Combined Diagnostic Accuracy of Diffusion and Perfusion MR Imaging to Differentiate Radiation-Induced Necrosis from Recurrence in Glioblastoma
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Technique
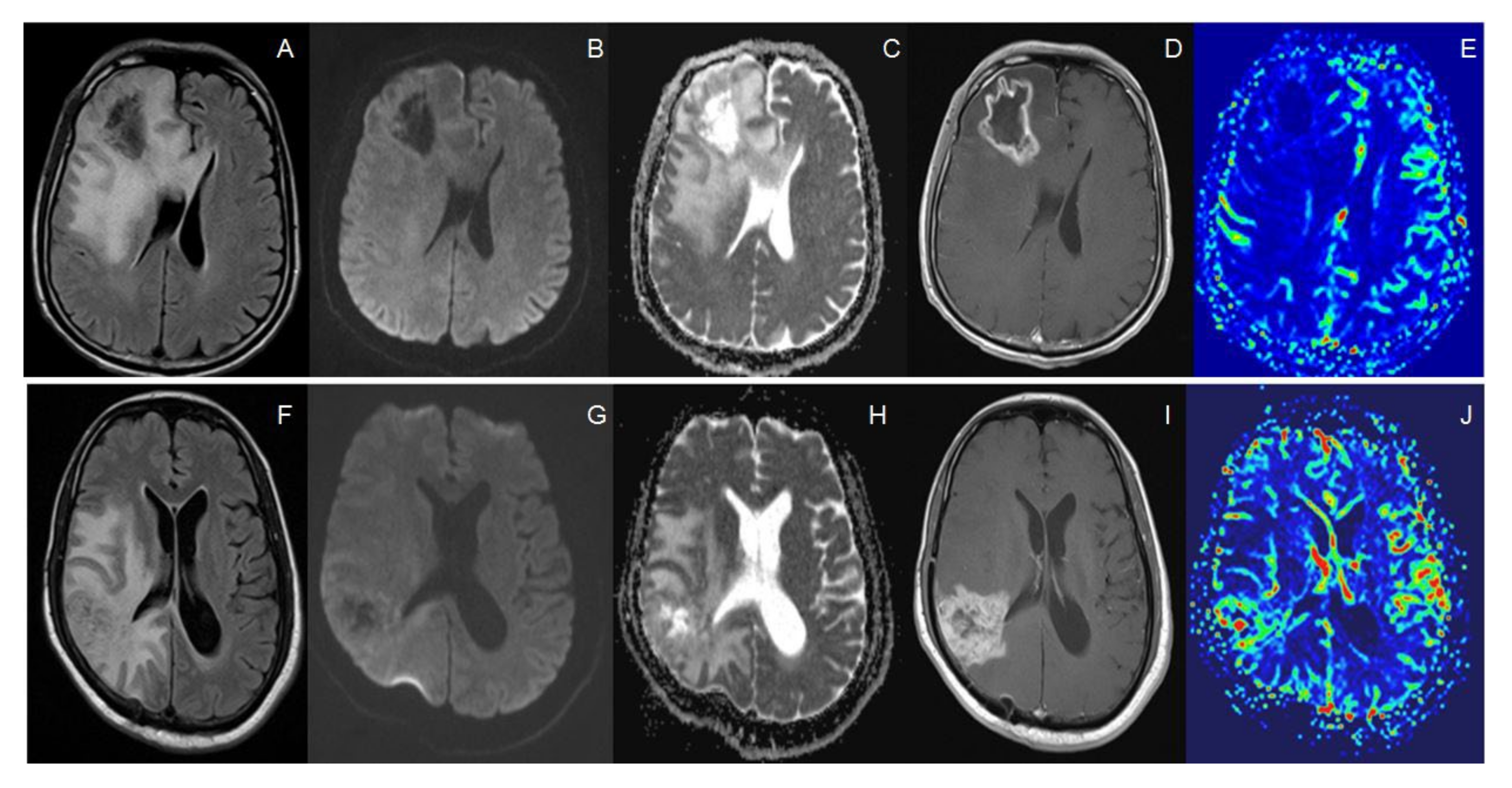
2.3. Imaging Analysis
2.4. Histopathology Interpretation
2.5. Statistical Analysis
3. Results
3.1. Baseline Characteristics
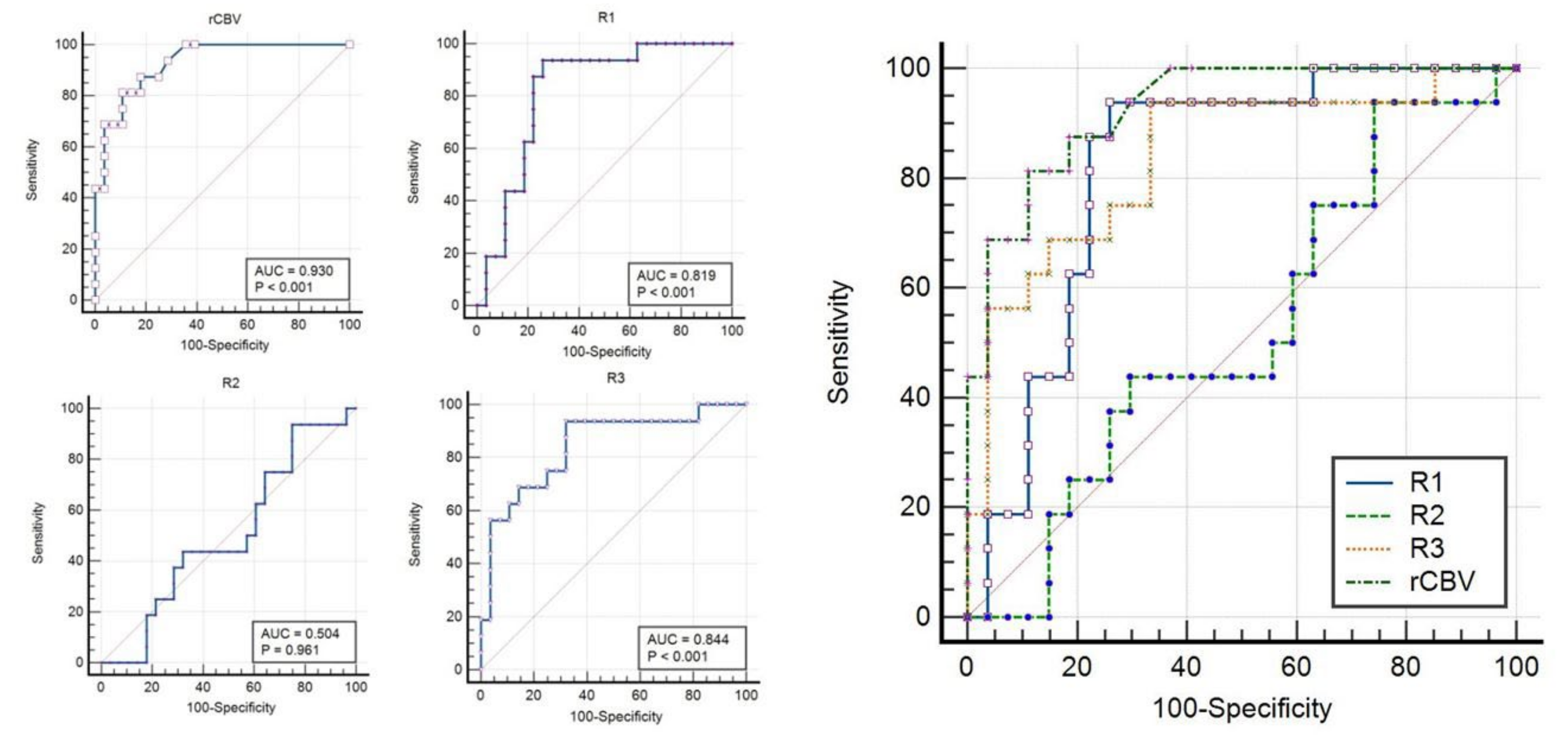
3.2. Quantitative MR Imaging Parameters with ROC Curve Analysis
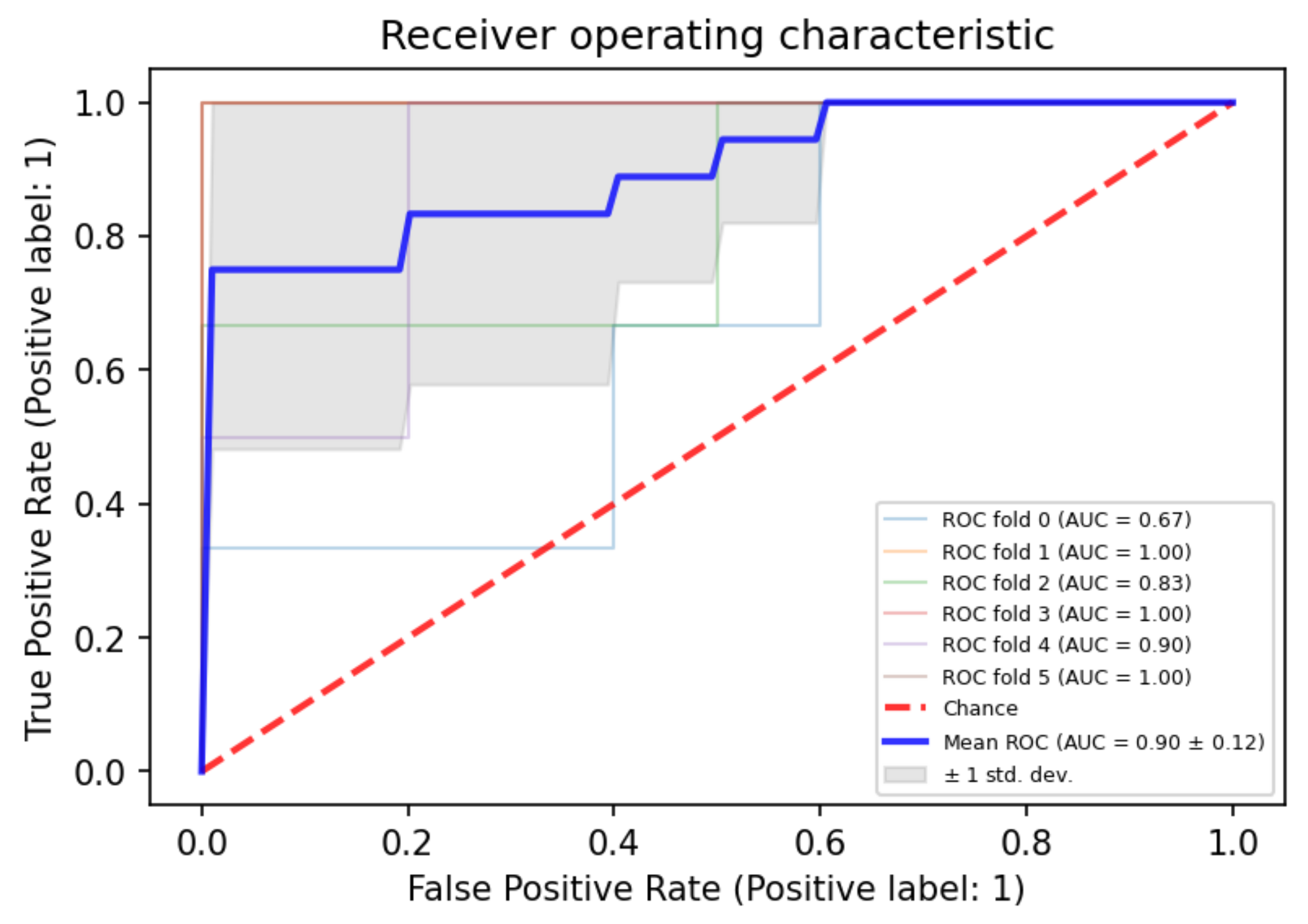
3.3. Combined Approach by Using Both PWI and ADC Parameters
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Han, Q.; Liang, H.; Cheng, P.; Yang, H.; Zhao, P. Gross Total vs. Subtotal Resection on Survival Outcomes in Elderly Patients with High-Grade Glioma: A Systematic Review and Meta-Analysis. Front. Oncol. 2020, 10, 151. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smoll, N.R.; Schaller, K.; Gautschi, O.P. Long-term survival of patients with glioblastoma multiforme (GBM). J. Clin. Neurosci. 2013, 20, 670–675. [Google Scholar] [CrossRef] [PubMed]
- Boonzaier, N.R.; Larkin, T.J.; Matys, T.; van der Hoorn, A.; Yan, J.-L.; Price, S.J. Multiparametric MR Imaging of Diffusion and Perfusion in Contrast-enhancing and Nonenhancing Components in Patients with Glioblastoma. Radiology 2017, 284, 180–190. [Google Scholar] [CrossRef] [PubMed]
- White, M.L.; Zhang, Y.; Yu, F.; Shonka, N.; Aizenberg, M.R.; Adapa, P.; Kazmi, S.A.J. Post-operative perfusion and diffusion MR imaging and tumor progression in high-grade gliomas. PLoS ONE 2019, 14, e0213905. [Google Scholar] [CrossRef] [Green Version]
- Verma, N.; Cowperthwaite, M.C.; Burnett, M.G.; Markey, M.K. Differentiating tumor recurrence from treatment necrosis: A review of neuro-oncologic imaging strategies. Neuro-Oncology 2013, 15, 515–534. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Ma, L.; Wang, Q.; Zheng, X.; Wu, C.; Xu, B. Role of magnetic resonance spectroscopy for the differentiation of recurrent glioma from radiation necrosis: A systematic review and meta-analysis. Eur. J. Radiol. 2014, 83, 2181–2189. [Google Scholar] [CrossRef]
- Chao, S.T.; Ahluwalia, M.S.; Barnett, G.H.; Stevens, G.H.J.; Murphy, E.S.; Stockham, A.L.; Shiue, K.; Suh, J.H. Challenges with the Diagnosis and Treatment of Cerebral Radiation Necrosis. Int. J. Radiat. Oncol. 2013, 87, 449–457. [Google Scholar] [CrossRef]
- Prager, A.J.; Martinez, N.; Beal, K.; Omuro, A.; Zhang, Z.; Young, R.J. Diffusion and Perfusion MRI to Differentiate Treatment-Related Changes Including Pseudoprogression from Recurrent Tumors in High-Grade Gliomas with Histopathologic Evidence. Am. J. Neuroradiol. 2015, 36, 877–885. [Google Scholar] [CrossRef]
- Alcaide-Leon, P.; Cluceru, J.; Lupo, J.M.; Yu, T.J.; Luks, T.L.; Tihan, T.; Bush, N.A.; Villanueva-Meyer, J.E. Centrally Reduced Diffusion Sign for Differentiation between Treatment-Related Lesions and Glioma Progression: A Validation Study. Am. J. Neuroradiol. 2020, 41, 2049–2054. [Google Scholar] [CrossRef]
- Zakhari, N.; Taccone, M.S.; Torres, C.; Chakraborty, S.; Sinclair, J.; Woulfe, J.; Jansen, G.H.; Nguyen, T.B. Diagnostic Accuracy of Centrally Restricted Diffusion in the Differentiation of Treatment-Related Necrosis from Tumor Recurrence in High-Grade Gliomas. Am. J. Neuroradiol. 2018, 39, 260–264. [Google Scholar] [CrossRef]
- Boxerman, J.L.; Schmainda, K.M.; Weisskoff, R.M. Relative Cerebral Blood Volume Maps Corrected for Contrast Agent Extravasation Significantly Correlate with Glioma Tumor Grade, Whereas Uncorrected Maps Do Not. Am. J. Neuroradiol. 2006, 27, 859–867. [Google Scholar] [PubMed]
- Sawlani, R.N.; Raizer, J.; Horowitz, S.W.; Shin, W.; Grimm, S.A.; Chandler, J.P.; Levy, R.; Getch, C.; Carroll, T.J. Glioblastoma: A Method for Predicting Response to Antiangiogenic Chemotherapy by Using MR Perfusion Imaging—Pilot Study. Radiology 2010, 255, 622–628. [Google Scholar] [CrossRef] [Green Version]
- Saraswathy, S.; Crawford, F.W.; Lamborn, K.R.; Pirzkall, A.; Chang, S.; Cha, S.; Nelson, S.J. Evaluation of MR markers that predict survival in patients with newly diagnosed GBM prior to adjuvant therapy. J. Neurooncol. 2009, 91, 69–81. [Google Scholar] [CrossRef] [PubMed]
- Barajas, R.F.; Phillips, J.J.; Parvataneni, R.; Molinaro, A.; Essock-Burns, E.; Bourne, G.; Parsa, A.T.; Aghi, M.K.; McDermott, M.W.; Berger, M.S.; et al. Regional variation in histopathologic features of tumor specimens from treatment-naive glioblastoma correlates with anatomic and physiologic MR Imaging. Neuro-Oncology 2012, 14, 942–954. [Google Scholar] [CrossRef] [Green Version]
- Young, R.J.; Gupta, A.; Shah, A.D.; Graber, J.J.; Chan, T.A.; Zhang, Z.; Shi, W.; Beal, K.; Omuro, A.M. MRI perfusion in determining pseudoprogression in patients with glioblastoma. Clin. Imaging 2013, 37, 41–49. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Law, M.; Young, R.J.; Babb, J.S.; Peccerelli, N.; Chheang, S.; Gruber, M.L.; Miller, D.C.; Golfinos, J.G.; Zagzag, D.; Johnson, G. Gliomas: Predicting Time to Progression or Survival with Cerebral Blood Volume Measurements at Dynamic Susceptibility-weighted Contrast-enhanced Perfusion MR Imaging. Radiology 2008, 247, 490–498. [Google Scholar] [CrossRef] [PubMed]
- Nael, K.; Bauer, A.H.; Hormigo, A.; Lemole, M.; Germano, I.M.; Puig, J.; Stea, B. Multiparametric MRI for Differentiation of Radiation Necrosis from Recurrent Tumor in Patients with Treated Glioblastoma. Am. J. Roentgenol. 2018, 210, 18–23. [Google Scholar] [CrossRef]
- Asao, C.; Korogi, Y.; Kitajima, M.; Hirai, T.; Baba, Y.; Makino, K.; Kochi, M.; Morishita, S.; Yamashita, Y. Diffusion-weighted imaging of radiation-induced brain injury for differentiation from tumor recurrence. AJNR Am. J. Neuroradiol. 2005, 26, 1455–1460. [Google Scholar]
- Fink, J.R.; Carr, R.B.; Matsusue, E.; Iyer, R.S.; Rockhill, J.K.; Haynor, D.R.; Maravilla, K.R. Comparison of 3 Tesla proton MR spectroscopy, MR perfusion and MR diffusion for distinguishing glioma recurrence from posttreatment effects. J. Magn. Reson. Imag. 2012, 35, 56–63. [Google Scholar] [CrossRef]
- Prah, M.A.; Al-Gizawiy, M.M.; Mueller, W.M.; Cochran, E.J.; Hoffmann, R.G.; Connelly, J.M.; Schmainda, K.M. Spatial discrimination of glioblastoma and treatment effect with histologically-validated perfusion and diffusion magnetic resonance imaging metrics. J. Neurooncol. 2018, 136, 13–21. [Google Scholar] [CrossRef]
- Iv, M.; Liu, X.; Lavezo, J.; Gentles, A.J.; Ghanem, R.; Lummus, S.; Born, D.E.; Soltys, S.G.; Nagpal, S.; Thomas, R.; et al. Perfusion MRI-Based Fractional Tumor Burden Differentiates between Tumor and Treatment Effect in Recurrent Glioblastomas and Informs Clinical Decision-Making. Am. J. Neuroradiol. 2019, 40, 1649–1657. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.Y.; Park, J.E.; Jo, Y.; Shim, W.H.; Nam, S.J.; Kim, J.H.; Yoo, R.-E.; Choi, S.H.; Kim, H.S. Incorporating diffusion- and perfusion-weighted MRI into a radiomics model improves diagnostic performance for pseudoprogression in glioblastoma patients. Neuro-Oncology 2019, 21, 404–414. [Google Scholar] [CrossRef] [PubMed]
- Park, J.E.; Ham, S.; Kim, H.S.; Park, S.Y.; Yun, J.; Lee, H.; Choi, S.H.; Kim, N. Diffusion and perfusion MRI radiomics obtained from deep learning segmentation provides reproducible and comparable diagnostic model to human in post-treatment glioblastoma. Eur. Radiol. 2021, 31, 3127–3137. [Google Scholar] [CrossRef] [PubMed]
- Ali, F.S.; Arevalo, O.; Zorofchian, S.; Patrizz, A.; Riascos, R.; Tandon, N.; Blanco, A.; Ballester, L.Y.; Esquenazi, Y. Cerebral Radiation Necrosis: Incidence, Pathogenesis, Diagnostic Challenges, and Future Opportunities. Curr. Oncol. Rep. 2019, 21, 66. [Google Scholar] [CrossRef]
| MRI Parameters | Total n = 44 (%) | Tumor (n = 16) [36%] | RIN (n = 28) [64%] | p-Value |
|---|---|---|---|---|
| rCBV | 1–14 (Median = 1.5) | 1–14 (Median = 5.25) | 1–5.6 (Median = 1) | p < 0.001 |
| ADC-R1 (Whole tumor including necrosis) (expressed as values × 10−6 mm2/s) | 900–2693 (Median = 1450) | 991–1923 (Median = 1334) | 900–2693 (Median =1639) | p < 0.001 |
| ADC-R2 (Only enhancing solid portion) (expressed as values × 10−6 mm2/s) | 536–2295 (Median = 1177) | 825–2060 (Median = 1219) | 536–2295 (Median =1155) | p = 0.709 |
| ADC-R3 (Only central necrosis portion) (expressed as values × 10−6 mm2/s) | 1379–3353 (Median = 2061) | 1643–3353 (Median = 2603) | 1379–2700 (Median =1843) | p < 0.001 |
| Variables | AUC | 95% CI | Optimal Cut-off | Sensitivity | Specificity | p-Value |
|---|---|---|---|---|---|---|
| rCBV | 0.930 | 0.811–0.985 | >3.4 | 81.25 | 89.29 | p < 0.001 |
| ADC-R1 (Whole tumor including necrosis) (expressed as values × 10−6 mm2/s) | 0.819 | 0.672–0.920 | ≤1416 | 87.50 | 77.78 | p < 0.001 |
| ADC-R2 (Only enhancing solid portion) (expressed as values × 10−6 mm2/s) | 0.504 | 0.350–0.659 | <536 | 0.00 | 100.00 | p = 0.9608 |
| ADC-R3 (Only central necrosis portion) (expressed as values × 10−6 mm2/s) | 0.844 | 0.703–0.935 | >2383 | 56.25 | 96.43 | p < 0.001 |
| Variables | AUC | 95% CI | p-Value for ROC Comparison | p-Value for ROC Comparison | p-Value for ROC Comparison |
|---|---|---|---|---|---|
| rCBV | 0.930 | 0.811–0.985 | rCBV & ADC-R2 p < 0.001 | ||
| ADC-R1 (Whole tumor including necrosis) (expressed as values × 10−6 mm2 /s) | 0.819 | 0.672–0.920 | ADC-R1 & ADC-R2 p < 0.001 | ||
| ADC-R2 (Only enhancing solid portion) (expressed as values × 10−6 mm2/s) | 0.504 | 0.350–0.659 | ADC-R2 & ADC-R3 p = 0.014 | ||
| ADC-R3 (Only central necrosis portion) (expressed as values × 10−6 mm2/s) | 0.844 | 0.703–0.935 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jajodia, A.; Goel, V.; Goyal, J.; Patnaik, N.; Khoda, J.; Pasricha, S.; Gairola, M. Combined Diagnostic Accuracy of Diffusion and Perfusion MR Imaging to Differentiate Radiation-Induced Necrosis from Recurrence in Glioblastoma. Diagnostics 2022, 12, 718. https://doi.org/10.3390/diagnostics12030718
Jajodia A, Goel V, Goyal J, Patnaik N, Khoda J, Pasricha S, Gairola M. Combined Diagnostic Accuracy of Diffusion and Perfusion MR Imaging to Differentiate Radiation-Induced Necrosis from Recurrence in Glioblastoma. Diagnostics. 2022; 12(3):718. https://doi.org/10.3390/diagnostics12030718
Chicago/Turabian StyleJajodia, Ankush, Varun Goel, Jitin Goyal, Nivedita Patnaik, Jeevitesh Khoda, Sunil Pasricha, and Munish Gairola. 2022. "Combined Diagnostic Accuracy of Diffusion and Perfusion MR Imaging to Differentiate Radiation-Induced Necrosis from Recurrence in Glioblastoma" Diagnostics 12, no. 3: 718. https://doi.org/10.3390/diagnostics12030718
APA StyleJajodia, A., Goel, V., Goyal, J., Patnaik, N., Khoda, J., Pasricha, S., & Gairola, M. (2022). Combined Diagnostic Accuracy of Diffusion and Perfusion MR Imaging to Differentiate Radiation-Induced Necrosis from Recurrence in Glioblastoma. Diagnostics, 12(3), 718. https://doi.org/10.3390/diagnostics12030718






