Chemical Diagnosis of Calcium Pyrophosphate Deposition Disease of the Temporomandibular Joint: A Case Report
Abstract
:1. Introduction
2. Case Presentation
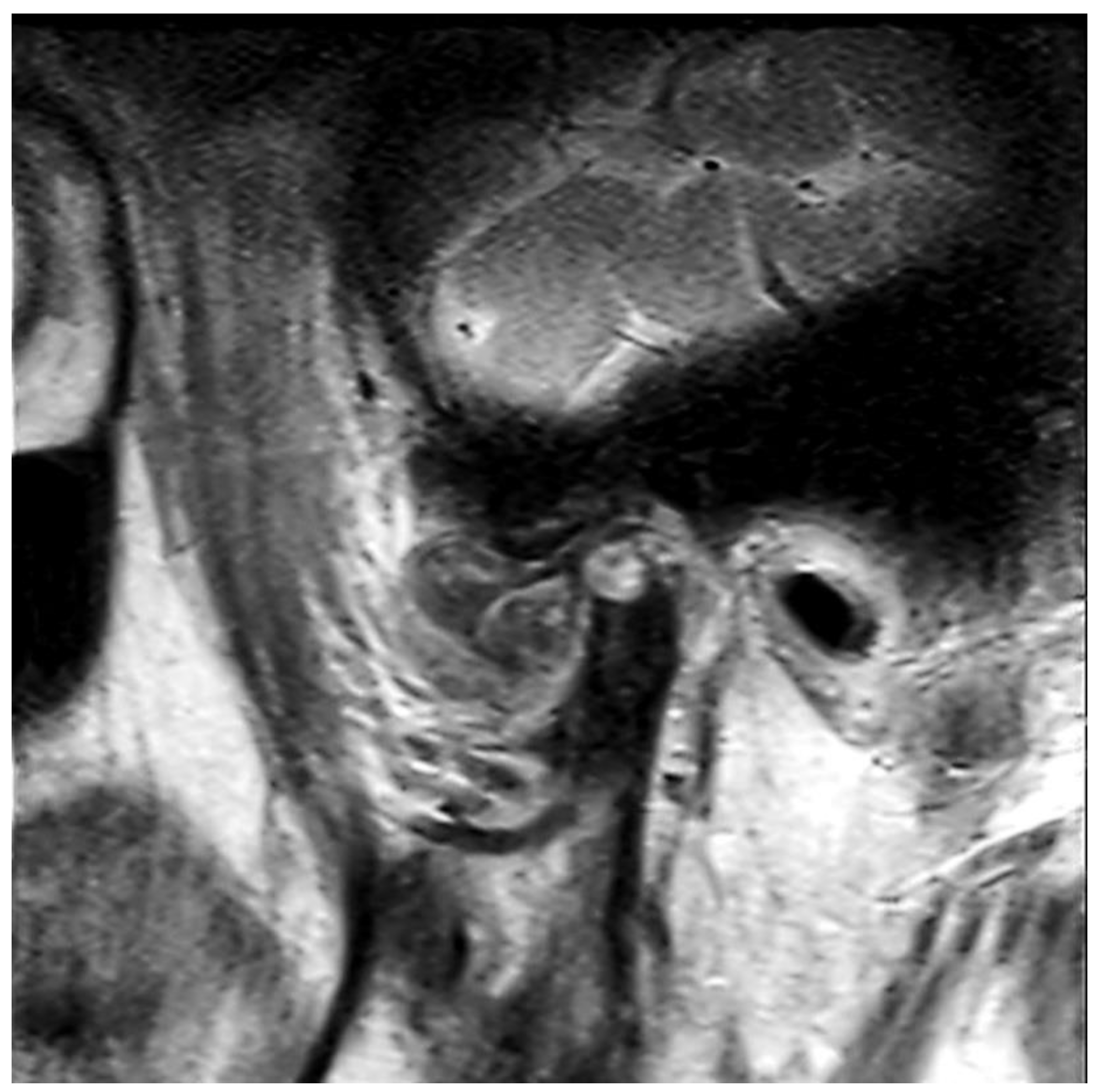
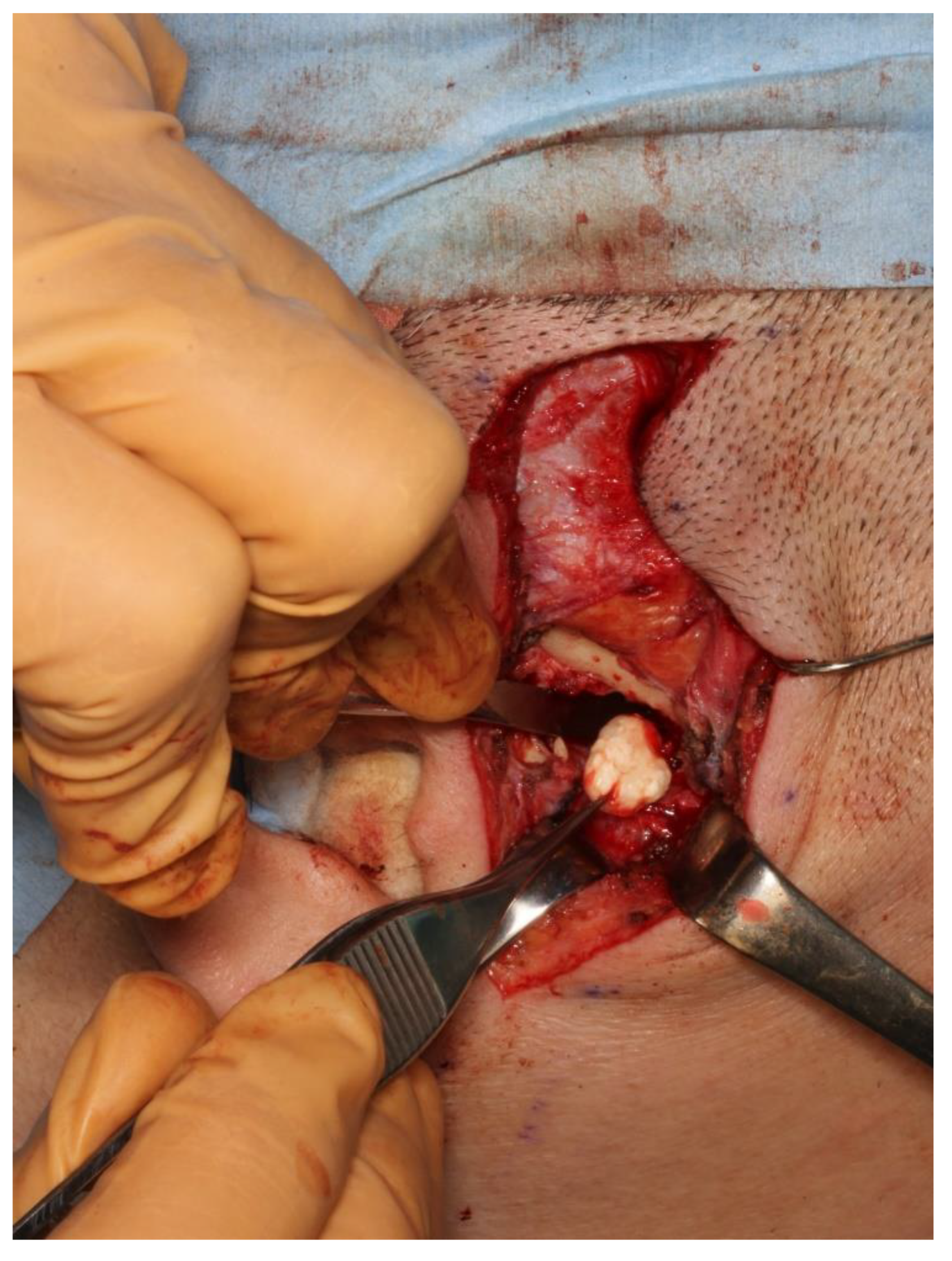
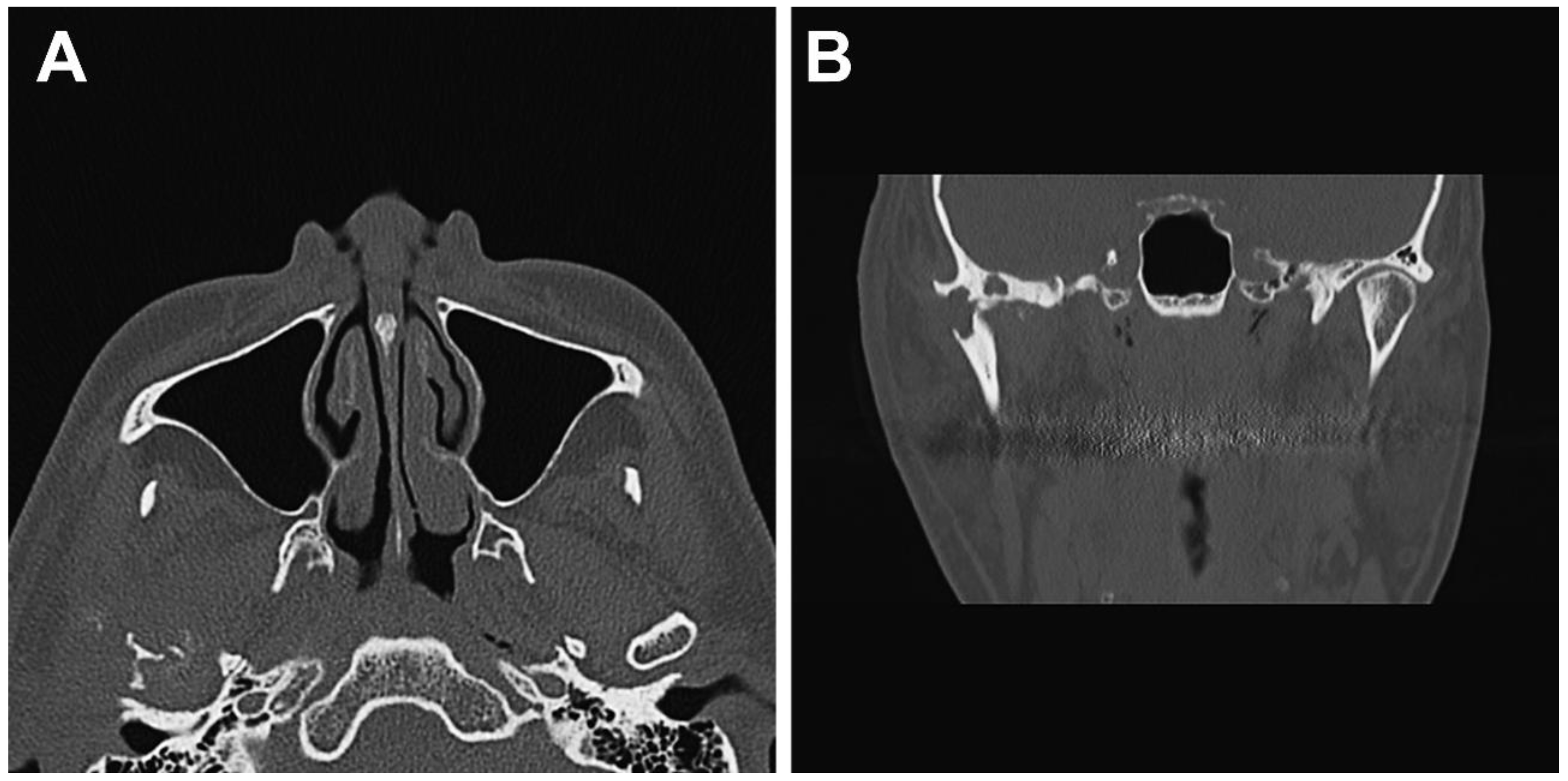
2.1. Clinical Summary
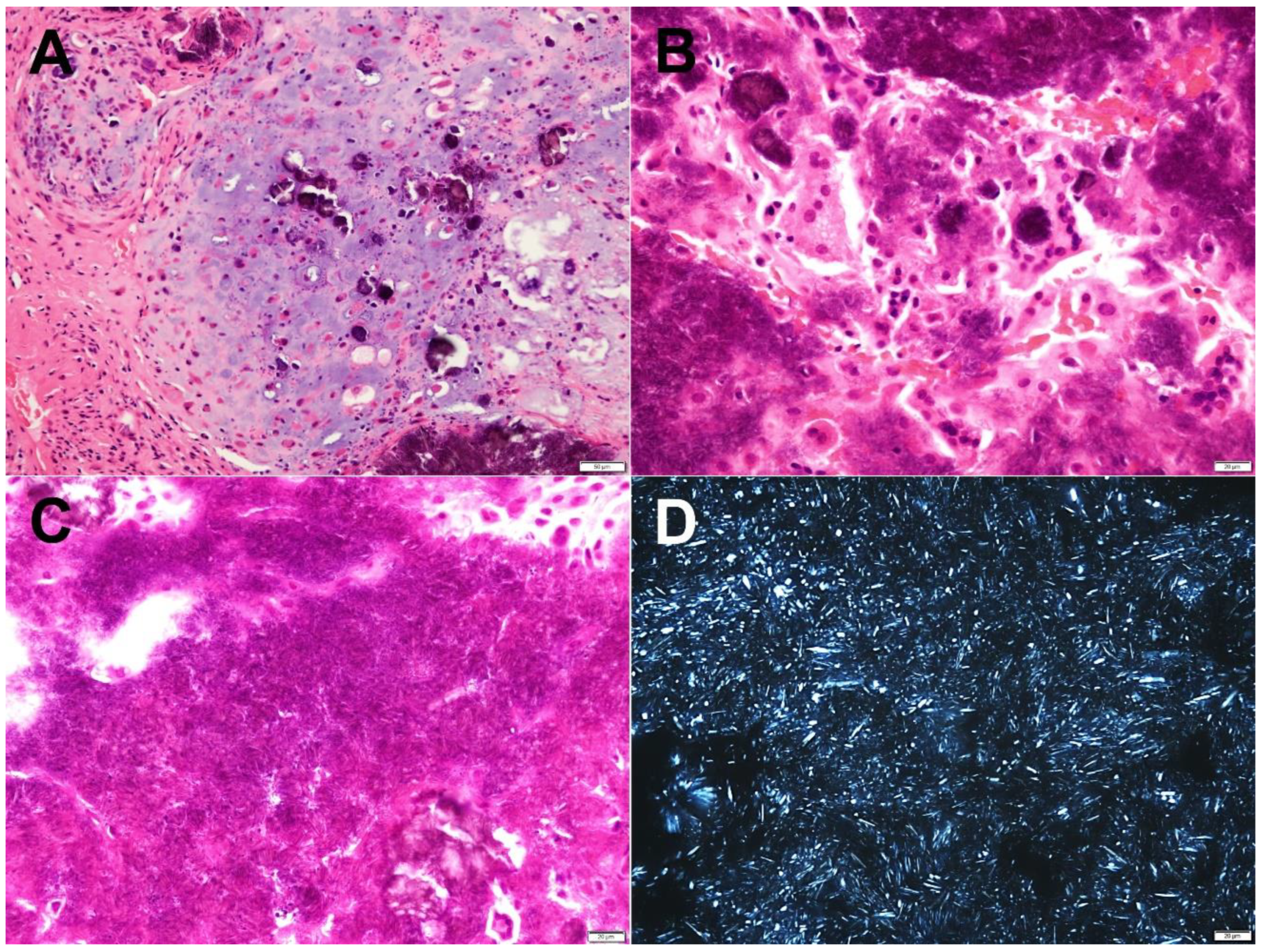
2.2. Pathological Findings
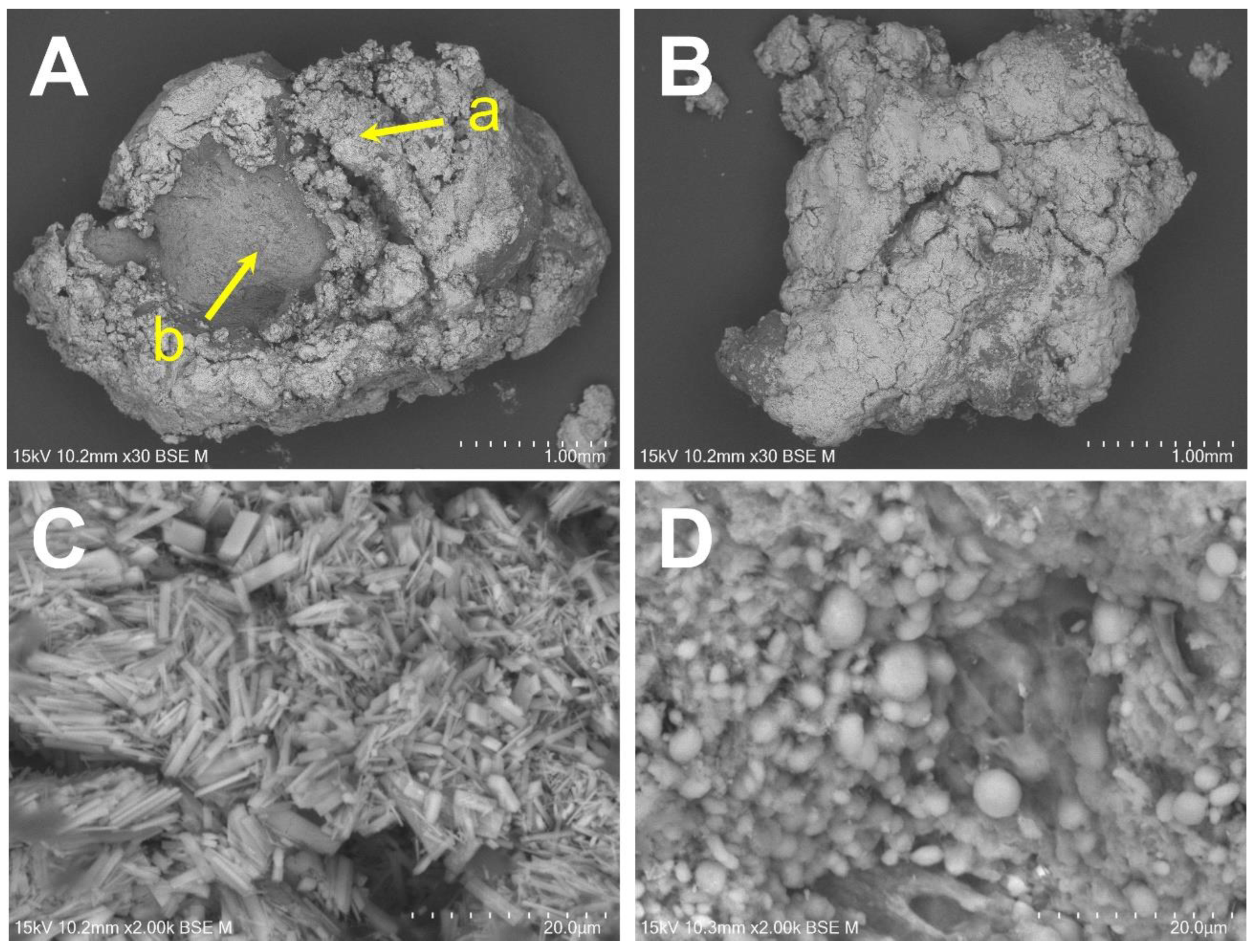
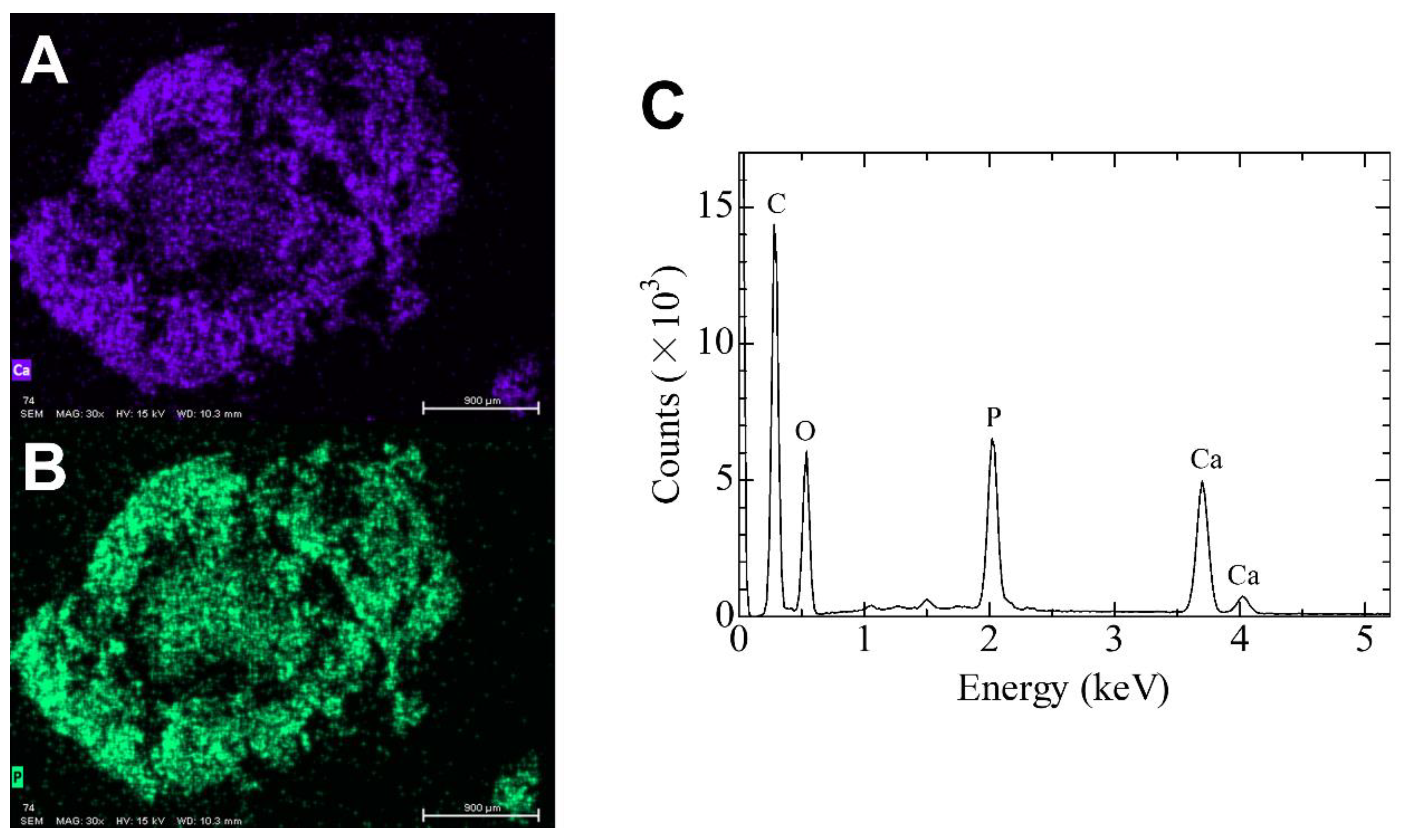
2.3. Elemental Analysis Using SEM/EDS
2.4. Crystal Phase Analysis Using XRD
2.5. Quantitative Elemental Analysis for ICP-AES
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- McCarty, D.J. Calcium pyrophosphate dihydrate crystal deposition disease: Pseudogout: Articular chondrocalcinosis. In Arthritis and Allied Conditions, 9th ed.; McCarty, D.J., Ed.; Lea & Febiger: Philadelphia, PA, USA, 1979; pp. 1276–1299. [Google Scholar]
- Kohn, N.N.; Hughes, R.E.; McCarty, D.R., Jr.; Faires, J.S. The significance of calcium phosphate crystals in the synovial fluid of arthritic patients: The “pseudogout syndrome”: II. Identification of crystals. Ann. Intern. Med. 1962, 56, 738–745. [Google Scholar] [CrossRef] [PubMed]
- Naqvi, A.H.; Abraham, J.L.; Kellman, R.M.; Khurana, K.K. Calcium pyrophosphate dihydrate deposition disease (CPPD)/pseudogout of the temporomandibular joint–FNA findings and microanalysis. Cytojournal 2008, 5, 8. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Guo, C.; Luo, H.; Chen, S.; Ma, X. A case of destructive calcium pyrophosphate dihydrate crystal deposition disease of the temporomandibular joint: A diagnostic challenge. Int. J. Oral Maxillofac. Surg. 2011, 40, 1431–1437. [Google Scholar] [CrossRef] [PubMed]
- Kwon, K.J.; Seok, H.; Lee, J.H.; Kim, M.K.; Kim, S.G.; Park, H.K.; Choi, H.M. Calcium Pyrophosphate Dihydrate Deposition Disease in the Temporomandibular Joint: Diagnosis and Treatment. Maxillofac. Plast. Reconstr. Surg. 2018, 40, 19. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, Y.; Ishibashi, K.; Kobayashi, K.; Westesson, P.L. Calcium pyrophosphate deposition disease in the temporomandibular joint: Report of two cases. J. Oral Maxillofac. Surg. 1999, 57, 1357–1363. [Google Scholar] [CrossRef]
- Abdelsayed, R.A.; Said-Al-Naief, N.; Salguerio, M.; Holmes, J.; El-Mofty, S.K. Tophaceous pseudogout of the temporomandibular joint: A series of 3 cases. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. 2014, 117, 369–375. [Google Scholar] [CrossRef] [PubMed]
- Scott, J.T. Copeman’s Textbook of the Rheumatic Diseases, 5th ed.; Churchill Livinstone: Edinburgh, UK, 1978. [Google Scholar]
- Resnick, D. Calcium pyrophosphate dihydrate (CPPD) crystal deposition disease. In Diagnosis of Bone and Joint Disorders, 4th ed.; Hotokezaka, Y., Ed.; W B Saunders: Philadelphia, PA, USA, 2002. [Google Scholar]
- Pritzker, K.P.; Phillips, H.; Luk, S.C.; Koven, I.H.; Kiss, A.; Houpt, J.B. Pseudotumor of temporomandibular joint: Destructive calcium pyrophosphate dihydrate arthropathy. J. Rheumatol. 1976, 3, 70–81. [Google Scholar] [PubMed]
- Aoyama, S.; Kino, K.; Amagasa, T.; Kayano, T.; Ichinose, S.; Kimijima, Y. Differential diagnosis of calcium pyrophosphate dihydrate deposition of the temporomandibular joint. Br. J. Oral Maxillofac. Surg. 2000, 38, 550–553. [Google Scholar] [CrossRef] [PubMed]
- Shidham, V.; Chivukula, M.; Basir, Z.; Shidham, G. Evaluation of crystals in formalin-fixed, paraffin-embedded tissue sections for the differential diagnosis of pseudogout, gout, and tumoral calcinosis. Mod. Pathol. 2001, 14, 806–810. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ann, K.; Rosenthal, A.K.; Ryan, L.M. Calcium pyrophosphate deposition disease. N. Engl. J. Med. 2016, 374, 2575–2584. [Google Scholar] [CrossRef]
- Kudoh, K.; Kudoh, T.; Tsuru, K.; Miyamoto, Y. A case of tophaceous pseudogout of the temporomandibular joint extending to the base of the skull. Int. J. Oral Maxillofac. Surg. 2017, 46, 355–359. [Google Scholar] [CrossRef] [PubMed]
- Dedhiya, M.G.; Young, F.; Higuchi, W.I. Mechanism for the retardation of the acid dissolution rate of hydroxyapatite by strontium. J. Dent. Res. 1973, 52, 1097–1109. [Google Scholar] [CrossRef] [PubMed]



| Element | Quantity | Unit | |
|---|---|---|---|
| Upper | Lower | ||
| Ca | 11.2 | 9.12 | wt% |
| P | 9.2 | 6.75 | |
| Fe | 24 | 22 | μg/g (ppm) |
| K | 153 | 102 | |
| Mg | 274 | 267 | |
| Na | 1920 | 2140 | |
| Zn | 7 | 16 | |
| Sr | 16 | 12 | |
| Composition Formula | Ca/P Ratio | Name | Abbreviation (Mineral Name) |
|---|---|---|---|
| Ca (H2PO4)2·H2O | 0.5 | Calcium bis(dihydrogenphosphate) monohydrate | MCPM |
| CaHPO4 | 1 | Calcium monohydrogen phosphate | DCPA (monetite) |
| CaHPO4·2H2O | 1 | Calcium hydrogen phosphate dihydrate | DCPD (brushite) |
| Ca2P2O7 | 1 | Calcium pyrophosphate | |
| Ca2P2O7·2H2O | 1 | Calcium pyrophosphate dihydrate | CPPD |
| Ca8(PO4)4(HPO4)2(OH)2 | 1.33 | Octacalcium phosphate | OCP |
| Ca3(PO4)2 | 1.5 | Tricalcium phosphate | TCP |
| Ca10(PO4)6(OH)2 | 1.66 | Hydroxyapatite | HAp |
| Ca4(PO4)2O | 2 | Tetracalcium phosphate | TTCP |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Terauchi, M.; Uo, M.; Fukawa, Y.; Yoshitake, H.; Tajima, R.; Ikeda, T.; Yoda, T. Chemical Diagnosis of Calcium Pyrophosphate Deposition Disease of the Temporomandibular Joint: A Case Report. Diagnostics 2022, 12, 651. https://doi.org/10.3390/diagnostics12030651
Terauchi M, Uo M, Fukawa Y, Yoshitake H, Tajima R, Ikeda T, Yoda T. Chemical Diagnosis of Calcium Pyrophosphate Deposition Disease of the Temporomandibular Joint: A Case Report. Diagnostics. 2022; 12(3):651. https://doi.org/10.3390/diagnostics12030651
Chicago/Turabian StyleTerauchi, Masahiko, Motohiro Uo, Yuki Fukawa, Hiroyuki Yoshitake, Rina Tajima, Tohru Ikeda, and Tetsuya Yoda. 2022. "Chemical Diagnosis of Calcium Pyrophosphate Deposition Disease of the Temporomandibular Joint: A Case Report" Diagnostics 12, no. 3: 651. https://doi.org/10.3390/diagnostics12030651
APA StyleTerauchi, M., Uo, M., Fukawa, Y., Yoshitake, H., Tajima, R., Ikeda, T., & Yoda, T. (2022). Chemical Diagnosis of Calcium Pyrophosphate Deposition Disease of the Temporomandibular Joint: A Case Report. Diagnostics, 12(3), 651. https://doi.org/10.3390/diagnostics12030651






