino, Sara Giannoni, Giovanni Palermo, Nicole Campese, Gloria Tognoni, Gabriele Siciliano, Roberto Ceravolo, Ciro De Luca, and et al. 2022. "Fluid Biomarkers in Alzheimer’s Disease and Other Neurodegenerative Disorders: Toward Integrative Diagnostic Frameworks and Tailored Treatments" Diagnostics 12, no. 4: 796. https://doi.org/10.3390/diagnostics12040796
APA StyleGiampietri, L., Belli, E., Beatino, M. F., Giannoni, S., Palermo, G., Campese, N., Tognoni, G., Siciliano, G., Ceravolo, R., De Luca, C., & Baldacci, F. (2022). Fluid Biomarkers in Alzheimer’s Disease and Other Neurodegenerative Disorders: Toward Integrative Diagnostic Frameworks and Tailored Treatments. Diagnostics, 12(4), 796. https://doi.org/10.3390/diagnostics12040796







