Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study
Abstract
:1. Introduction
2. Patients and Methods
2.1. Design of the Study
2.2. Measurement of Serum Biomarkers
2.3. Statistical Analysis
3. Results
3.1. Patients—Serum Samples
3.2. Histology
3.3. Serum Biomarkers Testing Results
3.4. Diagnostic Performance in Patients without PPI Therapy
3.5. Comparison between H. pylori-Positive and H. pylori-Negative Patients
3.6. Comparison between the Results of the Previous Study (Gastropanel®) and the Current Study (CLEIA Fujirebio®)
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Arnold, M.; Park, J.Y.; Camargo, M.C.; Lunet, N.; Forman, D.; Soerjomataram, I. Is gastric cancer becoming a rare disease? A global assessment of predicted incidence trends to 2035. Gut 2020, 69, 823–829. [Google Scholar] [CrossRef]
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries: Global Cancer Statistics 2018. CA. Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapelle, N.; Manfredi, S.; Lepage, C.; Faivre, J.; Bouvier, A.-M.; Jooste, V. Erratum to: Trends in gastric cancer incidence: A period and birth cohort analysis in a well-defined French population. Gastric Cancer 2016, 19, 682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapelle, N.; Bouvier, A.-M.; Manfredi, S.; Drouillard, A.; Lepage, C.; Faivre, J.; Jooste, V. Early Gastric Cancer: Trends in Incidence, Management, and Survival in a Well-Defined French Population. Ann. Surg. Oncol. 2016, 23, 3677–3683. [Google Scholar] [CrossRef]
- Correa, P.; Haenszel, W.; Cuello, C.; Tannenbaum, S.; Archer, M. A model for gastric cancer epidemiology. Lancet Lond. Engl. 1975, 2, 58–60. [Google Scholar] [CrossRef]
- Correa, P. A human model of gastric carcinogenesis. Cancer Res. 1988, 48, 3554–3560. [Google Scholar] [PubMed]
- de Vries, A.C.; van Grieken, N.C.T.; Looman, C.W.N.; Casparie, M.K.; de Vries, E.; Meijer, G.A.; Kuipers, E.J. Gastric cancer risk in patients with premalignant gastric lesions: A nationwide cohort study in the Netherlands. Gastroenterology 2008, 134, 945–952. [Google Scholar] [CrossRef]
- Song, H.; Ekheden, I.G.; Zheng, Z.; Ericsson, J.; Nyrén, O.; Ye, W. Incidence of gastric cancer among patients with gastric precancerous lesions: Observational cohort study in a low risk Western population. BMJ 2015, 351, h3867. [Google Scholar] [CrossRef] [Green Version]
- Chapelle, N.; Péron, M.; Mosnier, J.-F.; Quénéhervé, L.; Coron, E.; Bourget, A.; Cauchin, E.; Touchefeu, Y.; Matysiak-Budnik, T. Prevalence, Characteristics and Endoscopic Management of Gastric Premalignant Lesions in France. Dig. Dis. 2020, 38, 286–292. [Google Scholar] [CrossRef]
- den Hoed, C.; Holster, I.; Capelle, L.; de Vries, A.; den Hartog, B.; ter Borg, F.; Biermann, K.; Kuipers, E. Follow-up of premalignant lesions in patients at risk for progression to gastric cancer. Endoscopy 2013, 45, 249–256. [Google Scholar] [CrossRef]
- den Hollander, W.J.; Holster, I.L.; den Hoed, C.M.; Capelle, L.G.; Tang, T.J.; Anten, M.-P.; Prytz-Berset, I.; Witteman, E.M.; ter Borg, F.; den Hartog, G.; et al. Surveillance of premalignant gastric lesions: A multicentre prospective cohort study from low incidence regions. Gut 2019, 68, 585–593. [Google Scholar] [CrossRef] [PubMed]
- Miki, K. Gastric cancer screening by combined assay for serum anti-Helicobacter pylori IgG antibody and serum pepsinogen levels—“ABC method”. Proc. Jpn. Acad. Ser. B Phys. Biol. Sci. 2011, 87, 405–414. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pimentel-Nunes, P.; Libânio, D.; Marcos-Pinto, R.; Areia, M.; Leja, M.; Esposito, G.; Garrido, M.; Kikuste, I.; Megraud, F.; Matysiak-Budnik, T.; et al. Management of epithelial precancerous conditions and lesions in the stomach (MAPS II): European Society of Gastrointestinal Endoscopy (ESGE), European Helicobacter and Microbiota Study Group (EHMSG), European Society of Pathology (ESP), and Sociedade Portuguesa de Endoscopia Digestiva (SPED) guideline update 2019. Endoscopy 2019, 51, 365–388. [Google Scholar] [PubMed] [Green Version]
- Storskrubb, T.; Aro, P.; Ronkainen, J.; Sipponen, P.; Nyhlin, H.; Talley, N.J.; Engstrand, L.; Stolte, M.; Vieth, M.; Walker, M.; et al. Serum biomarkers provide an accurate method for diagnosis of atrophic gastritis in a general population: The Kalixanda study. Scand. J. Gastroenterol. 2008, 43, 1448–1455. [Google Scholar] [CrossRef] [PubMed]
- SyrjäNen, K. A Panel of Serum Biomarkers (GastroPanel®) in Non-invasive Diagnosis of Atrophic Gastritis. Systematic Review and Meta-analysis. Anticancer Res. 2016, 36, 5133–5144. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chapelle, N.; Petryszyn, P.; Blin, J.; Leroy, M.; Le Berre-Scoul, C.; Jirka, I.; Neunlist, M.; Moussata, D.; Lamarque, D.; Olivier, R.; et al. A panel of stomach-specific biomarkers (GastroPanel®) for the diagnosis of atrophic gastritis: A prospective, multicenter study in a low gastric cancer incidence area. Helicobacter 2020, 25, e12727. [Google Scholar] [CrossRef]
- Kelesidis, I.; Kelesidis, T.; Mantzoros, C.S. Adiponectin and cancer: A systematic review. Br. J. Cancer 2006, 94, 1221–1225. [Google Scholar] [CrossRef]
- Inagaki, Y.; Xu, H.; Nakata, M.; Seyama, Y.; Hasegawa, K.; Sugawara, Y.; Tang, W.; Kokudo, N. Clinicopathology of sialomucin: MUC1, particularly KL-6 mucin, in gastrointestinal, hepatic and pancreatic cancers. Biosci. Trends 2009, 3, 220–232. [Google Scholar]
- Chen, G.; Tang, N.; Wang, C.; Xiao, L.; Yu, M.; Zhao, L.; Cai, H.; Han, L.; Xie, C.; Zhang, Y. TNF-α-inducing protein of Helicobacter pylori induces epithelial-mesenchymal transition (EMT) in gastric cancer cells through activation of IL-6/STAT3 signaling pathway. Biochem. Biophys. Res. Commun. 2017, 484, 311–317. [Google Scholar] [CrossRef] [PubMed]
- Nozaki, K.; Ogawa, M.; Williams, J.A.; Lafleur, B.J.; Ng, V.; Drapkin, R.I.; Mills, J.C.; Konieczny, S.F.; Nomura, S.; Goldenring, J.R. A Molecular Signature of Gastric Metaplasia Arising in Response to Acute Parietal Cell Loss. Gastroenterology 2008, 134, 511–522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dixon, M.F.; Genta, R.M.; Yardley, J.H.; Correa, P. Classification and grading of gastritis. The updated Sydney System. International Workshop on the Histopathology of Gastritis, Houston 1994. Am. J. Surg. Pathol. 1996, 20, 1161–1181. [Google Scholar] [CrossRef] [PubMed]
- Osumi, H.; Fujisaki, J.; Suganuma, T.; Horiuchi, Y.; Omae, M.; Yoshio, T.; Ishiyama, A.; Tsuchida, T.; Miki, K. A significant increase in the pepsinogen I/II ratio is a reliable biomarker for successful Helicobacter pylori eradication. PLoS ONE 2017, 12, e0183980. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kishino, T.; Oyama, T.; Tomori, A.; Takahashi, A.; Shinohara, T. Usefulness and Limitations of a Serum Screening System to Predict the Risk of Gastric Cancer. Intern. Med. 2020, 59, 1473–1480. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zagari, R.M.; Rabitti, S.; Greenwood, D.C.; Eusebi, L.H.; Vestito, A.; Bazzoli, F. Systematic review with meta-analysis: Diagnostic performance of the combination of pepsinogen, gastrin-17 and anti- Helicobacter pylori antibodies serum assays for the diagnosis of atrophic gastritis. Aliment. Pharmacol. Ther. 2017, 46, 657–667. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cinquanta, L.; Fontana, D.E.; Bizzaro, N. Chemiluminescent immunoassay technology: What does it change in autoantibody detection? Autoimmun. Highlights 2017, 8, 9. [Google Scholar] [CrossRef] [PubMed]
- Leja, M.; Camargo, M.C.; Polaka, I.; Isajevs, S.; Liepniece-Karele, I.; Janciauskas, D.; Rudzite, D.; Kikuste, I.; Vanags, A.; Kojalo, I.; et al. Detection of gastric atrophy by circulating pepsinogens: A comparison of three assays. Helicobacter 2017, 22, e12393. [Google Scholar] [CrossRef] [PubMed]
- Mera, R.M.; Bravo, L.E.; Camargo, M.C.; Bravo, J.C.; Delgado, A.G.; Romero-Gallo, J.; Yepez, M.C.; Realpe, J.L.; Schneider, B.G.; Morgan, D.R.; et al. Dynamics of Helicobacter pylori infection as a determinant of progression of gastric precancerous lesions: 16-year follow-up of an eradication trial. Gut 2018, 67, 1239–1246. [Google Scholar] [CrossRef] [PubMed]
- Chapelle, N.; Péron, M.; Quénéhervé, L.; Bourget, A.; Leroy, M.; Touchefeu, Y.; Cauchin, E.; Coron, E.; Mosnier, J.F.; Matysiak-Budnik, T. Long-Term Follow-up of Gastric Precancerous Lesions in a Low GC Incidence Area. Clin. Transl. Gastroenterol. 2020, 11, e00237. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Sanz-Anquela, J.M.; Companioni, O.; Bonet, C.; Berdasco, M.; López, C.; Mendoza, J.; Martín-Arranz, M.D.; Rey, E.; Poves, E.; et al. Incomplete type of intestinal metaplasia has the highest risk to progress to gastric cancer: Results of the Spanish follow-up multicenter study: Incomplete type of intestinal metaplasia. J. Gastroenterol. Hepatol. 2016, 31, 953–958. [Google Scholar] [CrossRef] [PubMed]
- Dinis-Ribeiro, M.; Lopes, C.; da Costa-Pereira, A.; Guilherme, M.; Barbosa, J.; Lomba-Viana, H.; Silva, R.; Moreira-Dias, L. A follow up model for patients with atrophic chronic gastritis and intestinal metaplasia. J. Clin. Pathol. 2004, 57, 177–182. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leja, M.; Kupcinskas, L.; Funka, K.; Sudraba, A.; Jonaitis, L.; Ivanauskas, A.; Janciauskas, D.; Kuidelis, G.; Chiu, H.; Lin, J. Value of gastrin-17 in detecting antral atrophy. Adv. Med. Sci. 2011, 56, 145–150. [Google Scholar] [CrossRef] [PubMed]
- Kishida, K.; Funahashi, T.; Shimomura, I. Adiponectin as a routine clinical biomarker. Best Pract. Res. Clin. Endocrinol. Metab. 2014, 28, 119–130. [Google Scholar] [CrossRef] [PubMed]
- Ishikawa, M.; Kitayama, J.; Kazama, S.; Hiramatsu, T.; Hatano, K.; Nagawa, H. Plasma adiponectin and gastric cancer. Clin. Cancer Res. Off. J. Am. Assoc. Cancer Res. 2005, 11, 466–472. [Google Scholar]
- Akyürek, N.; Akyol, G.; Dursun, A.; Yamaç, D.; Günel, N. Expression of MUC1 and MUC2 Mucins in Gastric Carcinomas: Their Relationship with Clinicopathologic Parameters and Prognosis. Pathol. Res. Pract. 2002, 198, 665–674. [Google Scholar] [CrossRef] [PubMed]
- Kim, D.-K.; Oh, S.Y.; Kwon, H.-C.; Lee, S.; Kwon, K.A.; Kim, B.G.; Kim, S.-G.; Kim, S.-H.; Jang, J.S.; Kim, M.C.; et al. Clinical significances of preoperative serum interleukin-6 and C-reactive protein level in operable gastric cancer. BMC Cancer 2009, 9, 155. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sánchez-Zauco, N.; Torres, J.; Gómez, A.; Camorlinga-Ponce, M.; Muñoz-Pérez, L.; Herrera-Goepfert, R.; Medrano-Guzmán, R.; Giono-Cerezo, S.; Maldonado-Bernal, C. Circulating blood levels of IL-6, IFN-γ, and IL-10 as potential diagnostic biomarkers in gastric cancer: A controlled study. BMC Cancer 2017, 17, 384. [Google Scholar]
- Vainer, N.; Dehlendorff, C.; Johansen, J.S. Systematic literature review of IL-6 as a biomarker or treatment target in patients with gastric, bile duct, pancreatic and colorectal cancer. Oncotarget 2018, 9, 29820–29841. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, Y.-D.; Wang, J.-H.; Lu, H.; Li, X.-N.; Song, W.-W.; Zhang, X.-D.; Zhang, W.-M. The human epididymis protein 4 acts as a prognostic factor and promotes progression of gastric cancer. Tumor Biol. 2015, 36, 2457–2464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Neal, R.L.; Nam, K.T.; LaFleur, B.J.; Barlow, B.; Nozaki, K.; Lee, H.-J.; Kim, W.H.; Yang, H.-K.; Shi, C.; Maitra, A.; et al. Human epididymis protein 4 is up-regulated in gastric and pancreatic adenocarcinomas. Hum. Pathol. 2013, 44, 734–742. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weis, V.G.; Petersen, C.P.; Mills, J.C.; Tuma, P.L.; Whitehead, R.H.; Goldenring, J.R. Establishment of novel in vitro mouse chief cell and SPEM cultures identifies MAL2 as a marker of metaplasia in the stomach. Am. J. Physiol.-Gastrointest. Liver Physiol. 2014, 307, G777–G792. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Flores-Luna, L.; Camorlinga-Ponce, M.; Hernandez-Suarez, G.; Kasamatsu, E.; Martínez, M.E.; Murillo, R.; Lazcano, E.; Torres, J. The utility of serologic tests as biomarkers for Helicobacter pylori-associated precancerous lesions and gastric cancer varies between Latin American countries. Cancer Causes Control 2013, 24, 241–248. [Google Scholar] [CrossRef] [PubMed]
- Pittayanon, R.; Rerknimitr, R.; Klaikaew, N.; Sanpavat, A.; Chaithongrat, S.; Mahachai, V.; Kullavanijaya, P.; Barkun, A. The risk of gastric cancer in patients with gastric intestinal metaplasia in 5-year follow-up. Aliment. Pharmacol. Ther. 2017, 46, 40–45. [Google Scholar] [CrossRef] [PubMed]
- Miki, K.; Fujishiro, M. Cautious comparison between East and West is necessary in terms of the serum pepsinogen test. Dig. Endosc. 2009, 21, 134–135. [Google Scholar] [PubMed]
- Weck, M.N. Prevalence of Chronic Atrophic Gastritis in Different Parts of the World. Cancer Epidemiol. Biomarkers Prev. 2006, 15, 1083–1094. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Choi, C.E.; Sonnenberg, A.; Turner, K.; Genta, R.M. High Prevalence of Gastric Preneoplastic Lesions in East Asians and Hispanics in the USA. Dig. Dis. Sci. 2015, 60, 2070–2076. [Google Scholar] [CrossRef] [PubMed]
- Choi, A.Y.; Strate, L.L.; Fix, M.C.; Schmidt, R.A.; Ende, A.R.; Yeh, M.M.; Inadomi, J.M.; Hwang, J.H. Association of gastric intestinal metaplasia and East Asian ethnicity with the risk of gastric adenocarcinoma in a U.S. population. Gastrointest. Endosc. 2018, 87, 1023–1028. [Google Scholar] [CrossRef] [PubMed]
- den Hoed, C.M.; van Eijck, B.C.; Capelle, L.G.; van Dekken, H.; Biermann, K.; Siersema, P.D.; Kuipers, E.J. The prevalence of premalignant gastric lesions in asymptomatic patients: Predicting the future incidence of gastric cancer. Eur. J. Cancer 2011, 47, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filipe, M.I.; Muñoz, N.; Matko, I.; Kato, I.; Pompe-Kirn, V.; Jutersek, A.; Teuchmann, S.; Benz, M.; Prijon, T. Intestinal metaplasia types and the risk of gastric cancer: A cohort study in Slovenia. Int. J. Cancer 1994, 57, 324–329. [Google Scholar] [CrossRef] [PubMed]
- Kodama, M.; Murakami, K.; Okimoto, T.; Sato, R.; Uchida, M.; Abe, T.; Shiota, S.; Nakagawa, Y.; Mizukami, K.; Fujioka, T. Ten-year prospective follow-up of histological changes at five points on the gastric mucosa as recommended by the updated Sydney system after Helicobacter pylori eradication. J. Gastroenterol. 2012, 47, 394–403. [Google Scholar] [CrossRef] [PubMed]
- De Re, V.; Orzes, E.; Canzonieri, V.; Maiero, S.; Fornasarig, M.; Alessandrini, L.; Cervo, S.; Steffan, A.; Zanette, G.; Mazzon, C.; et al. Pepsinogens to Distinguish Patients With Gastric Intestinal Metaplasia and Helicobacter pylori Infection Among Populations at Risk for Gastric Cancer. Clin. Transl. Gastroenterol. 2016, 7, e183. [Google Scholar] [CrossRef] [PubMed]
- Peitz, U.; Wex, T.; Vieth, M.; Stolte, M.; Willich, S.; Labenz, J.; Jaspersen, D.; Lind, T.; Malfertheiner, P. Correlation of serum pepsinogens and gastrin-17 with atrophic gastritis in gastroesophageal reflux patients: A matched-pairs study. J. Gastroenterol. Hepatol. 2011, 26, 82–89. [Google Scholar] [CrossRef]
- Väänänen, H.; Vauhkonen, M.; Helske, T.; Kääriäinen, I.; Rasmussen, M.; Tunturi-Hihnala, H.; Koskenpato, J.; Sotka, M.; Turunen, M.; Sandström, R.; et al. Non-endoscopic diagnosis of atrophic gastritis with a blood test. Correlation between gastric histology and serum levels of gastrin-17 and pepsinogen I: A multicentre study. Eur. J. Gastroenterol. Hepatol. 2003, 15, 885–891. [Google Scholar] [CrossRef] [PubMed]
- González, C.A.; Sanz-Anquela, J.M.; Gisbert, J.P.; Correa, P. Utility of subtyping intestinal metaplasia as marker of gastric cancer risk. A review of the evidence: Subtypes of intestinal metaplasia and gastric cancer risk. Int. J. Cancer 2013, 133, 1023–1032. [Google Scholar] [CrossRef] [PubMed]
- Piazuelo, M.B.; Bravo, L.E.; Mera, R.M.; Camargo, M.C.; Bravo, J.C.; Delgado, A.G.; Washington, M.K.; Rosero, A.; Garcia, L.S.; Realpe, J.L.; et al. The Colombian Chemoprevention Trial: 20-Year Follow-Up of a Cohort of Patients With Gastric Precancerous Lesions. Gastroenterology 2021, 160, 1106–1117.e3. [Google Scholar] [CrossRef] [PubMed]
- Lansdorp-Vogelaar, I.; Meester, R.G.S.; Laszkowska, M.; Escudero, F.A.; Ward, Z.J.; Yeh, J.M. Cost-effectiveness of prevention and early detection of gastric cancer in Western countries. Best Pract. Res. Clin. Gastroenterol. 2021, 50–51, 101735. [Google Scholar] [CrossRef] [PubMed]


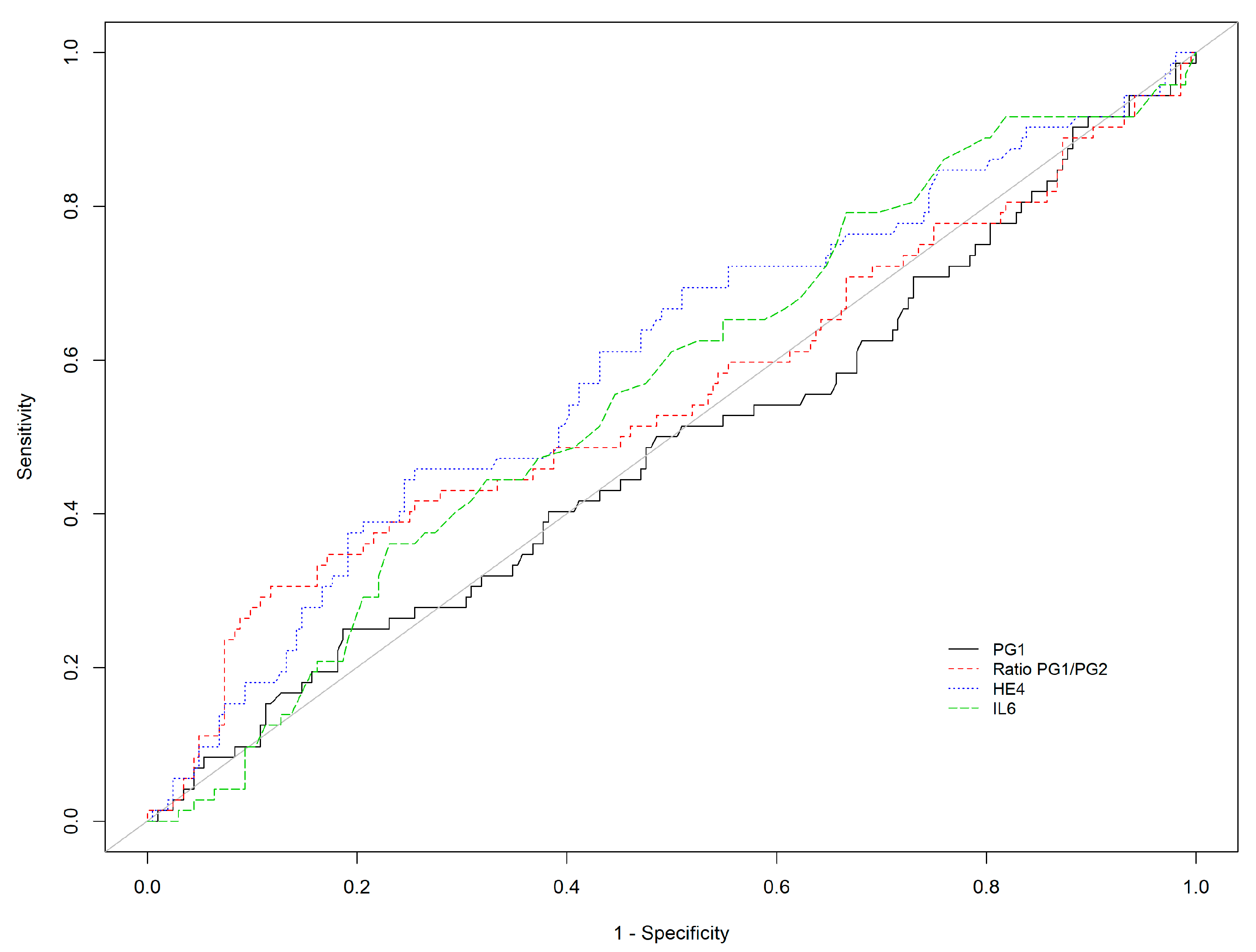
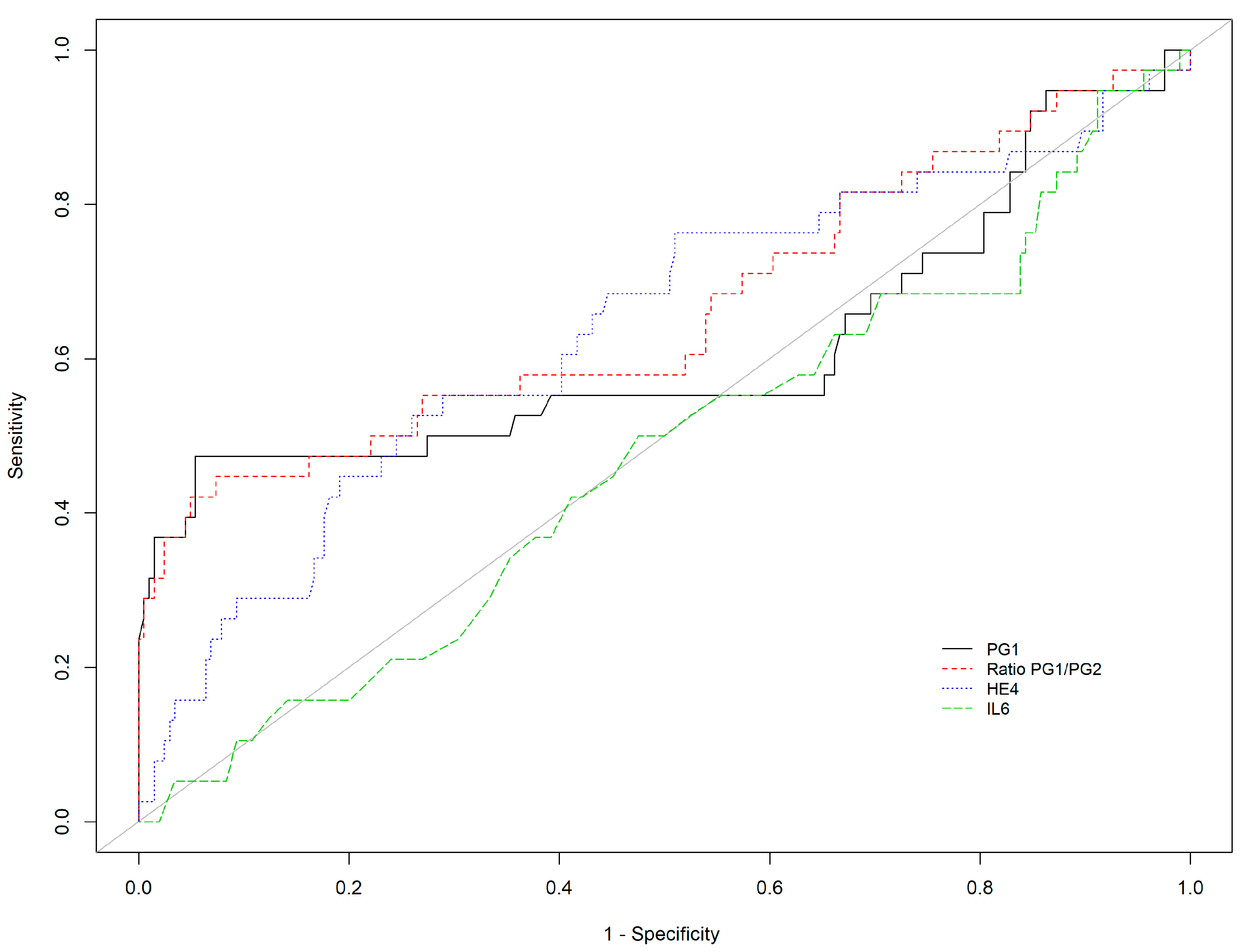
| N | NAG | AGA | AGC | AGAC | p-Value | |
|---|---|---|---|---|---|---|
| n = | 113 | 91 | 72 | 42 | 38 | |
| PG I | 70.93 (66.52) | 59.81 (44.40) | 70.70 (64.52) | 14.03 (33.25) | 48.45 (51.56) | <0.001 |
| PG II | 14.10 (11.52) | 13.63 (8.78) | 16.56 (16.09) | 10.36 (6.08) | 13.77 (8.92) | 0.027 |
| PGI/PGII | 4.86 (1.37) | 4.61 (1.75) | 4.54 (1.82) | 1.07 (1.54) | 3.30 (2.68) | <0.001 |
| Adiponectin | 5.07 (2.91) | 4.31 (2.81) | 4.92 (4.10) | 5.29 (3.47) | 5.31 (3.32) | 0.204 |
| Ferritin | 91.81 (88.67) | 81.22 (61.15) | 115.01 (121.68) | 68.58 (67.45) | 99.95 (98.58) | 0.105 |
| HE-4 | 75.70 (57.59) | 73.94 (42.49) | 86.42 (49.67) | 93.38 (83.34) | 115.34 (136.04) | 0.012 |
| IL-6 | 5.28 (11.44) | 4.80 (3.83) | 4.56 (2.83) | 6.86 (11.77) | 4.98 (4.62) | 0.249 |
| KL-6 | 291.63 (123.05) | 326.02 (181.11) | 328.81 (136.57) | 353.64 (157.71) | 337.21 (197.75) | 0.182 |
| n = | AUC | Cut-Off | Se (95%CI) | Sp (95%CI) | PPV (95%CI) | NPV (95%CI) | PLR (95%CI) | NLR (95%CI) | |
|---|---|---|---|---|---|---|---|---|---|
| PGI | 356 | 0.642 | ≤30 * | 46.7% (38.6; 55.0) | 83.8% (78.0; 88.6) | 68.3% (58.4; 77.1) | 67.9% (61.7; 73.6) | 2.89 (2.02; 4.12) | 0.64 (0.54; 0.75) |
| 356 | 0.642 | ≤21.1 # | 40.8% (32.9; 49.0) | 94.6% (90.6; 97.3) | 84.9% (74.6; 92.2) | 68.2% (62.4; 73.6) | 7.56 (4.13; 13.86) | 0.63 (0.55; 0.72) | |
| PGI/PGII | 356 | 0.685 | ≤3 * | 44.7% (36.7; 53.0) | 92.6% (88.2; 95.8) | 81.9% (72; 89.5) | 69.2% (63.4; 74.7) | 6.08 (3.62; 10.21) | 0.6 (0.51; 0.69) |
| 356 | 0.685 | ≤3.03 # | 46.7% (38.6; 55.0) | 92.6% (88.2; 95.8) | 82.6% (72.9; 89.9) | 70.0 % (64.2; 75.4) | 6.35 (3.79; 10.64) | 0.58 (0.49; 0.67) | |
| Adiponectin | 356 | 0.512 | ≥6.6 | 30.3% (23.1; 38.2) | 79.4% (73.2; 84.7) | 52.3% (41.4; 63.0) | 60.4% (54.3; 66.3) | 1.47 (1.02; 2.11) | 0.88 (0.77; 1.0) |
| Ferritin | 356 | 0.510 | ≥150 | 19.1% (13.2; 26.2) | 83.3% (77.5; 88.2) | 46.0 % (33.4; 59.1) | 58.0 % (52.1; 63.7) | 1.14 (0.73; 1.79) | 0.97 (0.88; 1.07) |
| HE4 | 356 | 0.606 | ≥75.8 | 47.4% (39.2; 55.6) | 74.0 % (67.4; 79.9) | 57.6% (48.4; 66.4) | 65.4% (58.8; 71.5) | 1.82 (1.37; 2.43) | 0.71 (0.6; 0.84) |
| IL6 | 356 | 0.555 | ≥4.5 | 41.4% (33.5; 49.7) | 69.1% (62.3; 75.4) | 50.0 % (41.0; 59.0) | 61.3% (54.7; 67.6) | 1.34 (1.02; 1.77) | 0.85 (0.72; 1.0) |
| KL6 | 356 | 0.564 | ≥322 | 50.7% (42.4; 58.9) | 62.3% (55.2; 68.9) | 50.0 % (41.8; 58.2) | 62.9% (55.8; 69.5) | 1.34 (1.06; 1.7) | 0.79 (0.65; 0.96) |
| PGI/PGII +/− HE-4 | 356 | 0.687 | PGI/PGII ≤ 3.03 OR HE4 ≥ 75.8 | 69.7% (61.8; 76.9) | 67.6% (60.8; 74.0) | 61.6% (53.9; 68.9) | 75.0 % (68.1; 81.1) | 2.16 (1.72; 2.7) | 0.45 (0.35; 0.58) |
| 356 | 0.614 | PGI/PGII ≤ 3.03 AND HE4 ≥ 75.8 | 23.7% (17.2; 31.3) | 99.0% (96.5; 99.9) | 94.7% (82.3; 99.4) | 63.5% (58.0; 68.8) | 24.16 (5.91; 98.78) | 0.77 (0.7; 0.84) | |
| PGI | 258 | 0.740 | ≤30 * | 55.6% (41.4; 69.1) | 83.8% (78.0; 88.6) | 47.6% (34.9; 60.6) | 87.7% (82.2; 92.0) | 3.43 (2.32; 5.09) | 0.53 (0.39; 0.72) |
| 258 | 0.740 | ≤20.2 # | 53.7% (39.6; 67.4) | 95.6% (91.8; 98.0) | 76.3% (59.8; 88.6) | 88.6% (83.7; 92.5) | 12.17 (6.14; 24.15) | 0.48 (0.36; 0.65) | |
| PGI/PGII | 258 | 0.758 | ≤3 * | 55.6% (41.4; 69.1) | 92.6% (88.2; 95.8) | 66.7% (51.0; 80.0) | 88.7% (83.7; 92.6) | 7.56 (4.39; 13.0) | 0.48 (0.36; 0.65) |
| 258 | 0.758 | ≤3.03 | 57.4% (43.2; 70.8) | 92.6% (88.2; 95.8) | 67.4% (52.0; 80.5) | 89.2% (84.2; 93.0) | 7.81 (4.56; 13.38) | 0.46 (0.34; 0.63) | |
| HE-4 | 258 | 0.637 | ≥63.2 | 70.4% (56.4–82.0) | 55.4% (48.3–62.3) | 29.5% (21.8–38.1) | 87.6% (80.6–92.7) | 1.58 (1.25–1.99) | 0.53 (0.35–0.82) |
| PGI/PGII +/− HE-4 | 258 | 0.686 | PGI/PGII ≤ 3.03 OR HE4 ≥ 63.2 | 85.2% (72.9; 93.4) | 52.0 % (44.9; 59.0) | 31.9% (24.4; 40.2) | 93.0 % (86.6; 96.9) | 1.77 (1.48; 2.12) | 0.29 (0.15; 0.55) |
| 0.684 | PGI/PGII ≤3.03 AND HE4 ≥ 63.2 | 40.7% (27.6; 55.0) | 96.1% (92.4; 98.3) | 73.3% (54.1; 87.7) | 86.0 % (80.8; 90.2) | 10.39 (4.9; 22.03) | 0.62 (0.49; 0.77) |
| n = | AUC | Cut-Off | Se (95%CI) | Sp (95%CI) | PPV (95%CI) | NPV (95%CI) | PLR (95%CI) | NLR (95%CI) | |
|---|---|---|---|---|---|---|---|---|---|
| PGI | 284 | 0.782 | ≤30 * | 71.2% (60.0; 80.8) | 83.8% (78.0; 88.6) | 63.3% (52.5; 73.2) | 88.1% (82.7; 92.3) | 4.4 (3.13; 6.2) | 0.34 (0.24; 0.49) |
| PGI | 284 | 0.782 | ≤21.1 # | 70.0% (58.7; 79.7) | 94.6% (90.6; 97.3) | 83.6% (72.5; 91.5) | 88.9% (84.0; 92.8) | 12.98 (7.18; 23.48) | 0.32 (0.23; 0.44) |
| PGI/PGII | 284 | 0.805 | ≤3 * | 67.5% (56.1; 77.6) | 92.6% (88.2; 95.8) | 78.3% (66.7; 87.3) | 87.9% (82.8; 91.9) | 9.18 (5.51; 15.29) | 0.35 (0.26; 0.48) |
| PGI/PGII | 284 | 0.805 | ≤2.59 # | 66.2% (54.8; 76.4) | 95.1% (91.2; 97.6) | 84.1% (72.7; 92.1) | 87.8% (82.7; 91.8) | 13.51 (7.24; 25.23) | 0.35 (0.26; 0.48) |
| Adiponectin | 284 | 0.540 | ≥6.66 | 37.5% (26.9; 49.0) | 79.4% (73.2; 84.7) | 41.7% (30.2; 53.9) | 76.4% (70.1; 82.0) | 1.82 (1.23; 2.69) | 0.79 (0.66; 0.95) |
| Ferritin | 284 | 0.463 | ≥150 | 15.0% (8.0; 24.7) | 83.3% (77.5; 88.2) | 26.1% (14.3; 41.1) | 71.4% (65.2; 77.1) | 0.9 (0.49; 1.65) | 1.02 (0.91; 1.14) |
| HE-4 | 284 | 0.616 | ≥63.2 | 67.5% (56.1; 77.6) | 55.4% (48.3; 62.3) | 37.2% (29.4; 45.7) | 81.3% (73.8; 87.4) | 1.51 (1.22; 1.88) | 0.59 (0.42; 0.82) |
| IL-6 | 284 | 0.549 | ≥4.2 | 47.5% (36.2; 59.0) | 64.2% (57.2; 70.8) | 34.2% (25.5; 43.8) | 75.7% (68.6; 81.9) | 1.33 (0.99; 1.78) | 0.82 (0.65; 1.03) |
| KL-6 | 284 | 0.564 | ≥421 | 35.0 % (24.7; 46.5) | 85.3% (79.7; 89.9) | 48.3% (35.0; 61.8) | 77.0 % (70.9; 82.3) | 2.38 (1.52; 3.72) | 0.76 (0.64; 0.9) |
| PGI | 240 | 0.856 | ≤30 * | 77.8% (60.8; 89.9) | 83.8% (78.0; 88.6) | 45.9% (33.1; 59.2) | 95.5% (91.4; 98.1) | 4.81 (3.36; 6.88) | 0.27 (0.14; 0.49) |
| PGI | 240 | 0.856 | ≤20.2 # | 77.8% (60.8; 89.9) | 95.6% (91.8; 98.0) | 75.7% (58.8; 88.2) | 96.1% (92.4; 98.3) | 17.63 (9.09; 34.18) | 0.23 (0.13; 0.43) |
| PGI/PGII | 240 | 0.859 | ≤3 * | 75.0 % (57.8; 87.9) | 92.6% (88.2; 95.8) | 64.3% (48.0; 78.4) | 95.5% (91.5; 97.9) | 10.2 (6.05; 17.2) | 0.27 (0.15; 0.48) |
| PGI/PGII | 240 | 0.859 | ≤0.96 # | 72.2% (54.8; 85.8) | 98.0 % (95.1; 99.5) | 86.7% (69.3; 96.2) | 95.2% (91.4; 97.7) | 36.83 (13.67; 99.25) | 0.28 (0.17; 0.48) |
| n = | AUC | Cut-off | Se (95%CI) | Sp (95%CI) | PPV (95%CI) | NPV (95%CI) | PLR (95%CI) | NLR (95%CI) | |
|---|---|---|---|---|---|---|---|---|---|
| Adiponectin | 276 | 0.520 | ≤4.22 | 58.3% (46.1; 69.8) | 50.5% (43.4; 57.5) | 29.4% (22.1; 37.6) | 77.4% (69.4; 84.2) | 1.18 (0.93; 1.5) | 0.83 (0.61; 1.12) |
| Ferritin | 276 | 0.563 | ≥150 | 23.6% (14.4; 35.1) | 83.3% (77.5; 88.2) | 33.3% (20.8; 47.9) | 75.6% (69.4; 81.0) | 1.42 (0.85; 2.37) | 0.92 (0.8; 1.06) |
| HE-4 | 276 | 0.595 | ≥77.6 | 45.8% (34.0; 58.0) | 74.5% (68.0; 80.3) | 38.8% (28.4; 50.0) | 79.6% (73.2; 85.1) | 1.8 (1.28; 2.54) | 0.73 (0.58; 0.91) |
| IL-6 | 276 | 0.561 | ≥5.1 | 36.1% (25.1; 48.3) | 77.0 % (70.6; 82.6) | 35.6% (24.7; 47.7) | 77.3% (71.0; 82.9) | 1.57 (1.05; 2.33) | 0.83 (0.69; 1.0) |
| KL-6 | 276 | 0.564 | ≥226 | 77.8% (66.4; 86.7) | 33.8% (27.4; 40.8) | 29.3% (23.0; 36.3) | 81.2% (71.2; 88.8) | 1.18 (1.0; 1.38) | 0.66 (0.41; 1.05) |
| Adiponectin | 258 | 0.501 | ≥8.47 | 22.2% (6.4; 47.6) | 88.2% (83.0; 92.3) | 14.3% (4.0; 32.7) | 92.8% (88.2; 96.0) | 1.89 (0.74; 4.85) | 0.88 (0.69; 1.13) |
| Ferritin | 258 | 0.550 | ≥150 | 16.7% (3.6; 41.4) | 83.3% (77.5; 88.2) | 8.1% (1.7; 21.9) | 91.9% (87.0; 95.4) | 1.0 (0.34; 2.94) | 1.0 (0.81; 1.24) |
| HE-4 | 258 | 0.600 | ≥64.8 | 66.7% (41.0; 86.7) | 56.9% (49.8; 63.8) | 12.0 % (6.4; 20.0) | 95.1% (89.6; 98.2) | 1.55 (1.08; 2.22) | 0.59 (0.3; 1.14) |
| IL-6 | 258 | 0.588 | ≥3.1 | 72.2% (46.5; 90.3) | 41.2% (34.4; 48.3) | 9.8% (5.3; 16.1) | 94.4% (87.4; 98.2) | 1.23 (0.9; 1.67) | 0.67 (0.31; 1.45) |
| KL6 | 258 | 0.565 | ≥192 | 94.4% (72.7; 99.9) | 22.5% (17.0; 28.9) | 9.7% (5.8; 15.1) | 97.9% (88.7; 99.9) | 1.22 (1.07; 1.39) | 0.25 (0.04; 1.68) |
| n | AUC | Cut-Off | Se (95%CI) | Sp (95%CI) | PPV (95%CI) | NPV (95%CI) | PLR (95%CI) | NLR (95%CI) | |
|---|---|---|---|---|---|---|---|---|---|
| PGI | 242 | 0.613 | ≤30 * | 47.4% (31.0; 64.2) | 83.8% (78.0; 88.6) | 35.3% (22.4; 49.9) | 89.5% (84.3; 93.5) | 2.93 (1.85; 4.63) | 0.63 (0.46; 0.85) |
| PGI | 242 | 0.613 | ≤21.1 # | 47.4% (31.0; 64.2) | 94.6% (90.6; 97.3) | 62.1% (42.3; 79.3) | 90.6% (85.9; 94.2) | 8.78 (4.52; 17.09) | 0.56 (0.41; 0.75) |
| PGI/PGII | 242 | 0.664 | ≤3 * | 44.7% (28.6; 61.7) | 92.6% (88.2; 95.8) | 53.1% (34.7; 70.9) | 90.0% (85.1; 93.7) | 6.08 (3.33; 11.11) | 0.6 (0.45; 0.8) |
| PGI/PGII | 242 | 0.664 | ≤2.86 # | 44.7% (28.6; 61.7) | 92.6% (88.2; 95.8) | 53.1% (34.7; 70.9) | 90.0% (85.1; 93.7) | 6.08 (3.33; 11.11) | 0.6 (0.45; 0.8) |
| Adiponectin | 242 | 0.542 | ≥6.79 | 44.7% (28.6; 61.7) | 79.9% (73.7; 85.2) | 29.3% (18.1; 42.7) | 88.6% (83.1; 92.8) | 2.23 (1.42; 3.48) | 0.69 (0.52; 0.93) |
| Ferritin | 242 | 0.527 | ≥150 | 21.1% (9.6; 37.3) | 83.3% (77.5; 88.2) | 19.0% (8.6; 34.1) | 85.0% (79.3; 89.6) | 1.26 (0.63; 2.51) | 0.95 (0.8; 1.13) |
| HE-4 | 242 | 0.638 | ≥75.8 | 52.6% (35.8; 69.0) | 74.0% (67.4; 79.9) | 27.4% (17.6; 39.1) | 89.3% (83.7; 93.6) | 2.03 (1.38; 2.96) | 0.64 (0.45; 0.9) |
| IL-6 | 242 | 0.529 | ≥6.4 | 31.6% (17.5–48.7) | 83.8% (78.0; 88.6) | 26.7% (14.6–41.9) | 86.8% (81.3–91.2) | 1.95 (1.11–3.43) | 0.82 (0.65–1.02) |
| KL-6 | 242 | 0.525 | ≥400 | 36.8% (21.8; 54.0) | 80.4% (74.3; 85.6) | 25.9% (15.0; 39.7) | 87.2% (81.6; 91.6) | 1.88 (1.14; 3.1) | 0.79 (0.61; 1.01) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chapelle, N.; Osmola, M.; Martin, J.; Blin, J.; Leroy, M.; Jirka, I.; Moussata, D.; Lamarque, D.; Olivier, R.; Tougeron, D.; et al. Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study. Diagnostics 2022, 12, 695. https://doi.org/10.3390/diagnostics12030695
Chapelle N, Osmola M, Martin J, Blin J, Leroy M, Jirka I, Moussata D, Lamarque D, Olivier R, Tougeron D, et al. Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study. Diagnostics. 2022; 12(3):695. https://doi.org/10.3390/diagnostics12030695
Chicago/Turabian StyleChapelle, Nicolas, Malgorzata Osmola, Jérôme Martin, Justine Blin, Maxime Leroy, Iva Jirka, Driffa Moussata, Dominique Lamarque, Raphael Olivier, David Tougeron, and et al. 2022. "Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study" Diagnostics 12, no. 3: 695. https://doi.org/10.3390/diagnostics12030695
APA StyleChapelle, N., Osmola, M., Martin, J., Blin, J., Leroy, M., Jirka, I., Moussata, D., Lamarque, D., Olivier, R., Tougeron, D., Hay-Lombardie, A., Bigot-Corbel, E., Masson, D., Mosnier, J.-F., & Matysiak-Budnik, T. (2022). Serum Pepsinogens Combined with New Biomarkers Testing Using Chemiluminescent Enzyme Immunoassay for Non-Invasive Diagnosis of Atrophic Gastritis: A Prospective, Multicenter Study. Diagnostics, 12(3), 695. https://doi.org/10.3390/diagnostics12030695






