Abstract
Early diagnosis of increased intracranial pressure (ICP) is crucial for prompt diagnosis and treatment of intracranial hypertension in critically ill pediatric patients, preventing secondary brain damage and mortality. Although the placement of an external ventricular drain coupled to an external fluid-filled transducer remains the gold standard for continuous ICP monitoring, other non-invasive approaches are constantly being improved and can provide reliable estimates. The use of point-of-care ultrasound (POCUS) for the assessment of ICP has recently become widespread in pediatric emergency and critical care settings, representing a valuable extension of the physical examination. The aim of this manuscript is to review and discuss the basic principles of ultra-sound measurement of the optic nerve sheath diameter (ONSD) and summarize current evidence on its diagnostic value in pediatric patients with ICP. There is increasing evidence that POCUS measurement of the ONSD correlates with ICP, thus appearing as a useful extension of the physical examination in pediatrics, especially in emergency medicine and critical care settings for the initial non-invasive assessment of patients with suspected raised ICP. Its role could be of value even to assess the response to therapy and in the follow-up of patients with diagnosed intracranial hypertension if invasive ICP monitoring is not available. Further studies on more homogeneous and extensive study populations should be performed to establish ONSD reference ranges in the different pediatric ages and to define cut-off values in predicting elevated ICP compared to invasive ICP measurement.
1. Introduction
Timely detection and treatment of elevated intracranial pressure (ICP) are essential for preventing secondary brain damage and related morbidity and mortality [1,2,3,4,5]. Increased intracranial pressure can emerge as a result of both neurological and non-neurological diseases. Traumatic brain injury (TBI) and its complications, including increased intracranial pressure, are the leading causes of mortality and morbidity in children [6,7,8,9]. Other causes of increased ICP include intracranial infections, stroke, intracranial hemorrhage, hydrocephalus, ventricular shunt malfunction, brain tumors, arachnoid cysts, craniosynostosis syndromes, impaired central nervous system venous outflow, idiopathic intracranial hypertension, or hepatic encephalopathy [6,7,8,9]. Secondary brain injury occurs within minutes to hours of the primary injury due to a pathophysiologic cascade of events reducing perfusion, oxygen and metabolite delivery, and clearance of metabolic waste and toxins [10,11,12,13].
Monitoring ICP is crucial in managing critically ill neurological patients. The gold standard for accurate ICP monitoring is the invasive positioning of ventricular or intraparenchymal devices. Among these, the external ventricular drain (EVD) coupled to an external fluid-filled transducer remains the best choice both for its measurement accuracy and for allowing therapeutic CSF drainage at the same time [14,15,16,17,18,19,20,21] Several non-invasive ICP monitoring techniques have been attempted repeatedly [22,23,24,25]. The use of point-of-care ultrasound (POCUS) for diagnostic assessment has recently become widespread in pediatric emergency and critical care services, representing a valuable extension of the physical examination [26,27,28,29]. The intraorbital portion of the optic nerve, ontogenetically part of the central nervous system, extends from the ocular bulb to the optic canal and is surrounded by cerebrospinal fluid and optic nerve sheath (ONS), a membrane continuous with the dura mater of the brain. The perioptic subarachnoid space is a prolongation of the intracranial subarachnoid space, specifically, the chiasmal cistern; as the ONS is distensible, acute variations of cerebrospinal fluid pressure determine changes occurring within minutes in optic nerve sheath diameter (ONSD) [30,31,32,33,34,35,36].
Optic nerve sheath diameter ultrasound has been shown to correlate with increased ICP, thus appearing as a promising non-invasive and radiation-free bedside tool to assess elevated ICP [34,37,38,39,40,41]. Optic nerve sonography has been applied in a variety of pediatric diseases at risk for intracranial hypertension, including: traumatic and nontraumatic brain injury, intracranial hemorrhage, diabetic ketoacidosis, metabolic disorders (hepatic failure), ventriculoperitoneal shunts, hydrocephalus, suspected intracranial lesions, hypoxic injury, meningoceles, spina bifida and craniosynostosis [34,42,43,44,45,46,47,48,49].
The aim of this narrative manuscript is to review and discuss the basic principles of ultrasound measurement of the ONSD and summarize current evidence on its diagnostic value in pediatric patients with ICP.
2. Pathophysiology of Raised Intracranial Pressure (ICP)
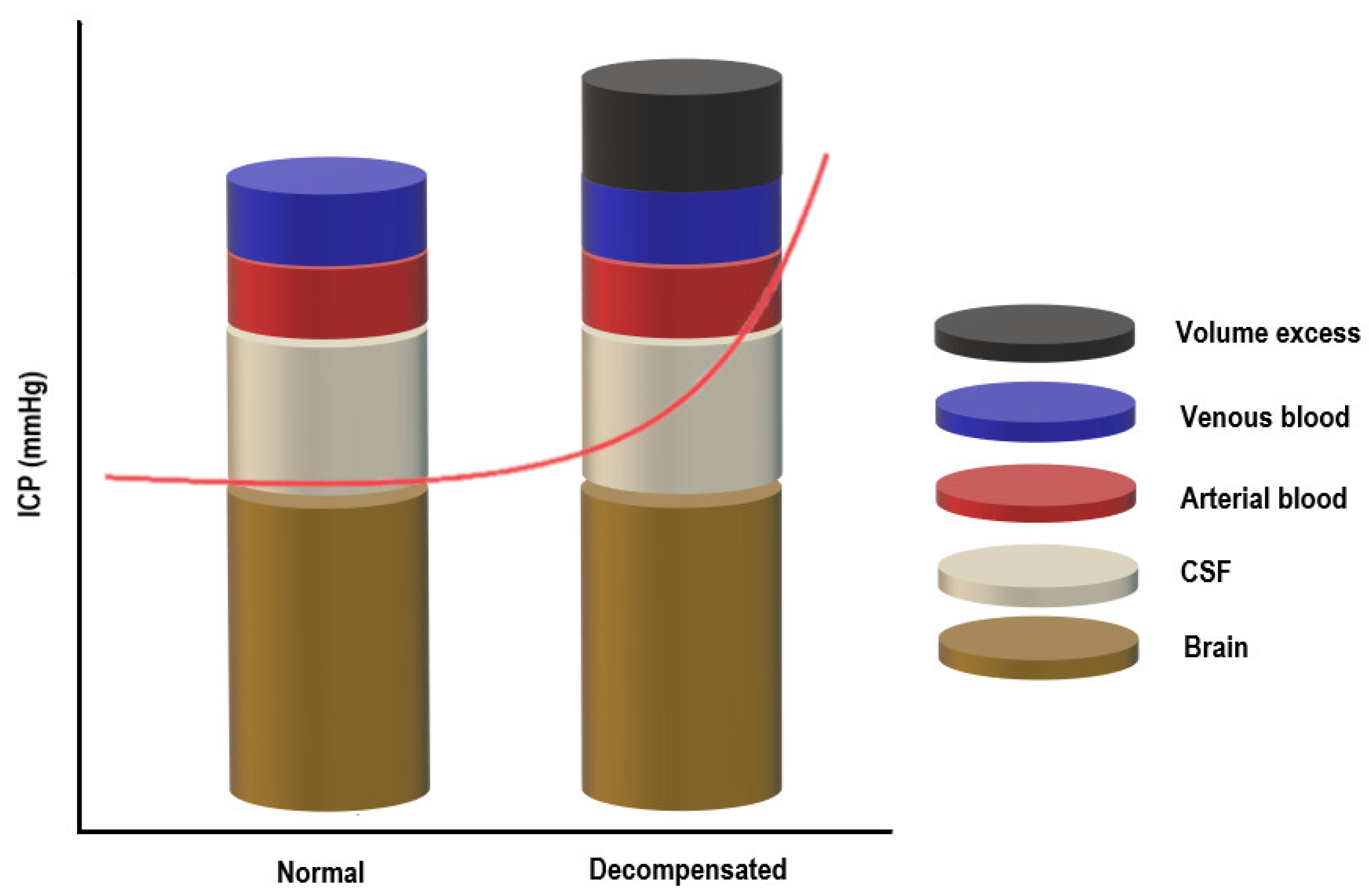
The skull can be imagined as a rigid box containing the following components: brain tissue, cerebrospinal fluid, and blood (arterial and venous). The Monro–Kellie model of ICP states that for ICP to remain constant, the sum of the volumes of the components mentioned above should remain constant [50,51]. Since brain tissue is assumed to be incompressible due to its high-water content, there must be a balance between the inflow and outflow of the intracranial fluids to keep ICP stable [52]. CSF secretion must be equal to the absorption rate, and at the same time, the arterial cerebral blood flow has to equal the effluent venous drainage (Figure 1) [50,51].
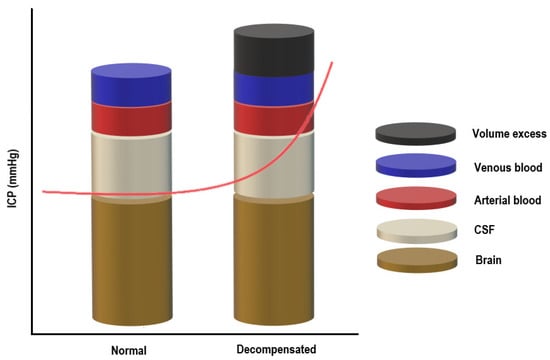
Figure 1.
The Monro–Kellie doctrine.
Under normal conditions, the intracranial volume is constant, and maintaining a steady ICP depends on the volume of the intracranial compartments (brain + CSF + blood): an increase in one component will cause a compensatory decrease in one or both of the others [53,54]. Raised ICP can result from any pathological condition increasing the volume of any of the three components or from the addition of a fourth component (e.g., intracranial hemorrhage, cerebral edema, or mass), overwhelming the compensatory mechanisms. Once the reserve is exhausted, the intracranial compliance will decrease, and slight elevations in the intracranial volume will lead to dramatic changes in ICP [53,54]. CO2, O2 and blood vessels size influence ICP in the critically ill patient [53,54].
Cerebral blood flow (CBF) is driven by cerebral perfusion pressure (CPP), which is defined as mean arterial pressure (MAP) minus intracranial pressure (CPP = MAP−ICP). Cerebrovascular autoregulation (CA) is tightly linked to CPP. It refers to the capacity of the cerebral circulation to alter the vascular arteriolar resistance to maintain a constant CBF as mean arterial pressure (MAP, and thus CPP) varies. In healthy adults, CA is normally operational across a wide range of MAPs, from 50 to 150 mm Hg. Beyond the limits of autoregulation, CBF becomes pressure passive. Few data are available in the pediatric population.
On the other end of the equation, ICP elevations can compromise the CPP leading to secondary ischemic brain injury. In the face of high ICP, brain ischemia can be partially counteracted by increasing the MAP through manipulation of the cardiac output and arterial pressure. Increased ICP can further compromise the brain parenchyma through herniation syndromes [55,56,57,58,59,60,61]. ICP fluctuates under physiologic conditions, including body posture (orthostatism vs. clinostatism), cardiorespiratory variations, electroencephalography (EEG) activity, and changes of the intrathoracic (ITP) and intra-abdominal pressure (IAP; if central venous pressure exceeds ICP) [62,63,64,65,66,67]. ICP is referenced at the level of the foramen of Monro. The normal suggested reference values for ICP vary with age (Table 1) [68,69,70,71,72].

Table 1.
Suggested age-related intracranial pressure (ICP) reference values.
It is currently accepted that physiologic mean ICP boundaries in healthy adult subjects resting in the horizontal position are 7–15 and −10 mm Hg but not exceeding −15 mm Hg in the vertical position. Normal mean ICP values are reported to be within the range of 3–7 mm Hg in young children and 1.5–6 mm Hg in term infants [68,69,70,71,72].
The definition of intracranial hypertension depends on each specific clinical condition. Acute intracranial hypertension (AIH) in adults has been classically defined as sustained ICP above 20 mm Hg for greater than five minutes. An ICP treatment threshold of 20 mm Hg is used in children since there is sufficient evidence in pediatric literature suggesting an association between ICP values greater than 20 mm Hg and poor outcome [6,15,68,73,74,75,76,77,78,79].
3. Optic Nerve and Its Measurement
3.1. Anatomy and Physiology of Optic Nerve
The intraorbital portion of the optic nerve, ontogenetically part of the central nervous system, extends from the ocular bulb to the optic canal and is surrounded by cerebrospinal fluid and optic nerve sheath (ONS), a membrane made up of leptomeninges in continuity with the dura mater of the brain.
The optic nerve is approximately 40 mm long and 4 mm wide, including the sheath, with an average diameter of 0.4 mm. The subarachnoid space features a structure of arachnoidal trabeculae, septa, and stout pillars. Under normal conditions, it holds approximately 0.1–0.2 mL of cerebrospinal fluid [30,80,81,82,83,84,85].
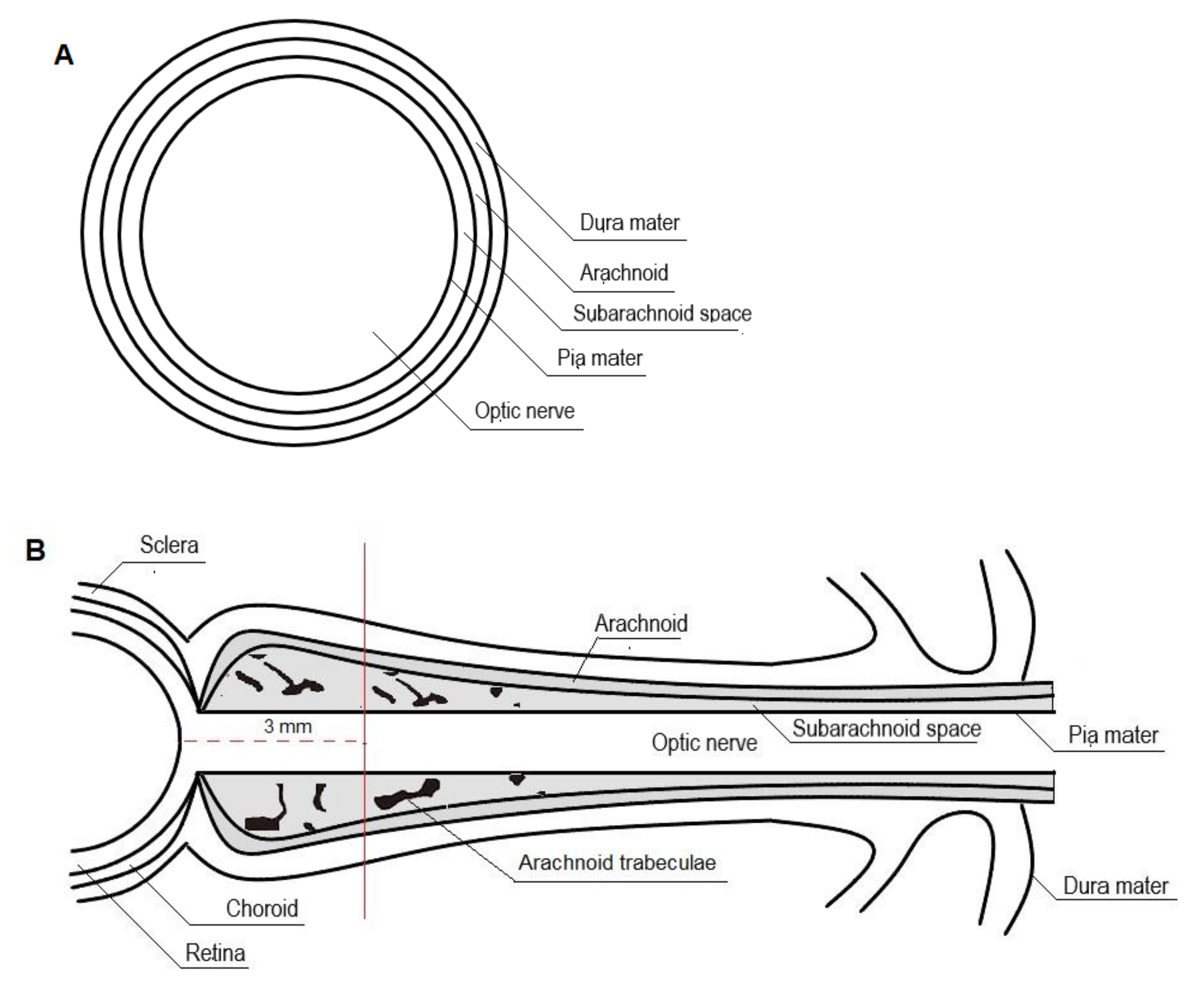
The perioptic subarachnoid space is a prolongation of the intracranial subarachnoid space, specifically, the chiasmal cistern [83,84,86,87]. It has been hypothesized that the perioptic CSF slowly percolates toward the bulbar portion of the nerve and that reversal flow occurs with eye movements squeezing the retrobulbar ONS (Figure 2) [30,84,86].
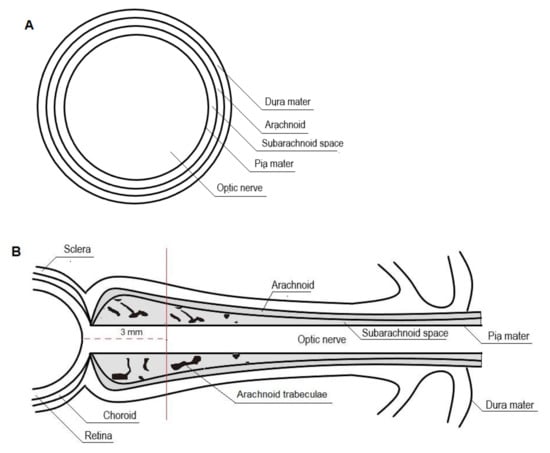
Figure 2.
Cross-section (A) and longitudinal section (B) of the optic nerve.
As the ONS is distensible, optic nerve sheath diameter (ONSD) changes rapidly with changing cerebrospinal fluid pressure. The ONSD is constant as long as the ICP remains within normal ranges. When ICP rises, maximum ONSD fluctuations occur in the anterior subarachnoid compartment, 3 mm behind the globe, rather than in the posterior perineural one. It has been suggested that this non-uniform enlargement may be the result of the asymmetrical distribution of the arachnoidal trabeculae, with lower density in the retrobulbar ONS. Moreover, the anterior compartment of the ONS is the thinnest of the entire segment and, therefore, the most distensible [30,31,33,88,89,90,91,92].
3.2. Ultrasonographic Technique for Optic Nerve Sheath Diameter (ONSD) Measurement
Optic nerve sonography is performed with a high-frequency linear transducer (>7.5 MHz), the patient lying supine, with the head in a neutral position and both eyes closed.
The probe is gently placed in an axial plane on the temporal side of the closed upper eyelid using a thick layer of sterile coupling ultrasound gel (lateral transbulbar approach). B-mode is selected. A transverse sonographic section allows for visualization of the globe and the structures of the retrobulbar area, including the optic nerve in its longitudinal course [32,39,47,89,93,94,95,96,97,98,99,100].
On images, the optic nerve complex is shown as a homogenous hypoechoic band extending posteriorly from the bulb′s base in the context of the echogenic retrobulbar fat. More specifically, the OND appears as a hypoechogenic structure surrounded by the more hyperechogenic ONSD, but still hypoechogenic compared to the retrobulbar fat. Color Doppler may be used to facilitate optic nerve identification through visualization of the central retinal artery and vein running inside [94,101].
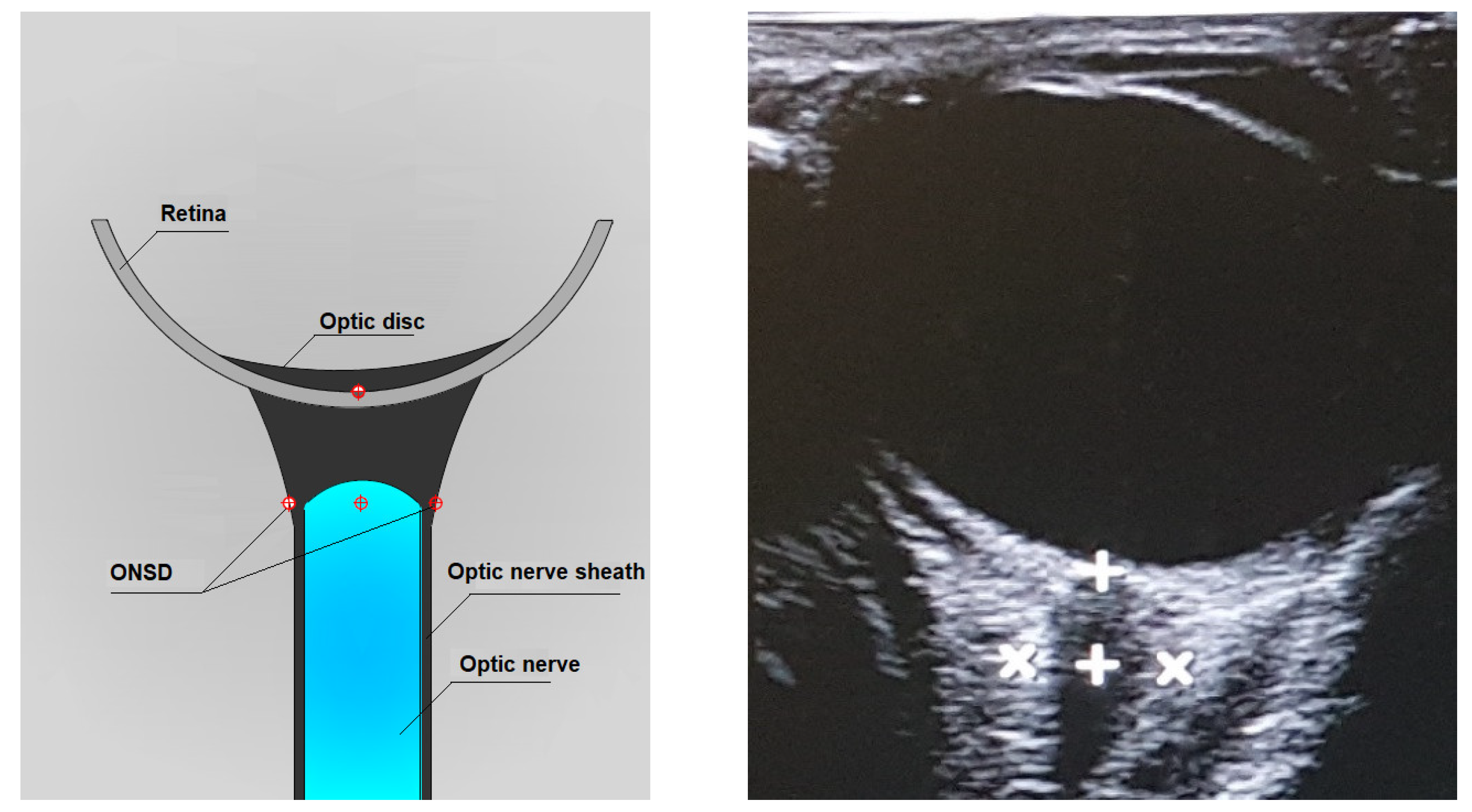
By convention, the ONSD measurement is performed 3 mm posterior to the papilla base by manual cursor placement on the outer contours of the optic nerve sheath. The zoom feature can be helpful for the correct display of the cursors (Figure 3) [30,32,33].
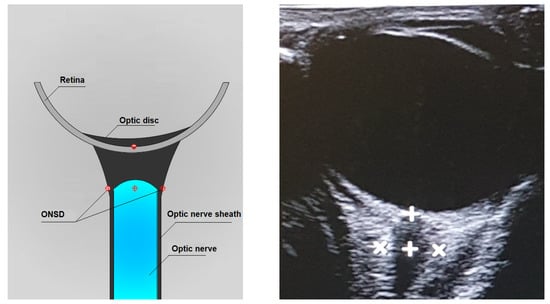
Figure 3.
Axial lateral transbulbar approach ONSD measurement.
4. Optic Nerve Sheath Diameter (ONSD) Measurements in Children
Knowledge of normal pediatric ONSD reference ranges is essential for detecting raised ICP in clinical practice.
Optic nerve sheath diameter reference ranges in children and neonates were first established in 1999 by Ballantyne et al. A total of 102 children aged from 0 to 15 years were included in the study; none suffered from neurological or ophthalmological disease. Optic nerve sheath diameter data were grouped by age: the range of normal values in children under 1 year was 2.1–4.0 mm, and the range for children over 1 year of age was 2–3 mm. The cut-off value for abnormal enlargement was above 4 mm in infants under 1 year of age and 4.5 mm in older children [102].
Rehman Siddiqui et al., identified an ONSD ultrasonographic threshold predictive of elevated ICP in various age groups [103]. Forty-eight children aged from one month to 16 years with the following inclusion criteria were enrolled in the study: traumatic brain injury defined as moderate (Glasgow coma scale 9–13) or severe (Glasgow coma scale <9), clinical signs and symptoms suggestive of raised ICP, progressive neurological deterioration and active malignancy history with new onset of neurological symptoms. Patients diagnosed with orbital trauma with orbital fractures, orbital tumors, or intraocular space-occupying lesions were excluded from the study. Cut-off ultrasonographic value for abnormal ONSD enlargement predictive of raised elevated ICP was above 4 mm in infants, 4.71 mm in children aged 1–10 years, and 5.43 mm in older children [103].
A total of 13 patients aged between 12 and 18 years as candidates for an elective lumbar puncture with the suspicion of idiopathic intracranial hypertension (IIH) were enrolled in the prospective study conducted by Irazuzta et al. [104]. Patients underwent ONSD ultrasound examination while awake immediately before sedation. A complete concordance was observed between the cut-off value for raised ICP (cerebrospinal fluid opening pressure above 20 cmH2O) and ONSD measured by ultrasonography (p < 0.01). An ONSD of >4.5 mm correlated with an increased ICP (sensitivity 100%, p < 0.01). Patients without elevated ICP had an ONSD <4.5 mm (specificity 100%) [104].
Aslan et al., evaluated the correlation between lumbar puncture opening pressure and ultrasonographic ONSD measures in seven patients diagnosed with pseudotumor cerebri syndrome (PTCS) [105]. This condition is characterized by raised ICP with no neuroradiological abnormalities. The control group included a total of 15 healthy children.
In the PTCS group, ultrasonographic ONSD) values of both eyes were statistically significantly higher than in the control group (p < 0.001). They also showed a significant correlation between the lumbar puncture opening pressure and ONSD baseline measures for both the right and the left eye (r = 0.882, p = 0.009 and r = 0.649, p = 0.004, respectively) [105].
Padayachy et al., analyzed the diagnostic accuracy of ONSD cut-off values compared to invasive ICP measurement at thresholds of 20, 15, 10, and 5 mm Hg in different age groups and taking into account the patency of the anterior fontanelle [106]. Data from 174 patients <14 years of age under general anesthesia were analyzed.
In children ≤1 year old, the ONSD measurement with the best diagnostic accuracy for detecting ICP ≥20 mm Hg was 5.16 mm (SE 80% (44.4–97.5), SP 76.1% (61.2–87.4), PPV 42.1% (20.3–66.5), NPV 94.6% (81.8–99.3), 95% CI). In children >1 year old, the ONSD measurement with the best diagnostic accuracy for detecting ICP ≥20 mm Hg was 5.75 mm (SE 85.9% (75–93.4), SP 70.4% (56.4–82), PPV 77.5% (66–86.5), NPV 80.9% (66.7–90.9), 95% CI) [106].
Likewise, Kerscher et al., compared ONSD cut-off values with invasively measured ICP values [107]. A total of 72 patients were enrolled in the study; 40% were investigated under general anesthesia, 39% were awake, 14% were sedated for lumbar or shunt reservoir puncture, and 7% were somnolent or comatose in the intensive care unit. Diagnostic accuracy of ONSD cut-off values have been compared to ICP measurement at thresholds of 5, 10, 15, 20, 25, and 30 mm Hg in different age groups and considering the patency of the anterior fontanelle. In children ≤1 year old, the ONSD measurement for detecting ICP ≥20 mm Hg was 4.99 mm (SE 50%, SP 58.8%, PPV 22.2%, NPV 83.3%); in children >1 year old was 5.75 mm (SE 91.7%, SP 66.7%, PPV 45.8%, NPV 96.3%). The authors also showed a significant correlation between ONSD values and intracranial pressure for children >1 year (r = 0.63, p < 0.01). The correlation was poor for patients ≤1 year (r = 0.21; open anterior fontanelle: r = 0.057, closed anterior fontanelle: r = 0.4) [107].
Robba et al., compared different non-invasive ultrasound-based methods of ICP evaluation with simultaneous direct readings from invasive ICP monitoring devices (either intraparenchymal or intraventricular catheters) [108]. A total of 10 children aged <16 years with an indication for invasive ICP monitoring were enrolled in the study. Among the non-invasive methods studied, ONSD ultrasound presented the best accuracy to assess ICP: ONSD measurements correlated with invasive ICP values (r = 0.852, p < 0.0001). The ONSD measurement with the best diagnostic accuracy for detecting ICP ≥ 20 mm Hg was 4.75 mm (SE 0.956, SP 0.938, AUC 0.976—95% CI = 0.948–1.00); considering a threshold of 15 mm Hg, the ONSD measurement with the best diagnostic accuracy was 3.85 mm (SE 0.811, SP 0.939, AUC 0.94—95% CI = 0.892–0.989) [108].
Fontanel et al., investigated normal ultrasonographic ONSD values in children aged 0 to 18 years and created an optic nerve growth curve [109]. The authors also defined the accuracy of ONSD cut-off values according to age group for intracranial hypertension (IHT) diagnosis. Two hundred fifteen children underwent ONSD ultrasound examination. The enrolled patients were divided into three groups: 165 healthy children, 29 children diagnosed with IHT (all >4 years of age), and 21 children with optic disc drusen. Ultrasound examination was performed on awake patients. Exclusion criteria were optic nerve disorders potentially influencing the ONSD measurement (congenital coloboma, microphthalmos, inflammation of the optic nerve such as papillitis, and diseases associated with abnormal intraocular pressure such as glaucoma). The authors detected a statistically significant difference between ultrasonographic ONSD values of both eyes between healthy subjects and IHT subjects and between IHT subjects and subjects with optic disc drusen (p < 0.001). Optic nerve growth curve for healthy subjects showed a progressive increase in ONSD values up to approximately 10 years of age, and then ONSD values remained constant until the age of 18, with an upper limit of 4.5 mm. In children >4 years old and for the subgroup 4–10 years, the ONSD cut-off value was 4.1 mm (SE 100%, SP 83.9 and SE 100%, SP 89.3% respectively), and 4.4 mm for the subgroup 11–18 years of age (SE 100%, SP 98.8%) [109].
Table 2 summarizes ONSD reference ranges and cut-off values for predicting elevated ICP for the pediatric population reported by different studies [102,103,104,105,106,107,108,109,110,111,112].

Table 2.
ONSD values in the pediatric population: study characteristics.
5. ONSD Measurements in Neonates
Published data about ONSD ultrasound measurements in the neonatal population are limited and mainly based on studies with a small sample size.
The first study defining reference ranges in neonates and children was carried out in 1999 by Ballantyne et al. [102]: 102 children aged 0 to 15 years admitted for abdominal or hip ultrasound evaluation underwent ONSD ultrasound examination. None suffered from neurological or ophthalmological diseases.
Optic nerve sheath diameter ultrasound data were grouped by age: the range of normal values for ONSD in children under 1 year was 2.1–4.0 mm, and the range for children over 1 year of age was 2.4–4.3 mm. The cut-off value for abnormal enlargement was above 4 mm in infants under 1 year of age and 4.5 mm in older children [102].
Gravendeel et al., established reference values for ONSD ultrasound measurements in 120 (boys 65, girls 55) healthy full-term neonates (gestational age between 37–42 weeks), with a birth weight more than 2500 g and uncomplicated postnatal course [113]. Ultrasound examinations were performed within 1–4 days of delivery; follow-up ultrasound re-examination was carried out at 4 months and 8 months of age. The mean ONSD value with 95% reference intervals reported was 3.9 mm (3.1–4.7) in healthy term boys aged 1 to 2 days and 3.7 mm (2.7–4.7) in healthy term girls. ONSD measurements and reference intervals markedly increased between birth and 4 months [113].
Ardell et al., carried out a pilot study to document ranges of ONSD ultrasound measurements in preterm infants [114]. Twelve patients had weekly serial scans between 29 and 36 weeks of corrected gestational age; a total of 114 scans were performed on both eyes. No patients with suspected or confirmed raised ICP or intraocular pressure were included in the study. They showed a significant correlation between ONSD measurements and corrected gestational age. On the contrary, weight and head circumference did not correlate strongly with ONSD [114].
Yapicioglu et al., have recently published the largest database of ONSD normal values in preterm and term neonates [115]. Overall, 554 newborns without intracranial pathology were enrolled in the study. Detailed reference intervals are given for any different gestational ages.
Optic nerve sheath diameter measurements at 3 mm from the papilla were impossible in some of the preterm babies, since cursors fell beyond the longitudinal ultrasonographic section of the optic nerve; measurements at 2 and 2.5 mm were possible in some cases. Moreover, the authors showed a significant and positive correlation between ONSD measurements and gestational age and somatic parameters (weight, height, and head circumference) [115].
The optic nerve sheath diameter (ONSD) values for neonates reported by different studies are summarized in Table 3 [102,113,114,115].

Table 3.
ONSD values in the neonatal population: study characteristics (SD, standard deviation).
6. Discussion and Future Directions
Increased ICP is a medical and often neurosurgical emergency. Real-time detection and dynamic evaluation of ICP in critically ill pediatric patients are crucial to warrant prompt diagnosis and management, thus preventing neurological morbidity and mortality [1,2,3,4,5].
Although the gold standard for ICP monitoring is invasive, with ventricular or intraparenchymal probes, the sonographic measurement of ONSD can provide valuable ICP estimates for the initial non-invasive assessment of patients with suspected raised ICP. Its role could be of value even to assess the response to raised ICP treatment and in the follow-up during the patient′s ICU stay and post-ICU care if more appropriate tools (invasive ICP monitoring) are not available or indicated [14,15,16,17,18,19,20,21,22,23,24,25].
In recent years, the use of point-of-care ultrasound (POCUS) for diagnostic assessment has become widespread in pediatric emergency and critical care medicine [26,27,28,29]. In comparison with conventional neuroimaging, such as magnetic resonance imaging (MRI) and computed tomography (CT), the advantages of POCUS of ONSD are low costs, short investigation times, repeatability, bedside availability, and radiation free. Contraindications to ONSD ultrasound examination are ocular trauma, diseases associated with abnormal intraocular pressure (e.g., glaucoma), optic nerve atrophy, and inflammatory lesions of the optic nerve affecting the ONSD [116].
However, in the current practice in pediatric neurocritical care, ONSD ultrasound appears to be underutilized because of several limitations affecting diagnostic accuracy in detecting raised ICP. Multiple studies reported significant variability in ONSD reference ranges and cut-off values predictive of elevated ICP in the different pediatric age groups. Most available data come from heterogeneous studies in terms of sample size, inclusion criteria (age, sex, somatic parameters, causes of increased ICP and comorbidities), reference standards (e.g., invasive ICP monitoring, CT, MRI, CSF OP at LP) and methodological approaches. Moreover, the ONSD ultrasonographic measurement technique should be standardized in the matter of probe lateral resolution and image acquisition [117,118,119]. By convention, the ONSD measurement is performed 3 mm posterior to the papilla base in adults. In pediatric patients, the maximal distensible part of the optic nerve sheath may be much more proximal than in adults, and further studies are needed. Furthermore, in some preterm ONSD, measurements 3 mm behind the papilla base are not always possible, as cursors could go beyond the longitudinal ultrasonographic section of the optic nerve [30,32,33,115,118].
Additional future research directions also include assessment of the learning curve and interobserver and intraobserver variability for POCUS of ONSD [120,121].
7. Conclusions
The optic nerve sheath diameter (ONSD) appears as a surrogate marker for the detection of raised ICP. POCUS of ONSD could represent a useful extension of the physical examination in pediatrics, especially in emergency medicine and critical care settings, for the initial non-invasive assessment of patients with suspected raised ICP. Repeat ONSD ultrasound measurements may be of value in an ICU setting to assess the response to raised ICP treatment and in the follow-up if invasive ICP monitoring is not available or indicated. Further studies on more homogeneous and extensive study populations should be performed to establish ONSD reference ranges in the different pediatric ages. The diagnostic accuracy of ONSD ultrasonographic cut-off values in predicting elevated ICP compared to invasive ICP measurement at different thresholds needs further investigation.
Author Contributions
G.C. designed the project, performed the literature review, and wrote the first draft of the manuscript. S.P. and A.M. gave substantial scientific contribution. S.E. supervised the project and revised the first draft of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
This review received no external funding. Costs for publication of the manuscript are covered by the Pediatric Clinic, Department of Medicine and Surgery, University of Parma, Parma, Italy.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Marmarou, A.; Anderson, R.L.; Ward, J.D.; Choi, S.C.; Young, H.F.; Eisenberg, H.M.; Foulkes, M.A.; Marshall, L.F.; Jane, J.A. Impact of ICP instability and hypotension on outcome in patients with severe head trauma. J. Neurosurg. 1991, 75, S59–S66. [Google Scholar] [CrossRef]
- Chesnut, R.M.; Marshall, L.F.; Klauber, M.R.; Blunt, B.A.; Baldwin, N.; Eisenberg, H.M.; Jane, J.A.; Marmarou, A.; Foulkes, M.A. The role of secondary brain injury in determining outcome from severe head injury. J. Trauma 1993, 34, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Juul, N.; Morris, G.F.; Marshall, S.B.; Marshall, L.F. Intracranial hypertension and cerebral perfusion pressure: Influence on neurological deterioration and outcome in severe head injury. J. Neurosurg. 2000, 92, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Balestreri, M.; Czosnyka, M.; Hutchinson, P.; Steiner, L.A.; Hiler, M.; Smielewski, P.; Pickard, J.D. Impact of Intracranial Pressure and Cerebral Perfusion Pressure on Severe Disability and Mortality After Head Injury. Neurocritical Care 2006, 4, 008–013. [Google Scholar] [CrossRef]
- Myburgh, J.A.; Cooper, D.J.; Finfer, S.R.; Venkatesh, B.; Jones, D.; Higgins, A.; Bishop, N.; Higlett, T. Epidemiology and 12-Month Outcomes from Traumatic Brain Injury in Australia and New Zealand. J. Trauma Acute Care Surg. 2008, 64, 854–862. [Google Scholar] [CrossRef]
- Kochanek, P.M.; Tasker, R.C.; Carney, N.; Totten, A.M.; Adelson, P.D.; Selden, N.R.; Davis-O’Reilly, C.; Hart, E.L.; Bell, M.J.; Bratton, S.L.; et al. Guidelines for the Management of Pediatric Severe Traumatic Brain Injury, Third Edition: Update of the Brain Trauma Foundation Guidelines, Executive Summary. Neurosurgery 2019, 84, 1169–1178. [Google Scholar] [CrossRef] [Green Version]
- Langlois, J.A.; Rutland-Brown, W.; Wald, M.M. The Epidemiology and Impact of Traumatic Brain Injury: A brief overview. J. Head Trauma Rehabil. 2006, 21, 375–378. [Google Scholar] [CrossRef] [Green Version]
- Bruce, D.A.; Alavi, A.; Bilaniuk, L.; Dolinskas, C.; Obrist, W.; Uzzell, B. Diffuse cerebral swelling following head injuries in children: The syndrome of “malignant brain edema”. J. Neurosurg. 1981, 54, 170–178. [Google Scholar] [CrossRef]
- Muizelaar, J.P.; Marmarou, A.; DeSalles, A.A.F.; Ward, J.D.; Zimmerman, R.S.; Li, Z.; Choi, S.C.; Young, H.F. Cerebral blood flow and metabolism in severely head-injured children. J. Neurosurg. 1989, 71, 63–71. [Google Scholar] [CrossRef]
- Hakim, S.; Venegas, J.G.; Burton, J.D. The physics of the cranial cavity, hydrocephalus and normal pressure hydrocephalus: Me-chanical interpretation and mathematical model. Surg. Neurol. 1976, 5, 187–210. [Google Scholar]
- Shapiro, H.M. Intracranial hypertension: Therapeutic and anesthetic considerations. J. Am. Soc. Anesthesiol. 1975, 43, 445–471. [Google Scholar] [CrossRef]
- Lidofsky, S.D.; Bass, N.M.; Prager, M.C.; Washington, D.E.; Read, A.E.; Wright, T.L.; Ascher, N.L.; Roberts, J.P.; Scharschmidt, B.F.; Lake, J. Intracranial pressure monitoring and liver transplantation for fulminant hepatic failure. Hepatology 1992, 16, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Bingaman, W.E.; Frank, J.I. Malignant Cerebral Edema and Intracranial Hypertension. Neurol. Clin. 1995, 13, 479–509. [Google Scholar] [CrossRef]
- Harary, M.; Dolmans, R.G.F.; Gormley, W.B. Intracranial Pressure Monitoring—Review and Avenues for Development. Sensors 2018, 18, 465. [Google Scholar] [CrossRef] [Green Version]
- Czosnyka, M. Monitoring and interpretation of intracranial pressure. J. Neurol. Neurosurg. Psychiatry 2004, 75, 813–821. [Google Scholar] [CrossRef]
- Padayachy, L.C.; Figaji, A.A.; Bullock, M.R. Intracranial pressure monitoring for traumatic brain injury in the modern era. Child’s Nerv. Syst. 2010, 26, 441–452. [Google Scholar] [CrossRef]
- Talving, P.; Karamanos, E.; Teixeira, P.G.; Skiada, D.; Lam, L.; Belzberg, H.; Inaba, K.; Demetriades, D. Intracranial pressure monitoring in severe head injury: Compliance with Brain Trauma Foundation guidelines and effect on outcomes: A prospective study. J. Neurosurg. 2013, 119, 1248–1254. [Google Scholar] [CrossRef] [Green Version]
- Bauer, D.F.; Razdan, S.N.; Bartolucci, A.A.; Markert, J.M. Meta-Analysis of Hemorrhagic Complications From Ventriculostomy Placement by Neurosurgeons. Neurosurgery 2011, 69, 255–260. [Google Scholar] [CrossRef] [Green Version]
- Binz, D.D.; Toussaint, L.G.; Friedman, J.A. Hemorrhagic Complications of Ventriculostomy Placement: A Meta-Analysis. Neurocritical Care 2009, 10, 253–256. [Google Scholar] [CrossRef]
- Bekar, A.; Doğan, Ş.; Abaş, F.; Caner, B.; Korfalı, G.; Kocaeli, H.; Yılmazlar, S. Risk factors and complications of intracranial pressure monitoring with a fiberoptic device. J. Clin. Neurosci. 2009, 16, 236–240. [Google Scholar] [CrossRef]
- Holloway, K.; Barnes, T.; Choi, S.; Bullock, R.; Marshall, L.F.; Eisenberg, H.M.; Jane, J.A.; Ward, J.D.; Young, H.F.; Marmarou, A. Ventriculostomy infections: The effect of monitoring duration and catheter exchange in 584 patients. J. Neurosurg. 1996, 85, 419–424. [Google Scholar] [CrossRef] [Green Version]
- Robba, C.; Bacigaluppi, S.; Cardim, D.; Donnelly, J.; Bertuccio, A.; Czosnyka, M. Non-invasive assessment of intracranial pressure. Acta Neurol. Scand. 2015, 134, 4–21. [Google Scholar] [CrossRef]
- Padayachy, L.C. Non-invasive intracranial pressure assessment. Child’s Nerv. Syst. 2016, 32, 1587–1597. [Google Scholar] [CrossRef]
- Kayhanian, S.; Young, A.M.H.; Piper, R.; Donnelly, J.; Scoffings, D.; Garnett, M.R.; Fernandes, H.M.; Smielewski, P.; Czosnyka, M.; Hutchinson, P.; et al. Radiological Correlates of Raised Intracranial Pressure in Children: A Review. Front. Pediatr. 2018, 6, 32. [Google Scholar] [CrossRef] [Green Version]
- Price, D.A.; Grzybowski, A.; Eikenberry, J.; Januleviciene, I.; Vercellin, A.C.V.; Mathew, S.; Siesky, B.; Harris, A. Review of non-invasive intracranial pressure measurement techniques for ophthalmology applications. Br. J. Ophthalmol. 2019, 104, 887–892. [Google Scholar] [CrossRef]
- Singh, Y.; Tissot, C.; Fraga, M.V.; Yousef, N.; Cortes, R.G.; Lopez, J.; Sanchez-De-Toledo, J.; Brierley, J.; Mayordomo-Colunga, J.; Raffaj, D.; et al. International evidence-based guidelines on Point of Care Ultrasound (POCUS) for critically ill neonates and children issued by the POCUS Working Group of the European Society of Paediatric and Neonatal Intensive Care (ESPNIC). Crit. Care 2020, 24, 65. [Google Scholar] [CrossRef] [Green Version]
- Bortcosh, W.; Shaahinfar, A.; Sojar, S.; Klig, J.E. New directions in point-of-care ultrasound at the crossroads of paediatric emergency and critical care. Curr. Opin. Pediatr. 2018, 30, 350–358. [Google Scholar] [CrossRef]
- O’Brien, A.J.; Brady, R.M. Point-of-care ultrasound in paediatric emergency medicine. J. Paediatr. Child Health 2016, 52, 174–180. [Google Scholar] [CrossRef]
- Moore, C.L.; Copel, J.A. Point-of-Care Ultrasonography. N. Engl. J. Med. 2011, 364, 749–757. [Google Scholar] [CrossRef] [Green Version]
- Liu, D.; Kahn, M. Measurement and Relationship of Subarachnoid Pressure of the Optic Nerve to Intracranial Pressures in Fresh Cadavers. Am. J. Ophthalmol. 1993, 116, 548–556. [Google Scholar] [CrossRef]
- Helmke, K.; Hansen, H.C. Fundamentals of transorbital sonographic evaluation of optic nerve sheath expansion under intracranial hypertension. Pediatr. Radiol. 1996, 26, 701–705. [Google Scholar] [CrossRef]
- Maude, R.R.; Hossain, M.A.; Hassan, M.U.; Osbourne, S.; Langan Abu Sayeed, K.; Rezaul Karim, M.; Samad, R.; Borooah, S.; Dhillon, B.; Day, N.P.J.; et al. Transorbital sonographic evaluation of normal optic nerve sheath diameter in healthy volunteers in Bangladesh. PLoS ONE 2013, 8, e81013. [Google Scholar] [CrossRef]
- Hansen, H.-C.; Helmke, K. Validation of the optic nerve sheath response to changing cerebrospinal fluid pressure: Ultrasound findings during intrathecal infusion tests. J. Neurosurg. 1997, 87, 34–40. [Google Scholar] [CrossRef]
- Moretti, R.; Pizzi, B. Ultrasonography of the optic nerve in neurocritically ill patients. Acta Anaesthesiol. Scand. 2011, 55, 644–652. [Google Scholar] [CrossRef]
- Launey, Y.; Nesseler, N.; Le Maguet, P.; Malledant, Y.; Seguin, P. Effect of Osmotherapy on Optic Nerve Sheath Diameter in Patients with Increased Intracranial Pressure. J. Neurotrauma 2014, 31, 984–988. [Google Scholar] [CrossRef]
- Toscano, M.; Spadetta, G.; Pulitano, P.; Rocco, M.; Di Piero, V.; Mecarelli, O.; Vicenzini, E. Optic Nerve Sheath Diameter Ultrasound Evaluation in Intensive Care Unit: Possible Role and Clinical Aspects in Neurological Critical Patients’ Daily Monitoring. BioMed Res. Int. 2017, 2017, 1621428. [Google Scholar] [CrossRef] [Green Version]
- Dubourg, J.; Javouhey, E.; Geeraerts, T.; Messerer, M.; Kassai, B. Ultrasonography of optic nerve sheath diameter for detection of raised intracranial pressure: A systematic review and meta-analysis. Intensiv. Care Med. 2011, 37, 1059–1068. [Google Scholar] [CrossRef]
- Rajajee, V.; Vanaman, M.; Fletcher, J.J.; Jacobs, T.L. Optic Nerve Ultrasound for the Detection of Raised Intracranial Pressure. Neurocritical Care 2011, 15, 506–515. [Google Scholar] [CrossRef]
- Geeraerts, T.; Merceron, S.; Benhamou, D.; Vigué, B.; Duranteau, J. Non-invasive assessment of intracranial pressure using ocular sonography in neurocritical care patients. Intensiv. Care Med. 2008, 34, 2062–2067. [Google Scholar] [CrossRef]
- Geeraerts, T.; Launey, Y.; Martin, L.; Pottecher, J.; Vigué, B.; Duranteau, J.; Benhamou, D. Ultrasonography of the optic nerve sheath may be useful for detecting raised intracranial pressure after severe brain injury. Intensiv. Care Med. 2007, 33, 1704–1711. [Google Scholar] [CrossRef]
- Tayal, V.S.; Neulander, M.; Norton, H.J.; Foster, T.; Saunders, T.; Blaivas, M. Emergency Department Sonographic Measurement of Optic Nerve Sheath Diameter to Detect Findings of Increased Intracranial Pressure in Adult Head Injury Patients. Ann. Emerg. Med. 2007, 49, 508–514. [Google Scholar] [CrossRef]
- Malayeri, A.A.; Bavarian, S.; Mehdizadeh, M. Sonographic Evaluation of Optic Nerve Diameter in Children with Raised Intracranial Pressure. J. Ultrasound Med. 2005, 24, 143–147. [Google Scholar] [CrossRef] [Green Version]
- Hansen, G.; Sellers, E.A.; Beer, D.L.; Vallance, J.K.; Clark, I. Optic Nerve Sheath Diameter Ultrasonography in Pediatric Patients with Diabetic Ketoacidosis. Can. J. Diabetes 2016, 40, 126–130. [Google Scholar] [CrossRef]
- Helmke, K.; Burdelski, M.; Hansen, H.-C. Detection and monitoring of intracranial pressure dysregulation in liver failure by ultrasound. Transplantation 2000, 70, 392–395. [Google Scholar] [CrossRef]
- Hall, M.K.; Spiro, D.M.; Sabbaj, A.; Moore, C.L.; Hopkins, K.L.; Meckler, G. Bedside optic nerve sheath diameter ultrasound for the evaluation of suspected pediatric ventriculoperitoneal shunt failure in the emergency department. Child’s Nerv. Syst. 2013, 29, 2275–2280. [Google Scholar] [CrossRef]
- McAuley, D.; Paterson, A.; Sweeney, L. Optic nerve sheath ultrasound in the assessment of paediatric hydrocephalus. Child’s Nerv. Syst. 2009, 25, 87–90. [Google Scholar] [CrossRef]
- Newman, W.D.; Hollman, A.S.; Dutton, G.N.; Carachi, R. Measurement of optic nerve sheath diameter by ultrasound: A means of detecting acute raised intracranial pressure in hydrocephalus. Br. J. Ophthalmol. 2002, 86, 1109–1113. [Google Scholar] [CrossRef] [Green Version]
- Driessen, C.; Van Veelen, M.-L.C.; Lequin, M.; Joosten, K.F.M.; Mathijssen, I.M.J. Nocturnal Ultrasound Measurements of Optic Nerve Sheath Diameter Correlate with Intracranial Pressure in Children with Craniosynostosis. Plast. Reconstr. Surg. 2012, 130, 448e–451e. [Google Scholar] [CrossRef]
- Murphy, S.; Cserti-Gazdewich, C.; Dhabangi, A.; Musoke, C.; Nabukeera-Barungi, N.; Price, D.; King, M.E.; Romero, J.; Noviski, N.; Dzik, W. Ultrasound findings in Plasmodium falciparum malaria: A pilot study. Pediatr. Crit. Care Med. 2011, 12, e58–e63. [Google Scholar] [CrossRef]
- Monro, A. Observations on the Structure and Functions of the Nervous System; Creech and Johnson: Edinbourgh, UK, 1783. [Google Scholar]
- Kellie, G. Appearances observed in the dissection of two individuals: Death from cold and congestion of the brain. Trans. Med. Chir. Soc. Edinbrugh 1824, 1, 84. [Google Scholar]
- Budday, S.; Ovaert, T.C.; Holzapfel, G.A.; Steinmann, P.; Kuhl, E. Fifty Shades of Brain: A Review on the Mechanical Testing and Modeling of Brain Tissue. Arch. Comput. Methods Eng. 2019, 27, 1187–1230. [Google Scholar] [CrossRef] [Green Version]
- Greenberg, M. (Ed.) Neuromonitoring. In Handbook of Neurosurgery, 8th ed.; Thieme: New York, NY, USA, 2016; pp. 856–881. [Google Scholar]
- Morton, R.; Ellenbogen, R. Intracranial hypertension. In Principles of Neurological Surgery, 3rd ed.; Saunders/Elsevier: Philadelphia, PA, USA, 2012; pp. 311–323. [Google Scholar]
- Lassen, N.A. Cerebral Blood Flow and Oxygen Consumption in Man. Physiol. Rev. 1959, 39, 183–238. [Google Scholar] [CrossRef] [Green Version]
- Lassen, N.A. Control of Cerebral Circulation in Health and Disease. Circ. Res. 1974, 34, 749–760. [Google Scholar] [CrossRef] [Green Version]
- Drummond, J.C. The Lower Limit of Autoregulation. Anesthesiologists 1997, 86, 1431–1433. [Google Scholar] [CrossRef]
- Latorre, J.G.S.; Greer, D.M. Management of Acute Intracranial Hypertension. Neurologist 2009, 15, 193–207. [Google Scholar] [CrossRef]
- Armstead, W.M. Cerebral Blood Flow Autoregulation and Dysautoregulation. Anesthesiol. Clin. 2016, 34, 465–477. [Google Scholar] [CrossRef] [Green Version]
- Meng, L.; Gelb, A.W. Regulation of Cerebral Autoregulation by Carbon Dioxide. Anesthesiology 2015, 122, 196–205. [Google Scholar] [CrossRef] [Green Version]
- Kinoshita, K. Traumatic brain injury: Pathophysiology for neurocritical care. J. Intensiv. Care 2016, 4, 29. [Google Scholar] [CrossRef] [Green Version]
- Youmans, J.R. (Ed.) Neurological Surgery, 4th ed.; WB Saunders: Philadelphia, PA, USA, 1996; Volume 3. [Google Scholar]
- Sanz-García, A.; Pérez-Romero, M.; Pastor, J.; Sola, R.G.; Vega-Zelaya, L.; Monasterio, F.; Torrecilla, C.; Vega, G.; Pulido, P.; Ortega, G.J. Identifying causal relationships between EEG activity and intracranial pressure changes in neurocritical care patients. J. Neural Eng. 2018, 15, 066029. [Google Scholar] [CrossRef]
- Donnelly, J.; Budohoski, K.P.; Smielewski, P.; Czosnyka, M. Regulation of the cerebral circulation: Bedside assessment and clinical implications. Crit. Care 2016, 20, 129. [Google Scholar] [CrossRef] [Green Version]
- Cavus, E.; Bein, B.; Dörges, V.; Stadlbauer, K.-H.; Wenzel, V.; Steinfath, M.; Hanss, R.; Scholz, J. Brain tissue oxygen pressure and cerebral metabolism in an animal model of cardiac arrest and cardiopulmonary resuscitation. Resuscitation 2006, 71, 97–106. [Google Scholar] [CrossRef]
- Bowton, D.L.; Bertels, N.H.; Prough, D.S.; Stump, D.A. Cerebral blood flow is reduced in patients with sepsis syndrome. Crit. Care Med. 1989, 17, 399–403. [Google Scholar] [CrossRef]
- Lundberg, N. Continuous recording and control of ventricular fluid pressure in neurosurgical practice. Acta Psychiatr. Scand. Suppl. 1960, 36, 13764297. [Google Scholar] [CrossRef] [Green Version]
- Hawryluk, G.W.J.; Nielson, J.L.; Huie, J.R.; Zimmermann, L.; Saigal, R.; Ding, Q.; Hirschi, R.; Zeiler, F.A.; Ferguson, A.R.; Manley, G.T. Analysis of Normal High-Frequency Intracranial Pressure Values and Treatment Threshold in Neurocritical Care Patients. JAMA Neurol. 2020, 77, 1150–1158. [Google Scholar] [CrossRef]
- Albeck, M.J.; Børgesen, S.E.; Gjerris, F.; Schmidt, J.F.; Sorensen, P.S. Intracranial pressure and cerebrospinal fluid outflow conductance in healthy subjects. J. Neurosurg. 1991, 74, 597–600. [Google Scholar] [CrossRef] [Green Version]
- Chapman, P.H.; Cosman, E.R.; Arnold, M.A. The relationship between ventricular fluid pressure and body position in normal subjects and subjects with shunts. Neurosurgery 1990, 26, 181–189. [Google Scholar] [CrossRef]
- Carney, N.; Totten, A.M.; O’Reilly, C.; Ullman, J.S.; Hawryluk, G.W.; Bell, M.J.; Bratton, S.L.; Chesnut, R.; Harris, O.A.; Kissoon, N.; et al. Guidelines for the Management of Severe Traumatic Brain Injury, Fourth Edition. Neurosurgery 2016, 80, 6–15. [Google Scholar] [CrossRef]
- Welch, K. The intracranial pressure in infants. J. Neurosurg. 1980, 52, 693–699. [Google Scholar] [CrossRef]
- Mohseni-Bod, H.; Drake, J.; Kukreti, V. Management of raised intracranial pressure in children with traumatic brain injury. J. Pediatr. Neurosci. 2014, 9, 207–215. [Google Scholar] [CrossRef]
- Cruz, J.; Nakayama, P.; Imamura, J.H.; Rosenfeld, K.G.; de Souza, H.S.; Giorgetti, G.V.F. Cerebral Extraction of Oxygen and Intracranial Hypertension in Severe, Acute, Pediatric Brain Trauma: Preliminary Novel Management Strategies. Neurosurgery 2002, 50, 774–780. [Google Scholar] [CrossRef]
- Adelson, P.D.; Ragheb, J.; Muizelaar, J.P.; Kanev, P.; Brockmeyer, D.; Beers, S.R.; Brown, S.D.; Cassidy, L.D.; Chang, Y.; Levin, H. Phase II Clinical Trial of Moderate Hypothermia after Severe Traumatic Brain Injury in Children. Neurosurgery 2005, 56, 740–754. [Google Scholar] [CrossRef]
- Chambers, I.R.; Treadwell, L.; Mendelow, A.D. Determination of threshold levels of cerebral perfusion pressure and intracranial pressure in severe head injury by using receiver operating—Characteristic curves: An observational study in 291 patients. J. Neurosurg. 2001, 94, 412–416. [Google Scholar] [CrossRef]
- Jagannathan, J.; Okonkwo, D.O.; Yeoh, H.K.; Dumont, A.S.; Saulle, D.; Haizlip, J.; Barth, J.T.; Jane, J.A. Long-term outcomes and prognostic factors in pediatric patients with severe traumatic brain injury and elevated intracranial pressure. J. Neurosurg. Pediatr. 2008, 2, 240–249. [Google Scholar] [CrossRef] [Green Version]
- Michaud, L.J.; Rivara, F.P.; Grady, M.S.; Reay, D.T. Predictors of Survival and Severity of Disability after Severe Brain Injury in Children. Neurosurgery 1992, 31, 254–264. [Google Scholar] [CrossRef]
- Esparza, J.; M-Portillo, J.; Sarabia, M.; Roger, R.; Lamas, E. Outcome in children with severe head injuries. Child’s Nerv. Syst. 1985, 1, 109–114. [Google Scholar] [CrossRef]
- Spencer, W.H. Ophthalmic Pathology: An Atlas and Textbook, 3rd ed.; WB Saunders: Philadelphia, PA, USA, 1986; pp. 2337–2458. [Google Scholar]
- Barr, R.; Gean, A. Craniofacial trauma. In Fundamentals of Diagnostic Radiology, 2nd ed.; Brant, W., Helms, C., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 1999; pp. 49–61. [Google Scholar]
- O’Rahilly, R. The early development of the eye in staged embryos. Contrib. Embryol. 1966, 38, 1–42. [Google Scholar]
- Rothman, M.I.; Zoarski, G.H. The orbit. In Textbook of Radiology and Imaging, 7th ed.; Sutton, D., Ed.; Churchill Living-Stone: London, UK, 2003; pp. 1573–1595. [Google Scholar]
- Killer, H.E.; Laeng, H.R.; Flammer, J.; Groscurth, P. Architecture of arachnoid trabeculae, pillars, and septa in the subarachnoid space of the human optic nerve: Anatomy and clinical considerations. Br. J. Ophthalmol. 2003, 87, 777–781. [Google Scholar] [CrossRef] [Green Version]
- Geeraerts, T.; Duranteau, J.; Benhamou, D. Ocular sonography in patients with raised intracranial pressure: The papilloedema revisited. Crit. Care 2008, 12, 150. [Google Scholar] [CrossRef]
- Wood, J.H. Physiology, pharmacology, and dynamics ofcerebrospinal fluid. In Neurobiology Ofcerebrospinal Fluid; Wood, J.H., Ed.; Plenum Press: New York, NJ, USA, 1989; pp. 1–16. [Google Scholar]
- Gausas, R.E.; Gonnering, R.; Lemke, B.N.; Dortzbach, R.K.; Sherman, D.D. Identification of Human Orbital Lymphatics. Ophthalmic Plast. Reconstr. Surg. 1999, 15, 252–259. [Google Scholar] [CrossRef]
- Hayreh, S.S. Pathogenesis of oedema of the optic disc (papilloedema): A preliminary report. Br. J. Ophthalmol. 1964, 48, 522–543. [Google Scholar] [CrossRef] [Green Version]
- Gangemi, M.; Cennamo, G.; Maiuri, F.; D’Andrea, F. Echographic measurement of the optic nerve in patients with intracranial hypertension. Min -Minim. Invasive Neurosurg. 1987, 30, 53–55. [Google Scholar] [CrossRef]
- Hansen, H.C.; Helmke, K. The subarachnoid space surrounding the optic nerves. An ultrasound study of the optic nerve sheath. Surg. Radiol. Anat. 1996, 18, 323–328. [Google Scholar] [CrossRef]
- Soldatos, T.; Karakitsos, D.; Chatzimichail, K.; Papathanasiou, M.; Gouliamos, A.; Karabinis, A. Optic nerve sonography in the diagnostic evaluation of adult brain injury. Crit. Care 2008, 12, R67. [Google Scholar] [CrossRef] [Green Version]
- Moretti, R.; Pizzi, B.; Cassini, F.; Vivaldi, N. Reliability of Optic Nerve Ultrasound for the Evaluation of Patients with Spontaneous Intracranial Hemorrhage. Neurocritical Care 2009, 11, 406–410. [Google Scholar] [CrossRef]
- Ossoinig, K.C. Standardized echography: Basic principles, clinical applications, and results. Int. Ophthalmol. Clin. 1979, 19, 127–210. [Google Scholar] [CrossRef]
- DiBernardo, C.W.; Greenberg, E. Ophthalmic Ultrasound: A Diagnostic Atlas; ThiemeMedical Publishers: New York, NY, USA, 2007. [Google Scholar]
- Romagnuolo, L.; Tayal, V.; Tomaszewski, C.; Saunders, T.; Norton, H.J. Optic nerve sheath diameter does not change with patient position. Am. J. Emerg. Med. 2005, 23, 686–688. [Google Scholar] [CrossRef]
- Soldatos, T.; Chatzimichail, K.; Papathanasiou, M.; Gouliamos, A. Optic nerve sonography: A new window for the non-invasive evaluation of intracranial pressure in brain injury. Emerg. Med. J. 2009, 26, 630–634. [Google Scholar] [CrossRef]
- Bäuerle, J.; Lochner, P.; Kaps, M.; Nedelmann, M. Intra- and Interobsever Reliability of Sonographic Assessment of the Optic Nerve Sheath Diameter in Healthy Adults. J. Neuroimaging 2012, 22, 42–45. [Google Scholar] [CrossRef]
- Ballantyne, S.; O’Neill, G.; Hamilton, R.; Hollman, A. Observer variation in the sonographic measurement of optic nerve sheath diameter in normal adults. Eur. J. Ultrasound 2002, 15, 145–149. [Google Scholar] [CrossRef]
- Steinborn, M.; Fiegler, J.; Ruedisser, K.; Hapfelmeier, A.; Denne, C.; Macdonald, E.; Hahn, H. Measurement of the Optic Nerve Sheath Diameter in Children: Comparison Between Transbulbar Sonography and Magnetic Resonance Imaging. Ultraschall Med. Eur. J. Ultrasound 2011, 33, 569–573. [Google Scholar] [CrossRef]
- Steinborn, M.; Fiegler, J.; Kraus, V.; Denne, C.; Hapfelmeier, A.; Wurzinger, L.; Hahn, H. High Resolution Ultrasound and Magnetic Resonance Imaging of the Optic Nerve and the Optic Nerve Sheath: Anatomic Correlation and Clinical Importance. Ultraschall Med. Eur. J. Ultrasound 2010, 32, 608–613. [Google Scholar] [CrossRef] [PubMed]
- Fielding, J. Ocular ultrasound. Clin. Radiol. 1996, 51, 533–544. [Google Scholar] [CrossRef]
- Ballantyne, J.; Hollman, A.; Hamilton, R.; Bradnam, M.; Carachi, R.; Young, D.; Dutton, G. Transorbital optic nerve sheath ultrasonography in normal children. Clin. Radiol. 1999, 54, 740–742. [Google Scholar] [CrossRef]
- Rehman Siddiqui, N.U.; Haque, A.; Abbas, Q.; Jurair, H.; Salam, B.; Sayani, R. Ultrasonographic optic nerve sheath diameter Meas-urement for raised intracranial pressure in a Tertiary care centre of a developing country. J. Ayub. Med. Coll Abbottabad. 2018, 30, 495–500. [Google Scholar]
- Irazuzta, J.E.; Brown, M.E.; Akhtar, J. Bedside Optic Nerve Sheath Diameter Assessment in the Identification of Increased Intracranial Pressure in Suspected Idiopathic Intracranial Hypertension. Pediatr. Neurol. 2016, 54, 35–38. [Google Scholar] [CrossRef]
- Aslan, N.; Yildizdas, D.; Ozcan, N.; Horoz, O.O.; Mert, G.G.; Sertdemir, Y.; Altunbasak, S. Optic Nerve Sheath Diameter and Retinal Artery Resistive Index Measurements with Bedside Ophthalmic Ultrasound in Pediatric Patients with Pseudotumor Cerebri Syndrome. J. Pediatr. Intensiv. Care 2020, 9, 181–187. [Google Scholar] [CrossRef]
- Padayachy, L.C.; Padayachy, V.; Galal, U.; Pollock, T.; Fieggen, A.G. The relationship between transorbital ultrasound measurement of the optic nerve sheath diameter (ONSD) and invasively measured ICP in children. Child’s Nerv. Syst. 2016, 32, 1779–1785. [Google Scholar] [CrossRef]
- Kerscher, S.R.; Schöni, D.; Hurth, H.; Neunhoeffer, F.; Haas-Lude, K.; Wolff, M.; Schuhmann, M.U. The relation of optic nerve sheath diameter (ONSD) and intracranial pressure (ICP) in pediatric neurosurgery practice—Part I: Correlations, age-dependency and cut-off values. Child’s Nerv. Syst. 2020, 36, 99–106. [Google Scholar] [CrossRef]
- Robba, C.; Cardim, D.; Czosnyka, M.; Abecasis, F.; Pezzato, S.; Buratti, S.; Moscatelli, A.; Sortica, C.; Racca, F.; Pelosi, P.; et al. Ultrasound non-invasive intracranial pressure assessment in paediatric neurocritical care: A pilot study. Child’s Nerv. Syst. 2019, 36, 117–124. [Google Scholar] [CrossRef]
- Fontanel, L.; Pensiero, S.; Ronfani, L.; Rosolen, V.; Barbi, E. Optic Nerve Sheath Diameter Ultrasound: Optic Nerve Growth Curve and Its Application to Detect Intracranial Hypertension in Children. Am. J. Ophthalmol. 2019, 208, 421–428. [Google Scholar] [CrossRef]
- Steinborn, M.; Friedmann, M.; Makowski, C.; Hahn, H.; Hapfelmeier, A.; Juenger, H. High resolution transbulbar sonography in children with suspicion of increased intracranial pressure. Child’s Nerv. Syst. 2016, 32, 655–660. [Google Scholar] [CrossRef] [PubMed]
- Aslan, N.; Yıldızdaş, D.; Horoz, Ö.Ö.; Özsoy, M.; Yöntem, A.; Çetinalp, E.; Mert, G.G. Evaluation of ultrasonographic optic nerve sheath diameter and central retinal artery Doppler indices by point-of-care ultrasound in pediatric patients with increased intracranial pressure. Turk. J. Pediatr. 2021, 63, 300–306. [Google Scholar] [CrossRef] [PubMed]
- Le, A.; Hoehn, M.E.; Smith, M.E.; Spentzas, T.; Schlappy, D.; Pershad, J. Bedside Sonographic Measurement of Optic Nerve Sheath Diameter as a Predictor of Increased Intracranial Pressure in Children. Ann. Emerg. Med. 2009, 53, 785–791. [Google Scholar] [CrossRef]
- Gravendeel, J.; Rosendahl, K. Cerebral biometry at birth and at 4 and 8 months of age. A prospective study using US. Pediatr. Radiol. 2010, 40, 1651–1656. [Google Scholar] [CrossRef] [PubMed]
- Ardell, S.; Daspal, S.; Holt, T.; Hansen, G. Optic Nerve Sheath Diameter for Preterm Infants: A Pilot Study. Neonatology 2019, 116, 1–5. [Google Scholar] [CrossRef]
- Yapicioglu, H.; Aslan, N.; Sertdemir, Y.; Yildizdas, D.; Gulasi, S.; Mert, K. Determination of normal values of optic nerve sheath diameter in newborns with bedside ultrasonography. Early Hum. Dev. 2020, 145, 104986. [Google Scholar] [CrossRef]
- Lochner, P.; Leone, M.A.; Coppo, L.; Nardone, R.; Zedde, M.L.; Cantello, R.; Brigo, F. B-mode transorbital ultrasononography for the diagnosis of acute optic neuritis. A systematic review. Clin. Neurophysiol. 2015, 127, 803–809. [Google Scholar] [CrossRef]
- Sargsyan, A.E.; Blaivas, M.; Geeraerts, T.; Karakitsos, D. Ocular ultrasound in the intensivecare unit. In Critical Care Ultrasound, 1st ed.; Lumb, P., Karakitsos, D., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2014; ISBN 978-1-4557-5357-4. [Google Scholar]
- Bloria, S.D.; Bloria, P.; Luthra, A. Is it the time to standardize the procedure of ultrasound guided optic nerve sheath diameter measurement? Saudi J. Anaesth. 2019, 13, 255–256. [Google Scholar] [CrossRef]
- Lochner, P.; Czosnyka, M.; Naldi, A.; Lyros, E.; Pelosi, P.; Mathur, S.; Fassbender, K.; Robba, C. Optic nerve sheath diameter: Present and future perspectives for neurologists and critical care physicians. Neurol. Sci. 2019, 40, 2447–2457. [Google Scholar] [CrossRef]
- Pansell, J.; Bell, M.; Rudberg, P.; Friman, O.; Cooray, C. Optic nerve sheath diameter measurement by ultrasound: Evaluation of a standardized protocol. J. Neuroimaging 2021, 32, 104–110. [Google Scholar] [CrossRef]
- Zeiler, F.A.; Ziesmann, M.T.; Goeres, P.; Unger, B.; Park, J.; Karakitsos, D.; Blaivas, M.; Vergis, A.; Gillman, L.M. A unique method for estimating the reliability learning curve of optic nerve sheath diameter ultrasound measurement. Crit. Ultrasound J. 2016, 8, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).