Working Algorithms and Detection Methods of Autoantibodies in Autoimmune Liver Disease: A Nationwide Study
Abstract
:1. Introduction
| Autoantibodies | Sensitivity | Specificity | Clinical Features | |
|---|---|---|---|---|
| Primary Biliary Cholangitis (PBC) | AMA-M2 | 84.5% [21] | 97.8% [21] | No difference in clinical features between AMA-positive and AMA-negative patients. |
| PDC-E2 | 80–90% [22,23] | |||
| OGDC-E2 | 20–60% [22,23] | |||
| BCOADC-E2 | 50–80% [22,23] | |||
| Sp100 | 21.3–24.9% [9] | 97.3–98.1% [9] | Severe disease and clinical outcome. Worse outcome and likely a more rapid progression of PBC. Marker of poor prognosis [9]. | |
| gp210 | 25.7–28.8% [9] | 98.2–98.8% [9] | Severe disease and clinical outcome. Hepatic failure-type progression [23]. Advanced stages of disease and faster disease progression rates. Marker of poor prognosis [9]. | |
| Autoimmune Hepatitis (AIH) | ANA | 23–83% [24] | 69–94% [24] | Diagnostic of AIH-1. Not associated with disease course or outcome. |
| SLA | 32.6% type 1 [25] | 100% [25] | Diagnostic of AIH-1. More severe disease and a worse outcome, propensity for relapse after corticosteroid withdrawal [26]. | |
| LKM-1 | 57% type 2 [27] | 100% [28] | Diagnostic of AIH-2 particularly in the absence of hepatitis C virus infection. Associated with younger age at presentation, more frequently associated with fulminant hepatic failure and with partial IgA deficiency [29]. | |
| LC-1 | 35% type 2 [30] | 98% [30] | Diagnostic of AIH-2 particularly in the absence of hepatitis C virus infection. Liver inflammation and rapid progression to cirrhosis [30]. | |
| SMA/F-Actin | 23–52% type 1 [24] | 93–100% [24] | SMA: Diagnostic of AIH type-1. Not associated with disease course or outcome; SMA/F-Actin: Associated with younger age at disease onset, treatment dependence in children. Predicts progression to liver failure and need for transplantation [31]. |
2. Materials and Methods
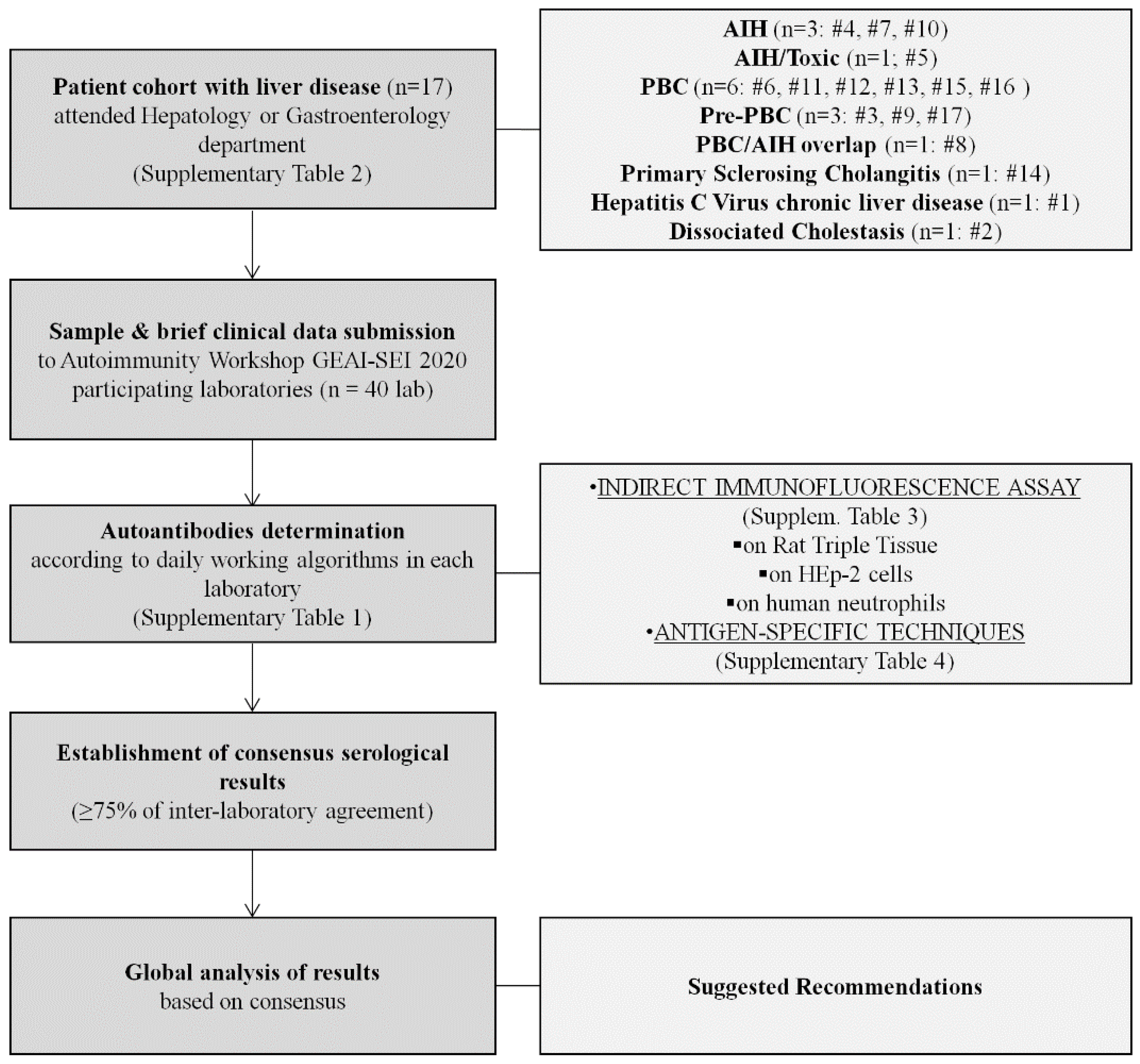
2.1. Autoimmunity Workshop GEAI-SEI 2020 Design
2.2. Patients
2.3. Methods
- (a)
- Indirect immunofluorescence assays:
- (b)
- Antigen-specific techniques (AgST):
2.4. Statistical Methods
3. Results
3.1. Working Algorithms
3.2. Autoimmune Hepatitis
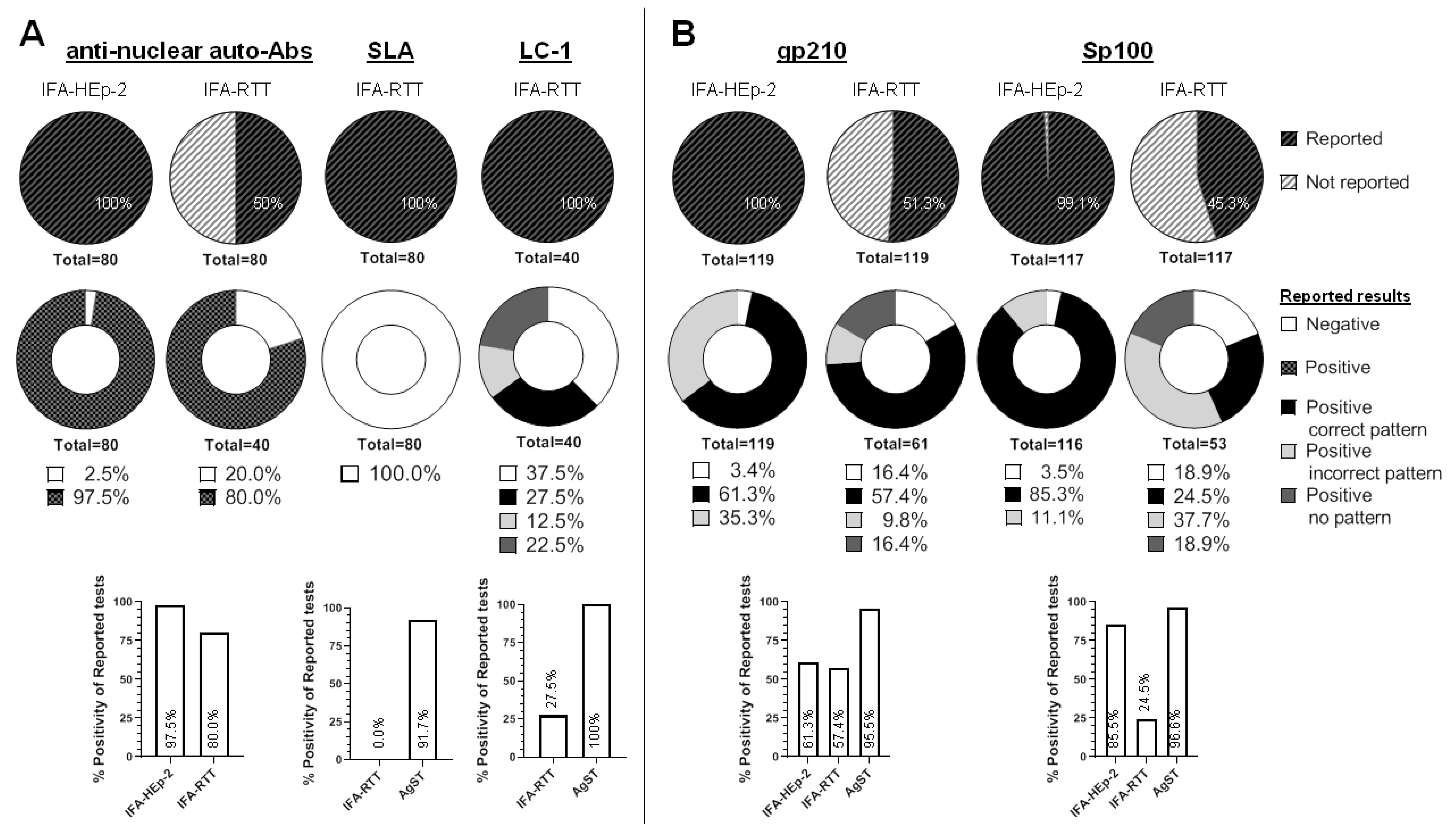
3.2.1. Anti-Nuclear Autoantibodies (ANA)
- Background
- Results
- Conclusions
3.2.2. Anti-Smooth Muscle/F-Actin (SMA/F-Actin) Autoantibodies
- Background
- Results
- Conclusions
3.2.3. Anti-Soluble Liver Antigen/Liver Pancreas (SLA) Autoantibodies
- Background
- Results
- Conclusions
3.2.4. Anti-Liver Cytosol Type-1 (LC1) Autoantibodies
- Background
- Results
- Conclusions
3.2.5. Anti-Liver Kidney Microsomal-1 Autoantibodies
3.3. Primary Biliary Cholangitis
3.3.1. Anti-gp210 Autoantibodies
- Background
- Results
- Conclusions
3.3.2. Anti-sp100 and Promyelocytic Leukaemia Protein (PML) Autoantibodies
- Background
- Results
- Conclusions
3.3.3. Anti-Mitochondrial (AMA) Autoantibodies
- Background
- Results
- Conclusions
3.4. Other Autoantibodies
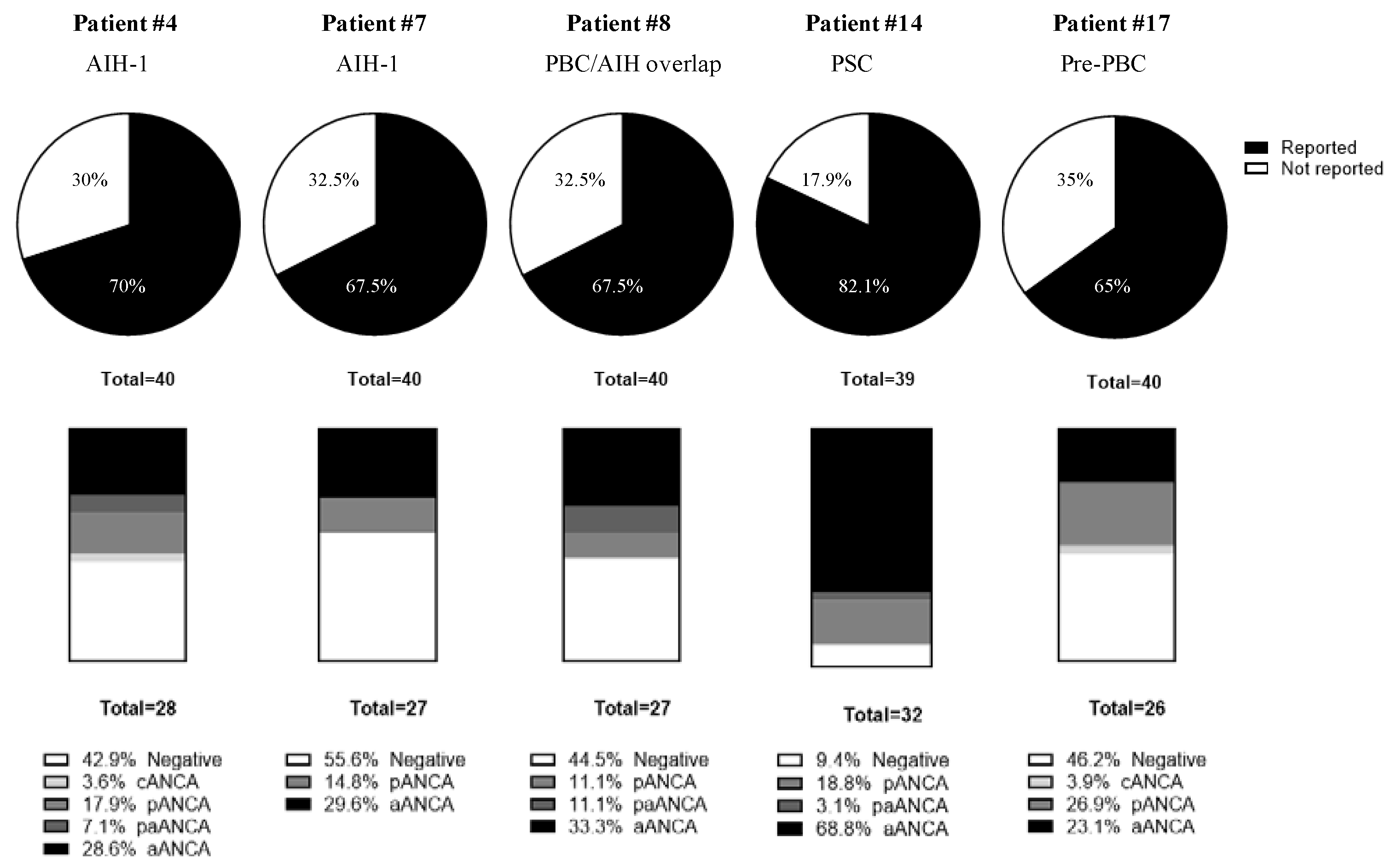
3.4.1. Anti-Neutrophil Cytoplasmic Autoantibodies
3.4.2. Anti-Ro52 Autoantibodies
3.4.3. Anti-Centromere Autoantibodies
4. Discussion
5. Conclusions
- Indirect immunofluorescence assays on rat triple tissue (IFA-RTT) and also on HEp-2 cells (IFA-HEp-2). The latter at least in the case of primary biliary cholangitis.
- Antigen-specific techniques, at least to detect the presence of anti-SLA autoantibodies, since it does not present a detectable pattern by IFA-RTT nor IFA-HEp-2, but ideally to confirm or rule out the presence of all autoantibodies whenever there is clinical suspicion.
- Study of anti-neutrophil cytoplasmic autoantibodies by IFA, if there is suspicion of autoimmune hepatitis or primary sclerosing cholangitis and the study performed at points 1 and 2 is negative.
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Christen, U.; Hintermann, E. Autoantibodies in autoimmune hepatitis: Can epitopes tell us about the etiology of the disease? Front. Immunol. 2018, 9, 163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sebode, M.; Weiler-Normann, C.; Liwinski, T.; Schramm, C. Autoantibodies in autoimmune liver disease-clinical and diagnostic relevance. Front. Immunol. 2018, 9, 609. [Google Scholar] [CrossRef] [Green Version]
- Younossi, Z.M.; Bernstein, D.; Shiffman, M.L.; Kwo, P.; Kim, W.R.; Kowdley, K.V.; Jacobson, I.M. Diagnosis and Management of Primary Biliary Cholangitis. Am. J. Gastroenterol. 2019, 114, 48–63. [Google Scholar] [CrossRef]
- Hirschfield, G.M.; Beuers, U.; Corpechot, C.; Invernizzi, P.; Jones, D.; Marzioni, M.; Schramm, C. EASL Clinical Practice Guidelines: The diagnosis and management of patients with primary biliary cholangitis. J. Hepatol. 2017, 67, 145–172. [Google Scholar] [CrossRef] [PubMed]
- Beenet, L. The major diagnostic role of autoantibodies in the diagnosis of autoimmune hepatitis, a disease of all ages. Clin. Mol. Hepatol. 2021, 27, 510–511. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, F.; Berg, P.A.; Bianchi, F.B.; Bianchi, L.; Burroughs, A.K.; Cancado, E.L.; Chapman, R.W.; Cooksley, W.G.E.; Czaja, A.J.; Desmet, V.J.; et al. International Autoimmune Hepatitis Group Report: Review of criteria for diagnosis of autoimmune hepatitis. J. Hepatol. 1999, 31, 929–938. [Google Scholar] [CrossRef]
- Taylor, S.A.; Assis, D.N.; MacK, C.L. The Contribution of B Cells in Autoimmune Liver Diseases. Semin. Liver Dis. 2019, 39, 422–431. [Google Scholar] [CrossRef]
- Mieli-Vergani, G.; Vergani, D.; Czaja, A.J.; Manns, M.P.; Krawitt, E.L.; Vierling, J.M.; Lohse, A.W.; Montano-Loza, A.J. Autoimmune hepatitis. Nature 2018, 4, 1–21. [Google Scholar] [CrossRef]
- Hu, S.L.; Zhao, F.R.; Hu, Q.; Chen, W.X. Meta-analysis assessment of GP210 and SP100 for the diagnosis of primary biliary cirrhosis. PLoS ONE 2014, 9, e0101916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Norman, G.L.; Reig, A.; Viñas, O.; Mahler, M.; Wunsch, E.; Milkiewicz, P.; Swain, M.G.; Mason, A.; Stinton, L.M.; Aparicio, M.B.; et al. The prevalence of anti-hexokinase-1 and anti-kelch-like 12 peptide antibodies in patients with primary biliary cholangitis is similar in Europe and North America: A large international, multi-center study. Front. Immunol. 2019, 10, 1–5. [Google Scholar] [CrossRef] [PubMed]
- Reig, A.; Norman, G.L.; Garcia, M.; Shums, Z.; Ruiz-Gaspà, S.; Bentow, C.; Mahler, M.; Romera, M.A.; Vinas, O.; Pares, A. Novel Anti–Hexokinase 1 Antibodies Are Associated With Poor Prognosis in Patients With Primary Biliary Cholangitis. Am. J. Gastroenterol. 2020, 115, 1634–1641. [Google Scholar] [CrossRef] [PubMed]
- Tabibian, J.H.; Bowlus, C.L. Primary sclerosing cholangitis: A review and update. Liver Res. 2017, 1, 221. [Google Scholar] [CrossRef] [PubMed]
- Nicoletti, A.; Maurice, J.B.; Thorburn, D. Guideline review: British Society of Gastroenterology/UK-PSC guidelines for the diagnosis and management of primary sclerosing cholangitis. Frontline Gastroenterol. 2021, 12, 62–66. [Google Scholar] [CrossRef] [PubMed]
- Terjung, B.; Söhne, J.; Lechtenberg, B.; Gottwein, J.; Muennich, M.; Herzog, V.; Mähler, M.; Sauerbruch, T.; Spengler, U. p-ANCAs in autoimmune liver disorders recognise human β-tubulin isotype 5 and cross-react with microbial protein FtsZ. Gut 2010, 59, 808–816. [Google Scholar] [CrossRef] [Green Version]
- Granito, A.; Muratori, P.; Muratori, L.; Pappas, G.; Cassani, F.; Worthington, J.; Ferri, S.; Quarneti, C.; Cipriano, V.; De Molo, C.; et al. Antibodies to SS-A/Ro-52kD and centromere in autoimmune liver disease: A clue to diagnosis and prognosis of primary biliary cirrhosis. Aliment. Pharmacol. Ther. 2007, 26, 831–838. [Google Scholar] [CrossRef] [PubMed]
- Dalekos, G.N.; Koskinas, J.; Papatheodoridis, G.V. Hellenic association for the study of the liver clinical practice guidelines: Autoimmune hepatitis. Ann. Gastroenterol. 2019, 32, 1–23. [Google Scholar] [CrossRef] [PubMed]
- Wunsch, E.; Norman, G.L.; Milkiewicz, M.; Krawczyk, M.; Bentow, C.; Shums, Z.; Mahler, M.; Lopens, S.; Reinhold, D.; Franke, A.; et al. Anti-glycoprotein 2 (anti-GP2) IgA and anti-neutrophil cytoplasmic antibodies to serine proteinase 3 (PR3-ANCA): Antibodies to predict severe disease, poor survival and cholangiocarcinoma in primary sclerosing cholangitis. Aliment. Pharmacol. Ther. 2021, 53, 302–313. [Google Scholar] [CrossRef]
- Crescente, J.G.; Dellavance, A.; Diniz, M.A.; Carrilho, F.J.; de Andrade, L.E.C.; Cancado, E.L.R. Antineutrophil cytoplasmic antibody profiles differ according to type of primary sclerosing cholangitis and autoimmune hepatitis. Clinics 2021, 76, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Peterson, L.K.; Tebo, A.E.; Wener, M.H.; Copple, S.S.; Fritzler, M.J. Assessment of antinuclear antibodies by indirect immunofluorescence assay: Report from a survey by the American Association of Medical Laboratory Immunologists. Clin. Chem. Lab. Med. 2020, 58, 1489–1497. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bogdanos, D.P.; Invernizzi, P.; Mackay, I.R.; Vergani, D. Autoimmune liver serology: Current diagnostic and clinical challenges. World J. Gastroenterol. 2008, 14, 3374. [Google Scholar] [CrossRef]
- Hu, S.; Zhao, F.; Wang, Q.; Chen, W.X. The accuracy of the anti-mitochondrial antibody and the M2 subtype test for diagnosis of primary biliary cirrhosis: A meta-analysis. Clin. Chem. Lab. Med. 2014, 52, 1533–1542. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M. Clinical significance of autoantibodies in primary biliary cirrhosis. Semin. Liver Dis. 2014, 34, 334–340. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Poyatos, E.; Morandeira, F.; Climent, J.; Mas, V.; Castellote, J.; Bas, J. Detection of anti-mitochondrial 2-oxoacid dehydrogenase complex subunit’s antibodies for the diagnosis of primary biliary cholangitis. Clin. Immunol. 2021, 108749. [Google Scholar] [CrossRef] [PubMed]
- Galaski, J.; Weiler-Normann, C.; Schakat, M.; Zachou, K.; Muratori, P.; Lampalzer, S.; Haag, F.; Schramm, C.; Lenzi, M.; Dalekos, G.N.; et al. Update of the simplified criteria for autoimmune hepatitis: Evaluation of the methodology for immunoserological testing. J. Hepatol. 2021, 74, 312–320. [Google Scholar] [CrossRef]
- Wies, I.; Brunner, S.; Henninger, J.; Herkel, J.; Kanzler, S.; zum Büschenfelde, K.-H.M.; Lohse, A.W. Identification of target antigen for SLA/LP autoantibodies in autoimmune hepatitis. Lancet 2000, 355, 1510–1515. [Google Scholar] [CrossRef]
- Liberal, R.; Mieli-Vergani, G.; Vergani, D. Clinical significance of autoantibodies in autoimmune hepatitis. J. Autoimmun. 2013, 46, 17–24. [Google Scholar] [CrossRef] [PubMed]
- Muratori, L.; Cataleta, M.; Muratori, P.; Lenzi, M.; Bianchi, F.B. Liver/kidney microsomal antibody type 1 and liver cytosol antibody type 1 concentrations in type 2 autoimmune hepatitis. Gut 1998, 42, 721–726. [Google Scholar] [CrossRef] [PubMed]
- Teubner, A.; Tillmann, H.L.; Schuppan, D.; Gericke, G.; Manns, M.P.; Stölzel, U. Prävalenz von zirkulierenden Autoantikörpern bei gesunden Individuen. Med. Klin. 2002, 97, 645–649. [Google Scholar] [CrossRef] [PubMed]
- Gregorio, G.V.; Portmann, B.; Reid, F.; Donaldson, P.T.; Doherty, D.G.; McCartney, M.; Mowat, A.P.; Vergani, D.; Mieli-Vergani, G. Autoimmune hepatitis in childhood: A 20-year experience. Hepatology 1997, 25, 541–547. [Google Scholar] [CrossRef] [PubMed]
- Martini, E.; Abuaf, N.; Cavalli, F.; Durand, V.; Johanet, C.; Homberg, J.-C. Antibody to liver cytosol (anti-LC1) in patients with autoimmune chronic active hepatitis type 2. Hepatology 1988, 8, 1662–1666. [Google Scholar] [CrossRef] [PubMed]
- Czaja, A.J.; Cassani, F.; Cataleta, M.; Valentini, P.; Bianchi, F.B. Frequency and significance of antibodies to actin in type 1 autoimmune hepatitis. Hepatology 1996, 24, 1068–1073. [Google Scholar] [CrossRef] [PubMed]
- Dellavance, A.; Cruvinel, W.M.; Francescantonio, P.L.C.; Coelho Andrade, L.E. Manual of Molecular and Clinical Laboratory Immunology, 8th ed.; ASM Press: Washington, DC, USA, 2016; Chapter 87; pp. 843–858. [Google Scholar]
- Chan, E.K.L.; Damoiseaux, J.; Carballo, O.G.; de Melo Cruvinel, W.; Conrad, K.; Herold, M.; von Mühlen, C.A.; Garcia-De La Torre, I.; Andrade, L.E.C.; Fritzler, M.J.; et al. Report of the First International Consensus on Standardized Nomenclature of Antinuclear Antibody HEp-2 Cell Patterns 2014–2015. Front. Immunol. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burlingame, R.W.; Buchner, C.E.; Hanly, J.G.; Walsh, N.M. Manual of Molecular and Clinical Laboratory Immunology, 8th ed.; ASM Press: Washington, DC, USA, 2016; Chapter 94; pp. 909–916. [Google Scholar]
- Chan, E.K.L.; Burlingame, R.W.; Fritzler, M.J. Manual of Molecular and Clinical Laboratory Immunology, 8th ed.; ASM Press: Washington, DC, USA, 2016; Chapter 88; pp. 859–867. [Google Scholar]
- Heneghan, M.A.; Yeoman, A.D.; Verma, S.; Smith, A.D.; Longhi, M.S. Autoimmune hepatitis. Lancet 2013, 382, 1433–1444. [Google Scholar] [CrossRef]
- Boberg, K.M.; Aadland, E.; Jahnsen, J.; Raknerud, N.; Stiris, M.; Bell, H. Incidence and Prevalence of Primary Biliary Cirrhosis, Primary Sclerosing Cholangitis, and Autoimmune Hepatitis in a Norwegian Population. Scand. J. Gastroenterol. 1998, 33, 99–103. [Google Scholar] [CrossRef] [PubMed]
- Mack, C.L.; Adams, D.; Assis, D.N.; Kerkar, N.; Manns, M.P.; Mayo, M.J.; Vierling, J.M.; Alsawas, M.; Murad, M.H.; Czaja, A.J. Diagnosis and Management of Autoimmune Hepatitis in Adults and Children: 2019 Practice Guidance and Guidelines From the American Association for the Study of Liver Diseases. Hepatology 2020, 72, 671–722. [Google Scholar] [CrossRef]
- Lohse, A.W.; Chazouillères, O.; Dalekos, G.; Drenth, J.; Heneghan, M.; Hofer, H.; Lammert, F.; Lenzi, M. EASL Clinical Practice Guidelines: Autoimmune hepatitis. J. Hepatol. 2015, 63, 971–1004. [Google Scholar] [CrossRef]
- Homberg, J.-C.; Abuaf, N.; Bernard, O.; Islam, S.; Alvarez, F.; Khalil, S.H.; Poupon, R.; Darnis, F.; Lévy, V.-G.; Grippon, P.; et al. Chronic active hepatitis associated with antiliver/kidney microsome antibody type 1: A second type of “autoimmune” hepatitis. Hepatology 1987, 7, 1333–1339. [Google Scholar] [CrossRef] [PubMed]
- Mackay, I.; Taft, L.I.; Cowling, D.C. Lupoid hepatitis. Lancet 1956, 268, 1323–1326. [Google Scholar] [CrossRef]
- Vergani, D.; Alvarez, F.; Bianchi, F.B.; Cançado, E.L.R.; Mackay, I.R.; Manns, M.P.; Nishioka, M.; Penner, E. Liver autoimmune serology: A consensus statement from the committee for autoimmune serology of the International Autoimmune Hepatitis Group. J. Hepatol. 2004, 41, 677–683. [Google Scholar] [CrossRef] [PubMed]
- Agmon-Levin, N.; Damoiseaux, J.; Kallenberg, C.; Sack, U.; Witte, T.; Herold, M.; Bossuyt, X.; Musset, L.; Cervera, R.; Plaza-Lopez, A.; et al. International recommendations for the assessment of autoantibodies to cellular antigens referred to as anti-nuclear antibodies. Ann. Rheum. Dis. 2014, 73, 17–23. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tan, E.M.; Feltkamp, T.E.W.; Smolen, J.S.; Butcher, B.; Dawkins, R.; Fritzler, M.J.; Gordon, T.; Hardin, J.A.; Kalden, J.R.; Lahita, R.G.; et al. Range of antinuclear antibodies in “healthy” individuals. Arthritis Rheum. 1997, 40, 1601–1611. [Google Scholar] [CrossRef] [PubMed]
- Johnson, G.D.; Holborow, E.J.; Glynn, L.E. Antibody to smooth muscle in patients with liver disease. Lancet 1965, 2, 878–879. [Google Scholar] [CrossRef]
- Johanet, C.; Ballot, E. Auto-antibodies in autoimmune hepatitis: Anti-smooth muscle antibodies (ASMA). Clin. Res. Hepatol. Gastroenterol. 2012, 36, 189–191. [Google Scholar] [CrossRef] [PubMed]
- Bottazzo, G.F.; Florin-Christensen, A.; Fairfax, A.; Swana, G.; Doniach, D.; Groeschel-Stewart, U. Classification of smooth muscle autoantibodies detected by immunofluorescence. J. Clin. Pathol. 1976, 29, 403–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liaskos, C.; Bogdanos, D.-P.; Davies, E.T.; Dalekos, G.N. Diagnostic relevance of anti-filamentous actin antibodies in autoimmune hepatitis. J. Clin. Pathol. 2007, 60, 107–108. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vitozzi, S.; Lapierre, P.; Djilali-Saiah, I.; Marceau, G.; Beland, K.; Alvarez, F. Anti-soluble liver antigen (SLA) antibodies in chronic HCV infection. Autoimmunity 2004, 37, 217–222. [Google Scholar] [CrossRef] [PubMed]
- Lapierre, P.; Hajoui, O.; Homberg, J.C.; Alvarez, F. Formiminotransferase cyclodeaminase is an organ-specific autoantigen recognized by sera of patients with autoimmune hepatitis. Gastroenterology 1999, 116, 643–649. [Google Scholar] [CrossRef]
- Lenzi, M.; Manotti, P.; Muratori, L.; Cataleta, M.; Ballardini, G.; Cassani, F.; Bianchi, F.B. Liver cytosolic 1 antigen-antibody system in type 2 autoimmune hepatitis and hepatitis C virus infection. Gut 1995, 36, 749–754. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lleo, A.; Wang, G.Q.; Gershwin, M.E.; Hirschfield, G.M. Primary biliary cholangitis. Lancet 2020, 396, 1915–1926. [Google Scholar] [CrossRef]
- Leung, P.S.C.; Choi, J.; Yang, G.; Woo, E.; Kenny, T.P.; Gershwin, M.E. A contemporary perspective on the molecular characteristics of mitochondrial autoantigens and diagnosis in primary biliary cholangitis. Expert Rev. Mol. Diagn. 2016, 16, 697–705. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tanaka, A. Current understanding of primary biliary cholangitis. Clin. Mol. Hepatol. 2021, 27, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Wesierska-Gadek, J.; Klima, A.; Komina, O.; Ranftler, C.; Invernizzi, P.; Penner, E. Characterization of Autoantibodies against Components of the Nuclear Pore Complexes: High Frequency of Anti-p62 Nucleoporin Antibodies. Ann. N. Y. Acad. Sci. 2007, 1109, 519–530. [Google Scholar] [CrossRef] [PubMed]
- Kraemer, D.M.; Tony, H.-P. Nuclear Pore Protein p62 Autoantibodies in Systemic Lupus Erythematosus~!2010-03-08~!2010-04-19~!2010-05-12~! Open Rheumatol. J. 2010, 4, 24–27. [Google Scholar] [CrossRef] [PubMed]
- Courvalin, J.C.; Lassoued, K.; Worman, H.J.; Blobel, G. Identification and characterization of autoantibodies against the nuclear envelope lamin B receptor from patients with primary biliary cirrhosis. J. Exp. Med. 1990, 172, 961–967. [Google Scholar] [CrossRef] [Green Version]
- Ou, Y.; Enarson, P.; Rattner, J.B.; Barr, S.G.; Fritzler, M.J. The nuclear pore complex protein Tpr is a common autoantigen in sera that demonstrate nuclear envelope staining by indirect immunofluorescence. Clin. Exp. Immunol. 2004, 136, 379–387. [Google Scholar] [CrossRef] [PubMed]
- Conrad, K.; WSchößler, F.; Hiepe, M.F. Autoantibodies in Organ Specific Autoimmune Diseases: A Diagnostic Reference; PABST, Wolfgang Science: Lengerich, Germany, 2011. [Google Scholar]
- Wichmann, I.; Montes-Cano, M.A.; Respaldiza, N.; Alvarez, A.; Walter, K.; Franco, E.; Núñez-Roldán, A. Clinical Significance of Anti-Multiple Nuclear Dots/Sp100 Autoantibodies. Scand. J. Gastroenterol. 2003, 38, 996–999. [Google Scholar] [CrossRef] [PubMed]
- Diagnóstico y Monitorización de las Enfermedades Autoinmunes: Sociedad Española de Inmunología; Fernández Pereira, L.; Plaza López, A. (Eds.) Elsevier: Marid, Spain, 2018; ISBN 9788491132448. [Google Scholar]
- Gkoutzourelas, A.; Liaskos, C.; Mytilinaiou, M.G.; Simopoulou, T.; Katsiari, C.; Tsirogianni, A.; Daoussis, D.; Scheper, T.; Meyer, W.; Bogdanos, D.P.; et al. Anti-Ro60 Seropositivity Determines Anti-Ro52 Epitope Mapping in Patients With Systemic Sclerosis. Front. Immunol. 2018, 9, 2835. [Google Scholar] [CrossRef] [Green Version]
- Zachou, K.; Gampeta, S.; Gatselis, N.K.; Oikonomou, K.; Goulis, J.; Manoussakis, M.N.; Renaudineau, Y.; Bogdanos, D.P.; Dalekos, G.N. Anti-SLA/LP alone or in combination with anti-Ro52 and fine specificity of anti-Ro52 antibodies in patients with autoimmune hepatitis. Liver Int. 2015, 35, 660–672. [Google Scholar] [CrossRef] [PubMed]
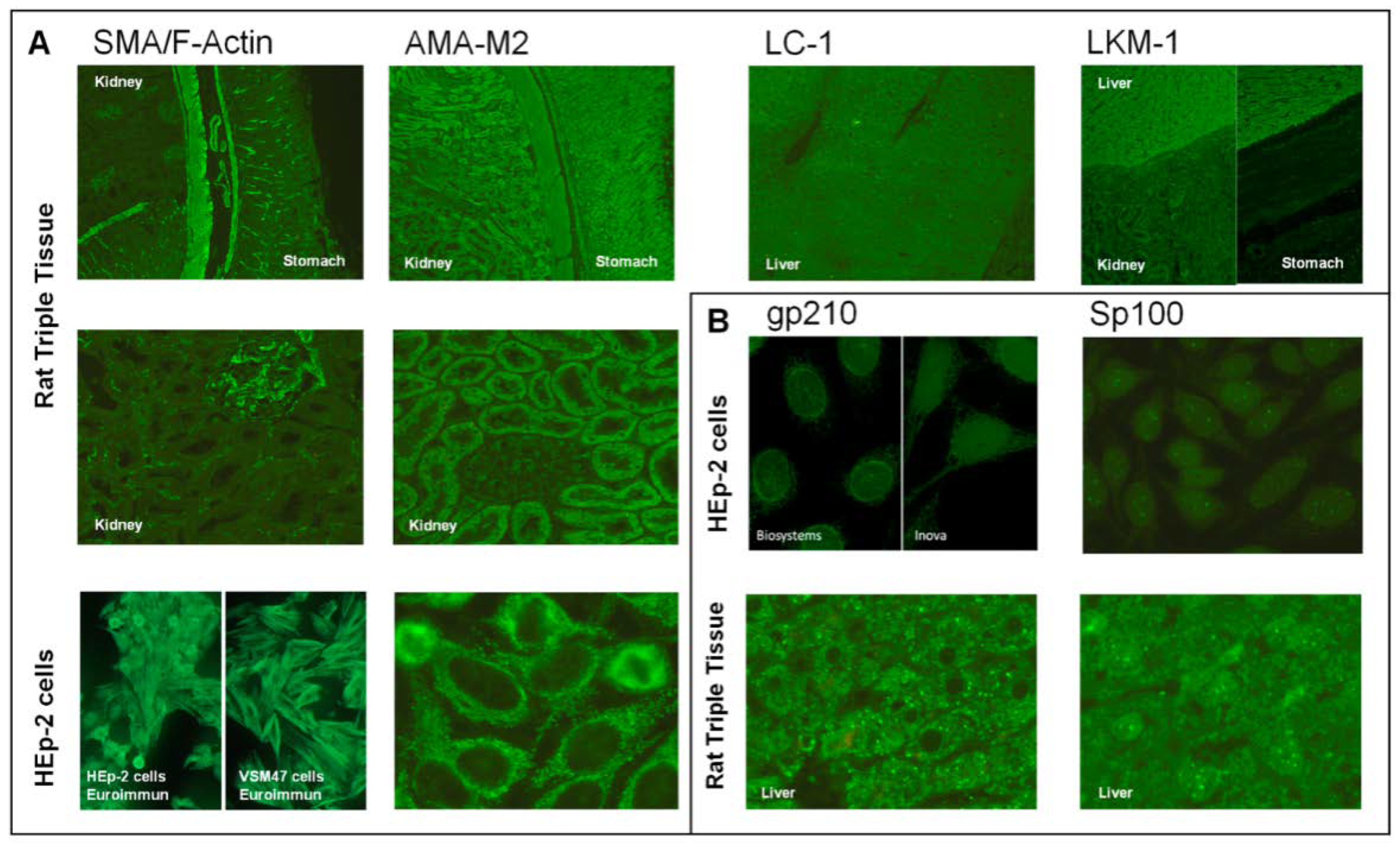
| Indirect Immunofluorescence Assay | Antigen-Specific Techniques | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Rat Triple Tissue | HEp-2 Cells | |||||||||||
| anti-SMA/F-Actin autoantibodies | Pt | Diagnosis | SMA | SMA/F-Actin | AC-15/16/17 | Total Performed | IFA EI VSM-47 | DB AP | DB OR | DB DT | ELISA WI | Unknown |
| % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | % (n) | |||
| 5 | Toxic AIH | 2 | 0 | 0 | 33 | 0% | 0 | 0 | 0 | 0 | 0 | |
| (1/39) | (0/39) | (0/38) | (13/39) | (0/2) | (0/2) | (0/1) | (0/4) | (0/2) | (0/2) | |||
| 4 | AIH-1 | 5 | 0 | 0 | 33 | 0 | 0 | 0 | 0 | 33 | 0 | |
| (2/40) | (0/40) | (0/38) | (13/39) | (0/2) | (0/2) | (0/0) | (0/4) | (1/3) | (0/2) | |||
| 7 | AIH-1 | 93 | 13 | 13 | 64 | 50 | 100 | 0 | 40 | 100 | 20 | |
| (37/40) | (5/40) | (5/39) | (25/40) | (4/8) | (2/2) | (0/1) | (2/5) | (4/4) | (1/5) | |||
| 2 | Dissociated cholestasis | 15 | 0 | 0 | 33 | 50 | 0 | 0 | 0 | 0 | 0 | |
| (6/39) | (0/39) | (0/39) | (13/39) | (1/2) | (0/2) | (0/1) | (0/4) | (0/2) | (0/2) | |||
| 1 | Chronic hepatopathy | 75 | 7 | 18 | 51 | 66 | 100 | 100 | 66 | 100 | 20 | |
| (29/39) | (3/39) | (7/39) | (20/39) | (4/6) | (2/2) | (1/1) | (2/3) | (3/3) | (1/5) | |||
| 10 | AIH-2 | 0 | 0 | 2 | 33 | 0 | 0 | 0 | 0 | 0 | 0 | |
| (0/40) | (0/40) | (1/38) | (13/39) | (0/2) | (0/2) | (0/1) | (0/4) | (0/2) | (0/2) | |||
| 8 | PBC/AIH-1 | 93 | 25 | 36 | 66 | 100 | 50 | 100 | 100 | 100 | 50 | |
| (37/40) | (10/40) | (14/39) | (26/40) | (9/9) | (1/2) | (1/1) | (4/4) | (4/4) | (3/6) | |||
| Anti-Mitochondrial Autoantibodies | Anti-Gastric Parietal Cells Autoantibodies | |||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Indirect Immunofluorescence Assay | Antigen-Specific Techniques | Rat Triple Tissue | Other Methods | |||||||||||||
| Rat Triple Tissue | HEp-2 Cells | |||||||||||||||
| Pt | Diagnosis | AMA % (n) | AMA-M2 % (n) | AC-21 % (n) | Total % (n) | AMA Native % (n) | AMA-M2 % (n) | M2-3E2 % (n) | PDC-E2 % (n) | BCOADC E2 % (n) | OGDC E2 % (n) | Negative % (n) | Positive % (n) | Not Valuable % (n) | Total % (n) | % (n) |
| 11 | PBC | 100 | 15 | 83 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 77 | 5 | 18 | 5 | 0 |
| (40/40) | (6/40) | (33/40) | (40/40) | (6/6) | (32/32) | (29/29) | (6/6) | (0/6) | (0/6) | (30/39) | (2/39) | (7/39) | (2/39) | (0/2) | ||
| 12 | PBC | 100 | 15 | 85 | 100 | 100 | 97 | 100 | 100 | 100 | 0 | 69 | 15 | 15 | 8 | 100 |
| (40/40) | (6/40) | (34/40) | (40/40) | (7/7) | (30/31) | (28/28) | (6/6) | (6/6) | (0/6) | (27/39) | (6/39) | (6/39) | (3/39) | (3/3) | ||
| 13 | PBC | 77 | 10 | 72 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 82 | 8 | 11 | 3 | 0 |
| (30/39) | (4/39) | (28/39) | (39/39) | (7/7) | (32/32) | (29/29) | (6/6) | (0/6) | (0/6) | (31/38) | (3/38) | (4/38) | (1/38) | (0/1) | ||
| 15 | PBC | 97 | 13 | 85 | 100 | 100 | 100 | 100 | 100 | 0 | 0 | 76 | 5 | 18 | 5 | 0 |
| (38/39) | (5/39) | (33/39) | (39/39) | (7/7) | (32/32) | (28/28) | (6/6) | (0/6) | (0/6) | (29/38) | (2/38) | (7/38) | (2/38) | (0/2) | ||
| 16 | PBC | 100 | 15 | 85 | 100 | 100 | 100 | 100 | 100 | 100 | 0 | 76 | 5 | 18 | 5 | 0 |
| (39/39) | (6/39) | (33/39) | (39/39) | (7/7) | (31/31) | (28/28) | (6/6) | (6/6) | (0/6) | (29/38) | (2/38) | (7/38) | (2/38) | (0/2) | ||
| 6 | PBC | 5 | 0 | 3 | 100 | 0 | 0 | 71 | 0 | 0 | 0 | 100 | 0 | 0 | 0 | - |
| (2/40) | (0/40) | (1/40) | (40/40) | (0/6) | (0/29) | (20/28) | (0/5) | (0/5) | (0/5) | (39/39) | (0/39) | (0/39) | (0/39) | |||
| 8 | PBC/AIH-1 | 25 | 0 | 45 | 100 | 0 | 0 | 77 | 0 | 100 | 0 | 87 | 8 | 5 | 3 | 100 |
| (10/40) | (0/40) | (18/40) | (40/40) | (0/7) | (0/30) | (23/30) | (0/6) | (6/6) | (0/6) | (34/39) | (3/39) | (2/39) | (1/39) | (1/1) | ||
| 3 | Pre-PBC | 33 | 5 | 8 | 100 | 0 | 0 | 0 | 0 | 50 | 0 | 87 | 10 | 3 | 0 | - |
| (13/40) | (2/40) | (3/40) | (40/40) | (0/5) | (0/32) | (0/28) | (0/4) | (2/4) | (0/4) | (34/39) | (4/39) | (1/39) | (0/39) | |||
| 9 | Pre-PBC | 67 | 8 | 26 | 100 | 14 | 28 | 59 | 0 | 100 | 0 | 32 | 58 | 11 | 18 | 100 |
| (26/39) | (3/39) | (10/39) | (39/39) | (1/7) | (9/32) | (17/29) | (0/6) | (6/6) | (0/6) | (12/38) | (22/38) | (4/38) | (7/38) | (7/7) | ||
| 17 | Pre-PBC | 13 | 3 | 15 | 100 | 60 | 20 | 37 | 0 | 25 | 50 | 100 | 0 | 0 | 0 | - |
| (5/40) | (1/40) | (6/40) | (40/40) | (3/5) | (6/30) | (11/30) | (0/4) | (1/4) | (2/4) | (39/39) | (0/39) | (0/39) | (0/39) | |||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Muñoz-Sánchez, G.; Pérez-Isidro, A.; Ortiz de Landazuri, I.; López-Gómez, A.; Bravo-Gallego, L.Y.; Garcia-Ormaechea, M.; Julià, M.R.; Viñas, O.; Ruiz-Ortiz, E.; on behalf of the 2020 GEAI-SEI Workshop Participants. Working Algorithms and Detection Methods of Autoantibodies in Autoimmune Liver Disease: A Nationwide Study. Diagnostics 2022, 12, 697. https://doi.org/10.3390/diagnostics12030697
Muñoz-Sánchez G, Pérez-Isidro A, Ortiz de Landazuri I, López-Gómez A, Bravo-Gallego LY, Garcia-Ormaechea M, Julià MR, Viñas O, Ruiz-Ortiz E, on behalf of the 2020 GEAI-SEI Workshop Participants. Working Algorithms and Detection Methods of Autoantibodies in Autoimmune Liver Disease: A Nationwide Study. Diagnostics. 2022; 12(3):697. https://doi.org/10.3390/diagnostics12030697
Chicago/Turabian StyleMuñoz-Sánchez, Guillermo, Albert Pérez-Isidro, Iñaki Ortiz de Landazuri, Antonio López-Gómez, Luz Yadira Bravo-Gallego, Milagros Garcia-Ormaechea, Maria Rosa Julià, Odette Viñas, Estíbaliz Ruiz-Ortiz, and on behalf of the 2020 GEAI-SEI Workshop Participants. 2022. "Working Algorithms and Detection Methods of Autoantibodies in Autoimmune Liver Disease: A Nationwide Study" Diagnostics 12, no. 3: 697. https://doi.org/10.3390/diagnostics12030697
APA StyleMuñoz-Sánchez, G., Pérez-Isidro, A., Ortiz de Landazuri, I., López-Gómez, A., Bravo-Gallego, L. Y., Garcia-Ormaechea, M., Julià, M. R., Viñas, O., Ruiz-Ortiz, E., & on behalf of the 2020 GEAI-SEI Workshop Participants. (2022). Working Algorithms and Detection Methods of Autoantibodies in Autoimmune Liver Disease: A Nationwide Study. Diagnostics, 12(3), 697. https://doi.org/10.3390/diagnostics12030697






