Semiautomated Segmentation and Volume Measurements of Cervical Carotid High-Signal Plaques Using 3D Turbo Spin-Echo T1-Weighted Black-Blood Vessel Wall Imaging: A Preliminary Study
Abstract
:1. Introduction
2. Materials and Methods
2.1. Patients
2.2. MRI Protocol
2.3. Segmentation of HSP by Software
2.4. Image Analyses
2.5. Statistical Analyses of Data
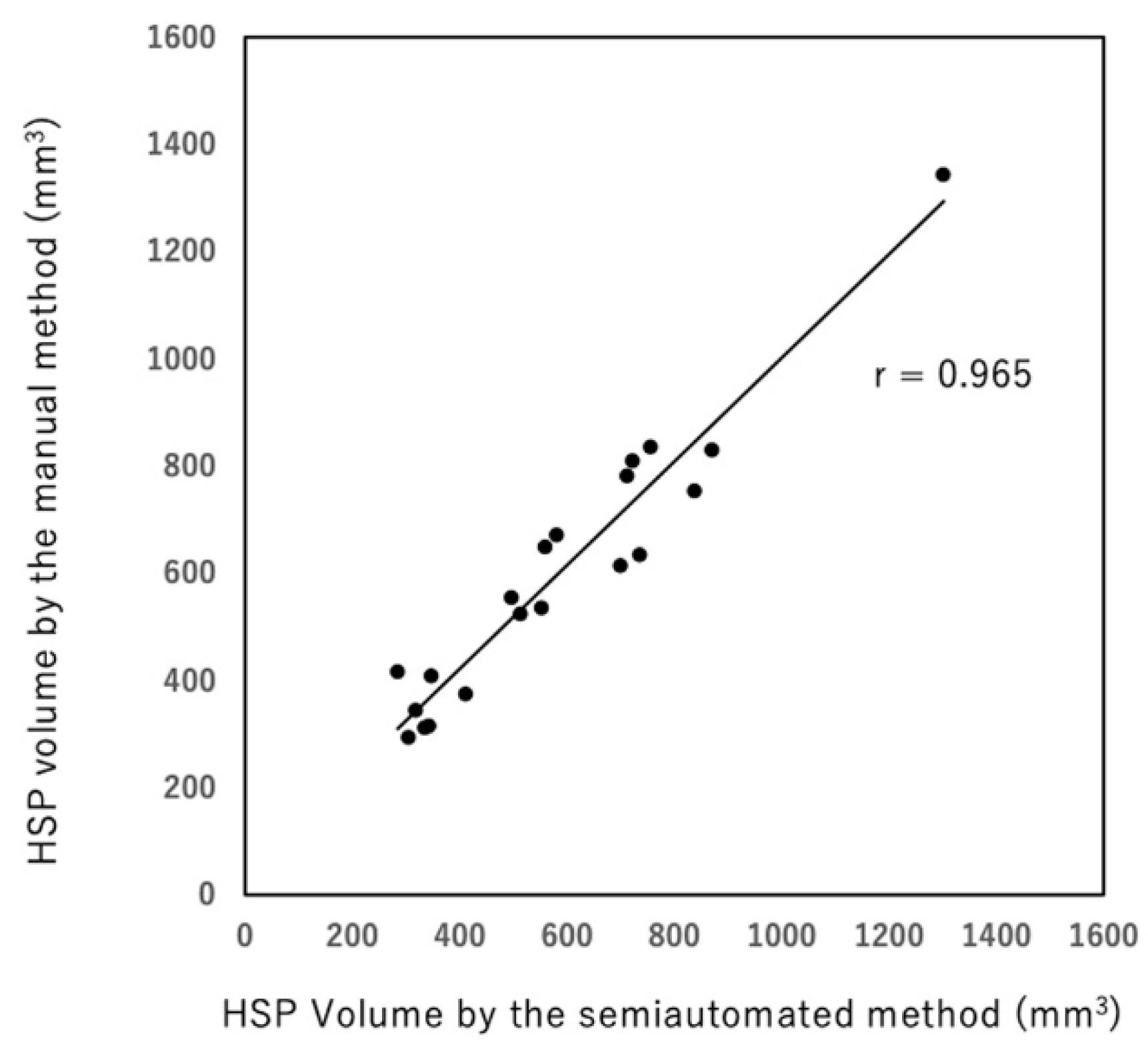
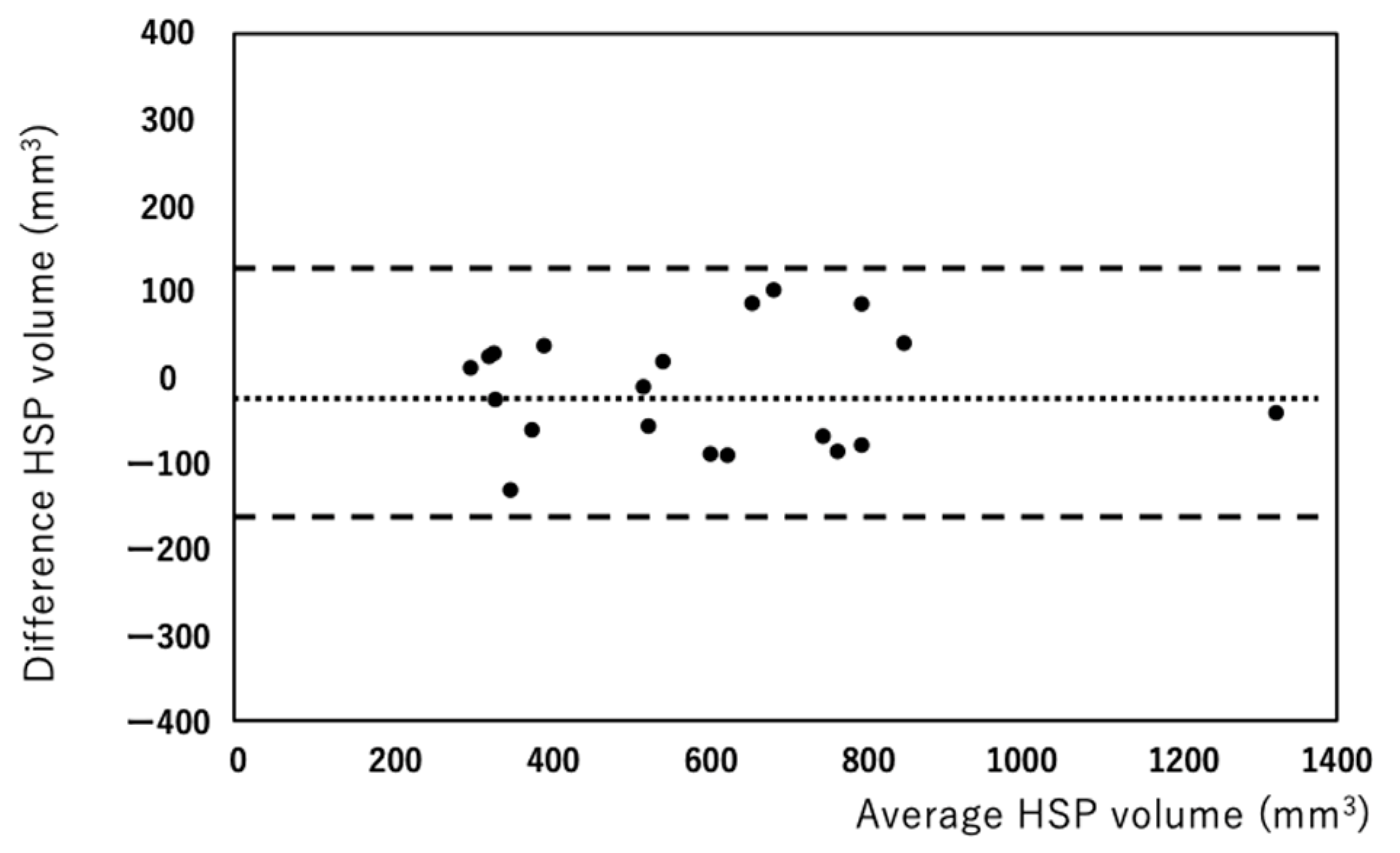
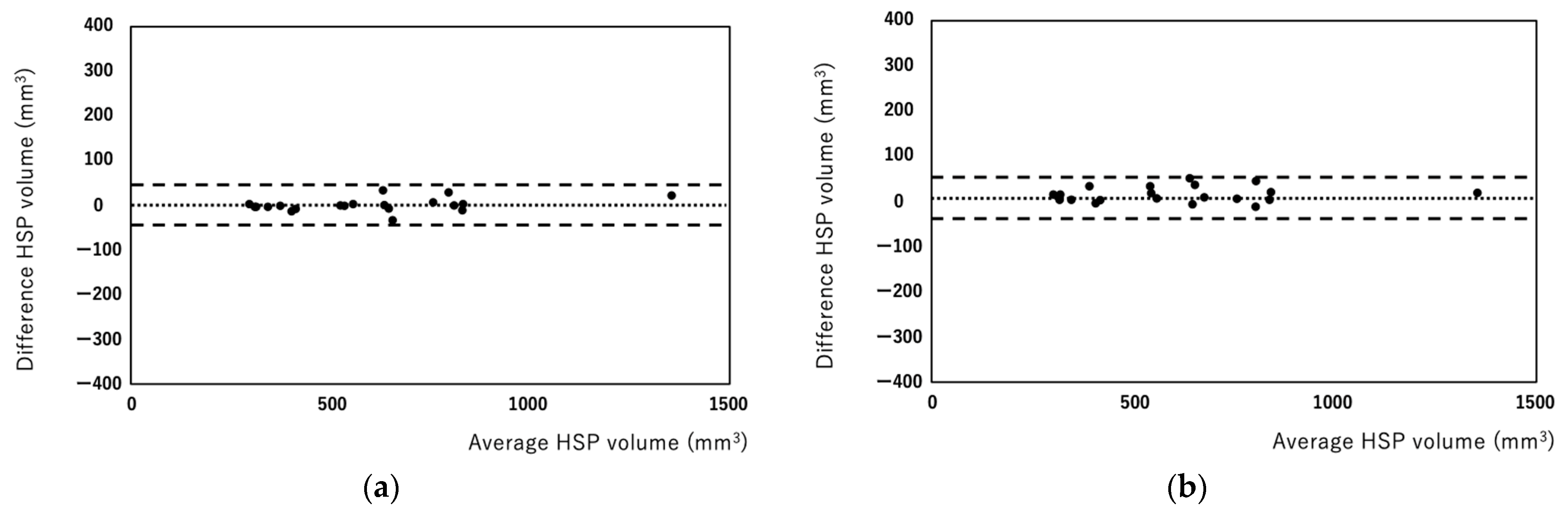
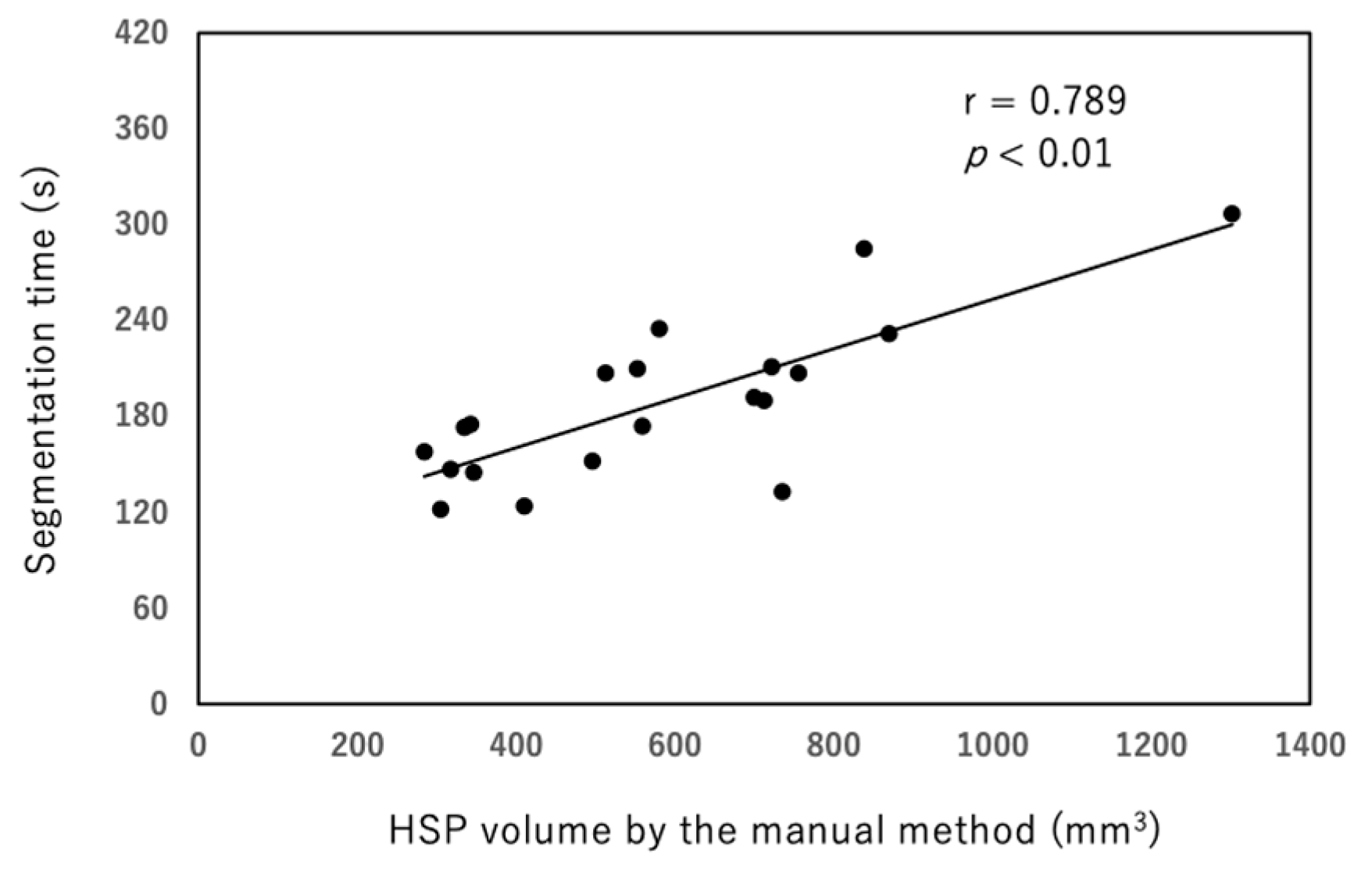
3. Results
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Altaf, N.; MacSweeney, S.T.; Gladman, J.; Auer, D.P. Carotid intraplaque hemorrhage predicts recurrent symptoms in patients with high-grade carotid stenosis. Stroke 2007, 38, 1633–1635. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Singh, N.; Moody, A.R.; Gladstone, D.J.; Leung, G.; Ravikumar, R.; Zhan, J.; Maggisano, R. Moderate carotid artery stenosis: MR imaging-depicted intraplaque hemorrhage predicts risk of cerebrovascular ischemic events in asymptomatic men. Radiology 2009, 252, 502–508. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ota, H.; Yarnykh, V.L.; Ferguson, M.S.; Underhill, H.R.; Demarco, J.K.; Zhu, D.C.; Oikawa, M.; Dong, L.; Zhao, X.; Collar, A.; et al. Carotid intraplaque hemorrhage imaging at 3.0-T MR imaging: Comparison of the diagnostic performance of three T1-weighted sequences. Radiology 2010, 254, 551–563. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigovan, M.; Bidet, C.; Bros, S.; Boussel, L.; Mechtouff, L.; Robson, P.M.; Fayad, Z.A.; Millon, A.; Douek, P. 3D black blood MR angiography of the carotid arteries. A simple sequence for plaque hemorrhage and stenosis evaluation. Magn. Reason. Imag. 2017, 42, 95–100. [Google Scholar] [CrossRef]
- Wu, Y.; Li, F.; Wang, Y.; Hu, T.; Xiao, L. Utility of minimum intensity projection images based on three-dimensional CUBE T1 weighted imaging for evaluating middle cerebral artery stenosis. Br. J. Radiol. 2021, 94, 20210145. [Google Scholar] [CrossRef]
- Scheffler, M.; Pellaton, A.; Boto, J.; Barnaure, I.; Delattre, B.M.; Remuinan, J.; Sztajzel, R.; Lövblad, K.O.; Vargas, M.I. Hemorrhagic plaques in mild carotid stenosis: The risk of stroke. Can. J. Neurol. Sci. 2021, 48, 218–225. [Google Scholar] [CrossRef]
- Inoue, K.; Maeda, M.; Umino, M.; Takase, S.; Yamahata, T.; Sakuma, H. Cervical carotid plaque evaluation using 3D T1-weighted black-blood magnetic resonance imaging: Comparison of turbo field-echo and turbo spin-echo sequences. Eur. J. Radiol. 2016, 85, 1035–1039. [Google Scholar] [CrossRef]
- Narumi, S.; Sasaki, M.; Natori, T.; Oura, M.Y.; Ogasawara, K.; Kobayashi, M.; Sato, Y.; Ogasawara, Y.; Hitomi, J.; Terayama, Y. Carotid plaque characterization using 3D T1-weighted MR imaging with histopathologic validation: A comparison with 2D technique. Am. J. Neuroradiol. 2015, 36, 751–756. [Google Scholar] [CrossRef] [Green Version]
- Tanemura, H.; Maeda, M.; Ichikawa, N.; Miura, Y.; Umeda, Y.; Hatazaki, S.; Toma, N.; Asakura, F.; Suzuki, H.; Sakaida, H.; et al. High-risk plaque for carotid artery stenting evaluated with 3-dimensional T1-weighted gradient echo sequence. Stroke 2013, 44, 105–110. [Google Scholar] [CrossRef]
- Tang, H.; Selwaness, M.; Hameeteman, R.; van Dijk, A.; van der Lugt, A.; Witteman, J.C.; Niessen, W.J.; van Vliet, L.J.; van Walsum, T. Semi-automatic MRI segmentation and volume quantification of intra-plaque hemorrhage. Int. J. Comp. Assist. Radiol. Surg. 2015, 10, 67–74. [Google Scholar] [CrossRef]
- Liu, J.; Sun, J.; Balu, N.; Ferguson, M.S.; Wang, J.; Kerwin, W.S.; Hippe, D.S.; Wang, A.; Hatsukami, T.S.; Yuan, C. Semiautomatic carotid intraplaque hemorrhage volume measurement using 3D carotid MRI. J. Magn. Reason. Imag. 2019, 50, 1055–1062. [Google Scholar] [CrossRef] [PubMed]
- Song, Y.J.; Kwak, H.S.; Chung, G.H.; Jo, S. Quantification of carotid intraplaque hemorrhage: Comparison between manual segmentation and semi-automatic segmentation on magnetization-prepared rapid acquisition with gradient-echo sequences. Diagnostics 2019, 9, 184. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Koo, T.K.; Li, M.Y. A guideline of selecting and reporting intraclass correlation Coefficients for reliability research. J. Chiropr. Med. 2016, 15, 155–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Zhao, X.Q.; Balu, N.; Hippe, D.S.; Hatsukami, T.S.; Isquith, D.A.; Yamada, K.; Neradilek, M.B.; Cantón, G.; Xue, Y.; et al. Carotid magnetic resonance imaging for monitoring atherosclerotic plaque progression: A multicenter reproducibility study. Int. J. Cardiovasc. Imag. 2015, 31, 95–103. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Liu, Y.; Wang, M.; Zhang, B.; Wang, W.; Xu, Y.; Han, Y.; Yuan, C.; Zhao, X. Size of carotid artery intraplaque hemorrhage and acute ischemic stroke: A cardiovascular magnetic resonance Chinese atherosclerosis risk evaluation study. J. Cardiovasc. Magn. Reson. 2019, 21, 36. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Liu, Y.; Han, Y.; Li, D.; Wang, W.; Li, R.; Yuan, C.; Zhao, X. Signal of carotid intraplaque hemorrhage on MR T1-weighted imaging: Association with acute cerebral infarct. Am. J. Neuroradiol. 2020, 41, 836–843. [Google Scholar] [CrossRef] [Green Version]
- Boussel, L.; Arora, S.; Rapp, J.; Rutt, B.; Huston, J.; Parker, D.; Yuan, C.; Bassiouny, H.; Saloner, D. MAPP Investigators. Atherosclerotic plaque progression in carotid arteries: Monitoring with high-spatial-resolution MR imaging. Multicenter trial. Radiology 2009, 252, 789–796. [Google Scholar] [CrossRef]
- Saba, L.; Saam, T.; Jäger, H.R.; Yuan, C.; Hatsukami, T.S.; Saloner, D.; Wasserman, B.A.; Bonati, L.H.; Wintermark, M. Imaging biomarkers of vulnerable carotid plaques for stroke risk prediction and their potential clinical implications. Lancet Neurol. 2019, 18, 559–572. [Google Scholar] [CrossRef]
- Ota, H.; Yu, W.; Underhill, H.R.; Oikawa, M.; Dong, L.; Zhao, X.; Polissar, N.L.; Neradilek, B.; Gao, T.; Zhang, Z.; et al. Hemorrhage and large lipid-rich necrotic cores are independently associated with thin or ruptured fibrous caps: An in vivo 3T MRI study. Atherioscler. Thromb. Vasc. Biol. 2009, 29, 1696–1701. [Google Scholar] [CrossRef] [Green Version]
- Takaya, N.; Yuan, C.; Chu, B.; Saam, T.; Polissar, N.L.; Jarvik, G.P.; Isaac, C.; McDonough, J.; Natiello, C.; Small, R.; et al. Presence of intraplaque hemorrhage stimulates progression of carotid atherosclerotic plaques: A high-resolution magnetic resonance imaging study. Circulation 2005, 111, 2768–2775. [Google Scholar] [CrossRef] [Green Version]
- Schindler, A.; Schinner, R.; Altaf, N.; Hosseini, A.A.; Simpson, R.J.; Esposito-Bauer, L.; Singh, N.; Kwee, R.M.; Kurosaki, Y.; Yamagata, S.; et al. Prediction of stroke risk by detection of hemorrhage in carotid plaques: Meta-analysis of individual patient data. JACC Cardiovasc. Imag. 2020, 13, 395–406. [Google Scholar] [CrossRef] [PubMed]
- Musialek, P.; Pieniazek, P.; Tracz, W.; Tekieli, L.; Przewlocki, T.; Kablak-Ziembicka, A.; Motyl, R.; Moczulski, Z.; Stepniewski, J.; Trystula, M.; et al. Safety of embolic protection device-assisted and unprotected intravascular ultrasound in evaluating carotid artery atherosclerotic lesions. Med. Sci. Monit. 2012, 18, MT7–MT18. [Google Scholar] [CrossRef] [PubMed]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Inoue, K.; Nakayama, R.; Isoshima, S.; Takase, S.; Yamahata, T.; Umino, M.; Maeda, M.; Sakuma, H. Semiautomated Segmentation and Volume Measurements of Cervical Carotid High-Signal Plaques Using 3D Turbo Spin-Echo T1-Weighted Black-Blood Vessel Wall Imaging: A Preliminary Study. Diagnostics 2022, 12, 1014. https://doi.org/10.3390/diagnostics12041014
Inoue K, Nakayama R, Isoshima S, Takase S, Yamahata T, Umino M, Maeda M, Sakuma H. Semiautomated Segmentation and Volume Measurements of Cervical Carotid High-Signal Plaques Using 3D Turbo Spin-Echo T1-Weighted Black-Blood Vessel Wall Imaging: A Preliminary Study. Diagnostics. 2022; 12(4):1014. https://doi.org/10.3390/diagnostics12041014
Chicago/Turabian StyleInoue, Katsuhiro, Ryohei Nakayama, Shiho Isoshima, Shinichi Takase, Tsunehiro Yamahata, Maki Umino, Masayuki Maeda, and Hajime Sakuma. 2022. "Semiautomated Segmentation and Volume Measurements of Cervical Carotid High-Signal Plaques Using 3D Turbo Spin-Echo T1-Weighted Black-Blood Vessel Wall Imaging: A Preliminary Study" Diagnostics 12, no. 4: 1014. https://doi.org/10.3390/diagnostics12041014
APA StyleInoue, K., Nakayama, R., Isoshima, S., Takase, S., Yamahata, T., Umino, M., Maeda, M., & Sakuma, H. (2022). Semiautomated Segmentation and Volume Measurements of Cervical Carotid High-Signal Plaques Using 3D Turbo Spin-Echo T1-Weighted Black-Blood Vessel Wall Imaging: A Preliminary Study. Diagnostics, 12(4), 1014. https://doi.org/10.3390/diagnostics12041014





