Role of Anterior Segment-Optical Coherence Tomography Angiography in Acute Ocular Burns
Abstract
:1. Introduction
2. Materials and Methods
3. Principle and Technology of Optical Coherence Tomography Angiography (OCTA)
3.1. Principle
3.2. Technology
4. Comparison with Fluorescein Angiography (FA) and Indocyanine Green Angiography (ICG)
5. Normal Limbal Vasculature
5.1. Clinical Anatomy
5.2. OCTA of Normal Limbus
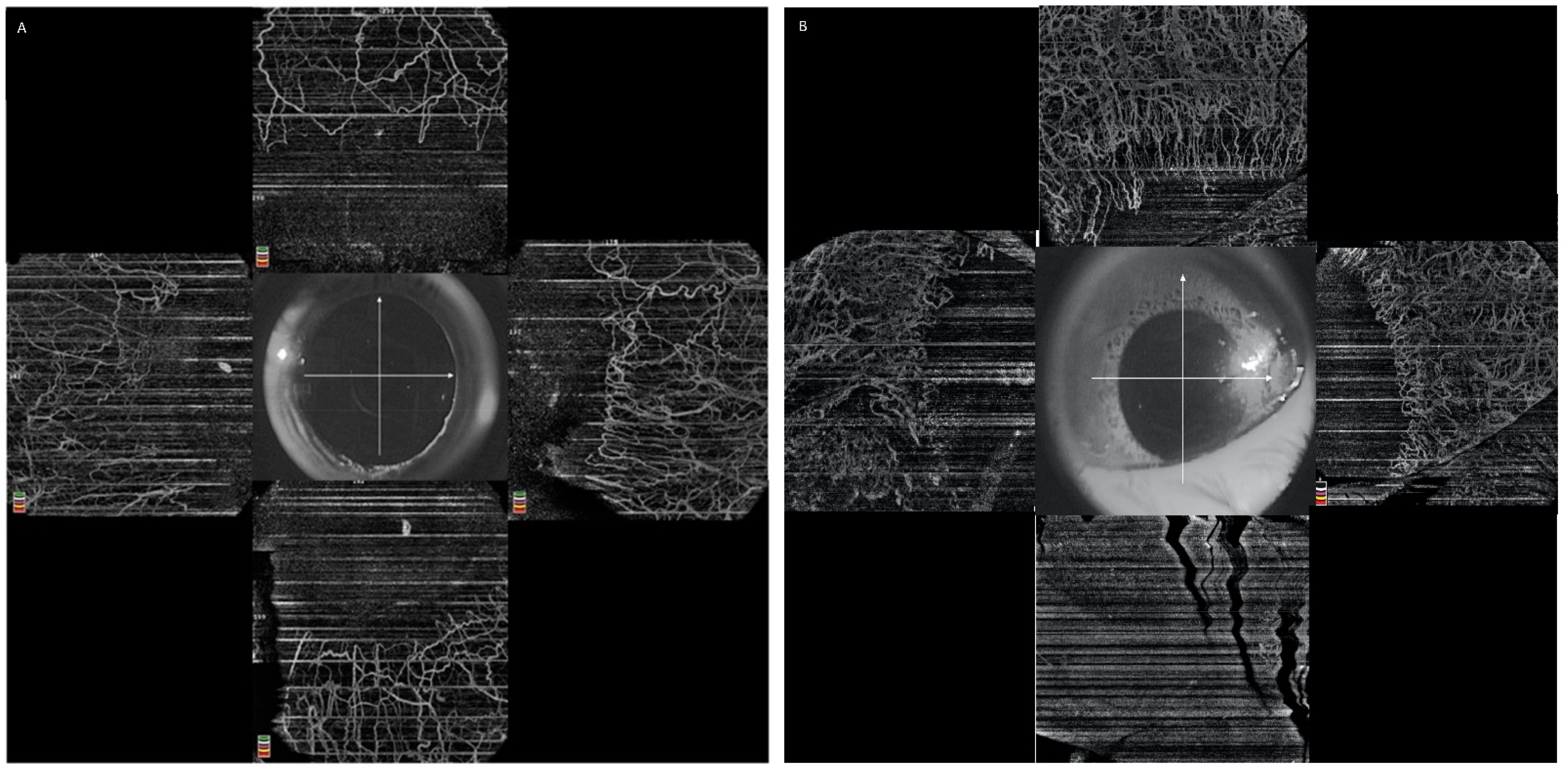
5.2.1. Image Acquisition
5.2.2. Normal Limbal Vasculature on OCTA
5.2.3. Qualitative Assessment of Limbal Vasculature
5.2.4. Quantitative Assessment of Limbal Vasculature
6. OCTA in Acute Ocular Burns
6.1. Role of AS-OCTA
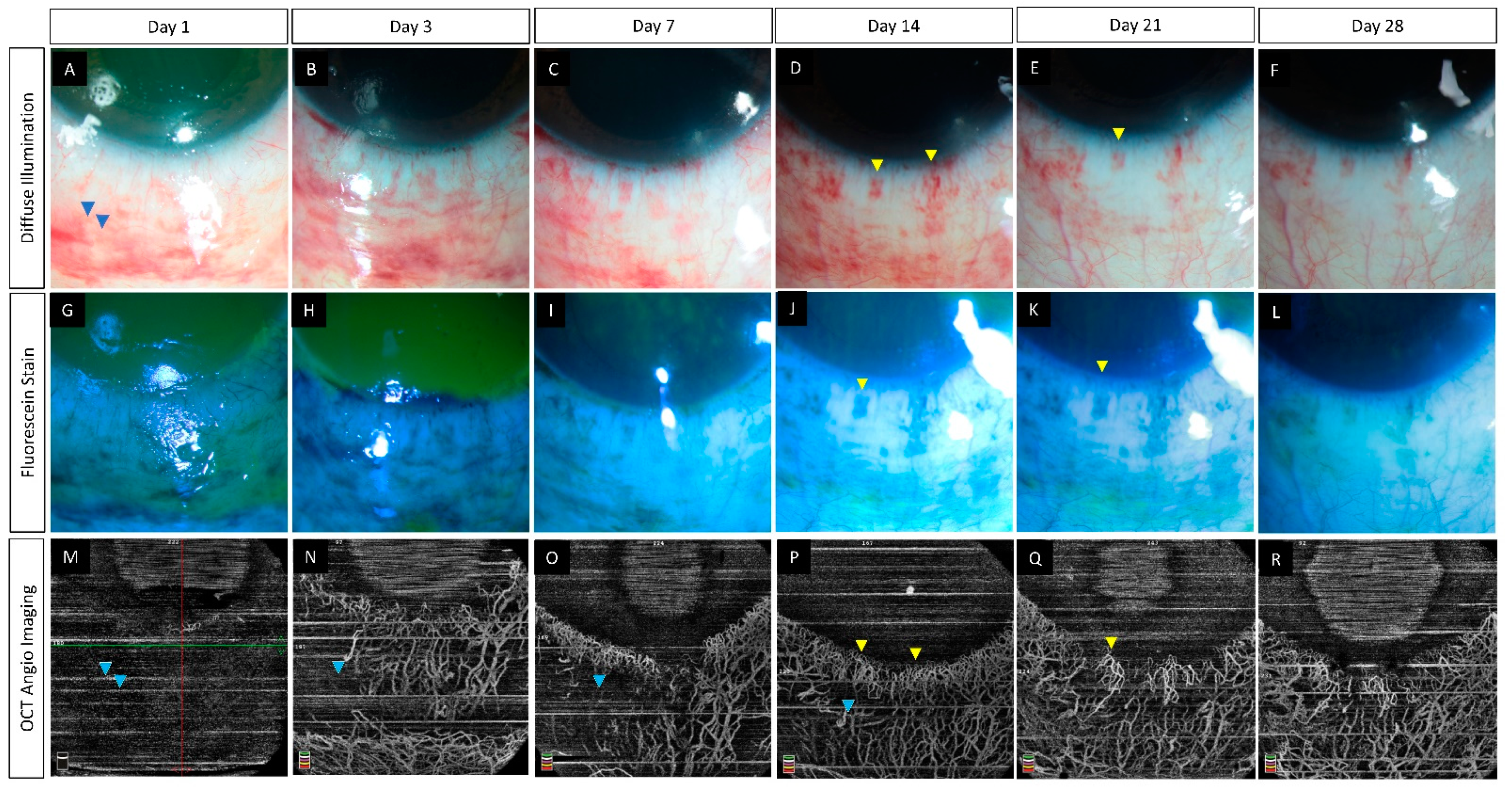
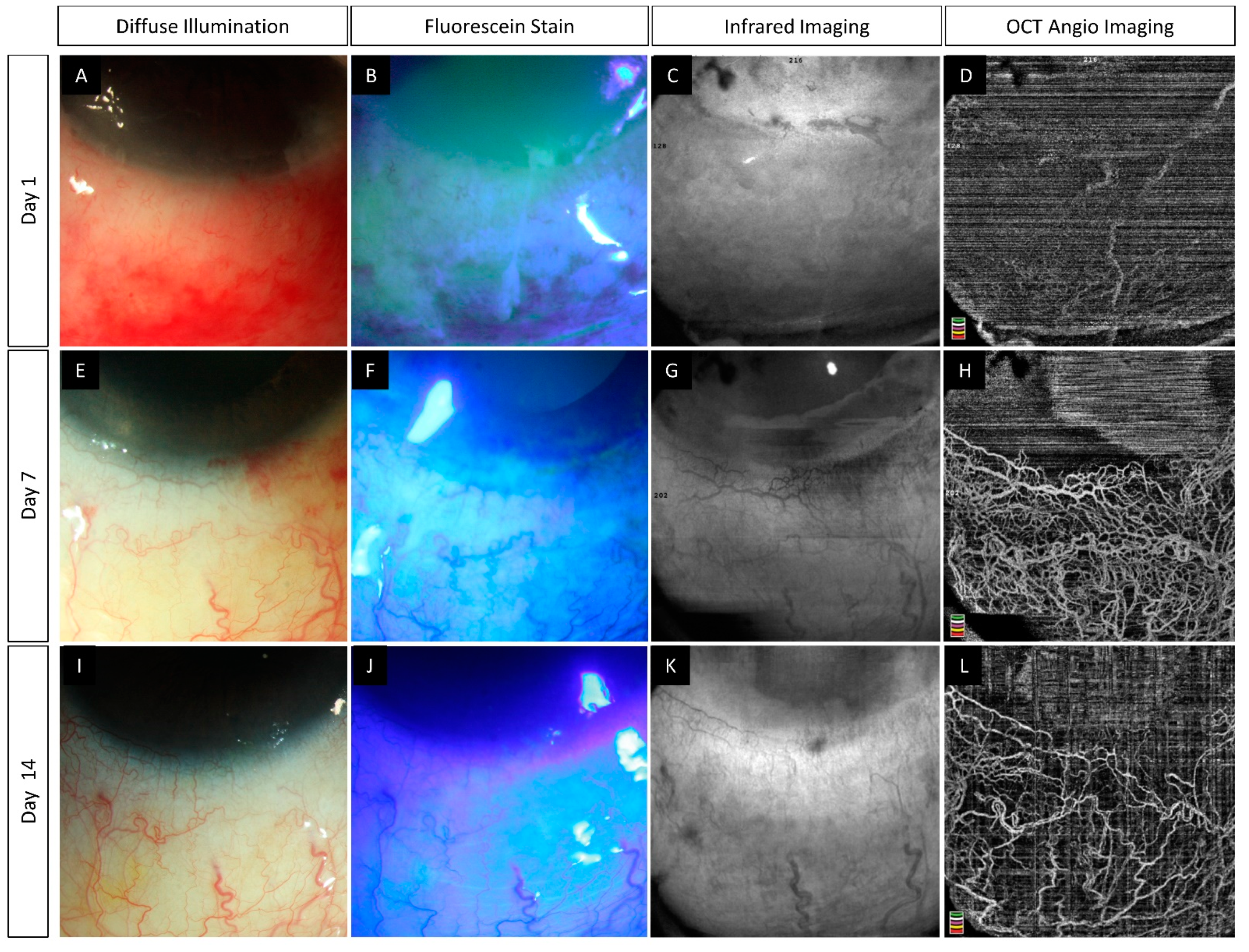
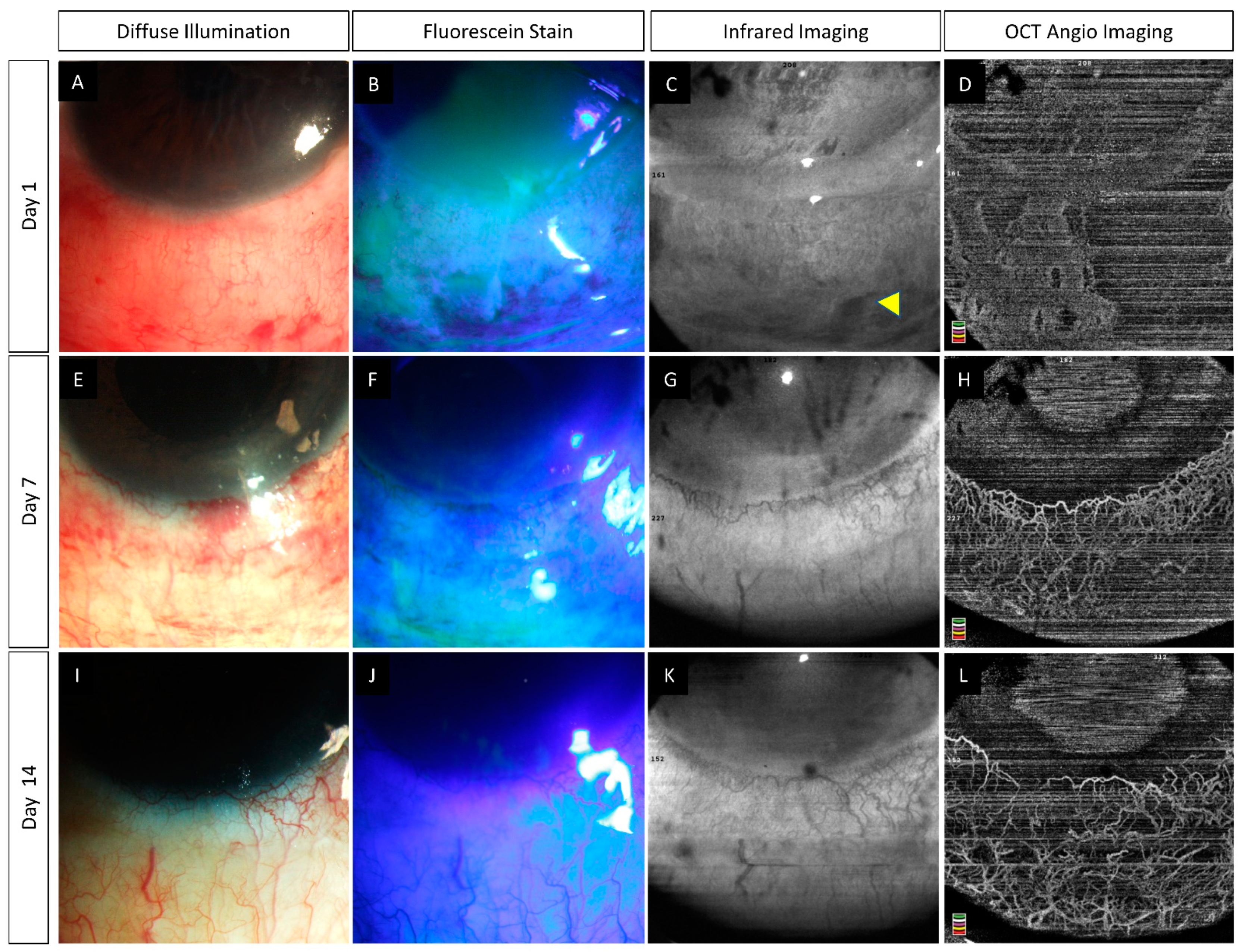
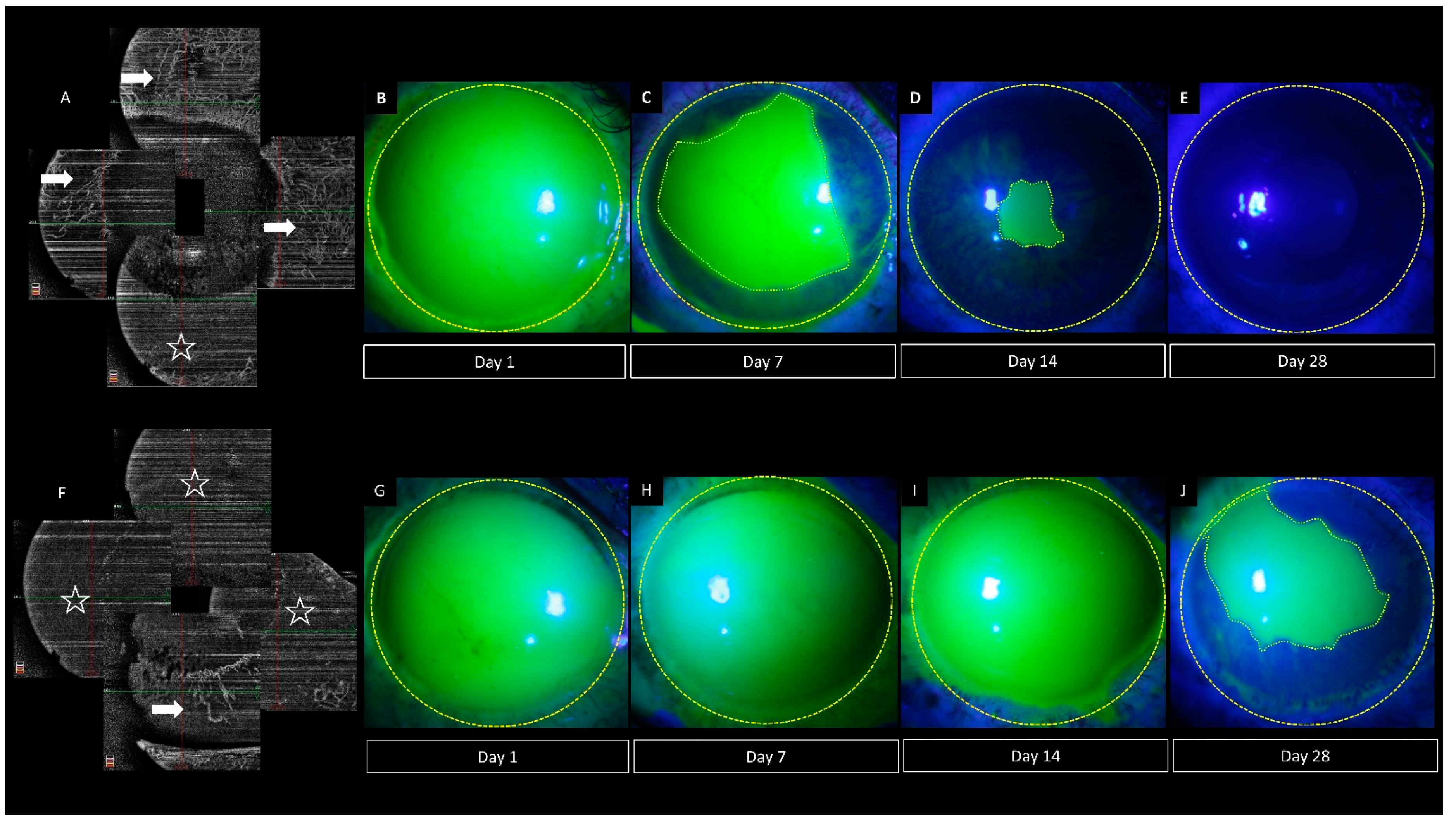
6.2. Pre-Clinical Studies
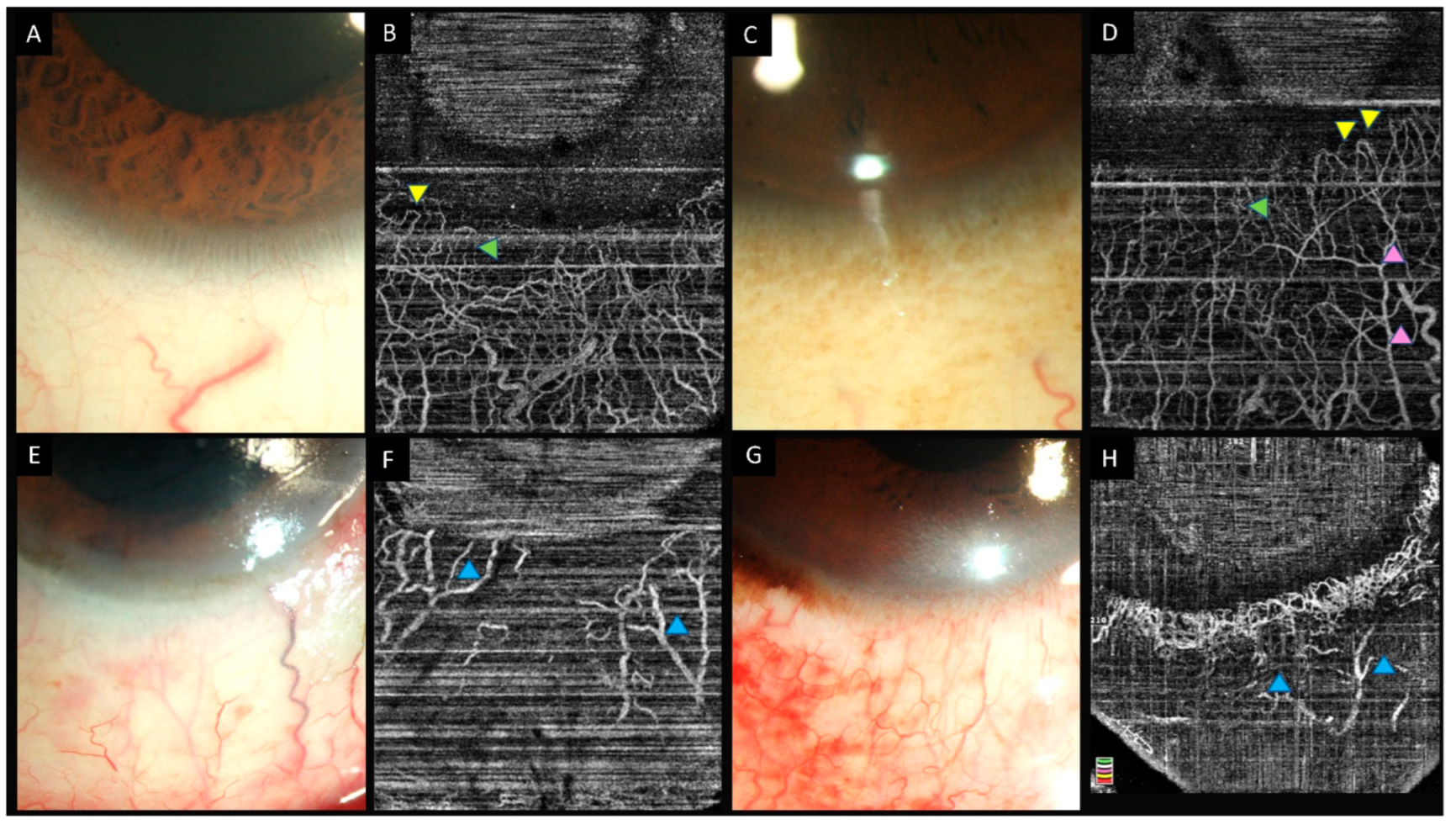
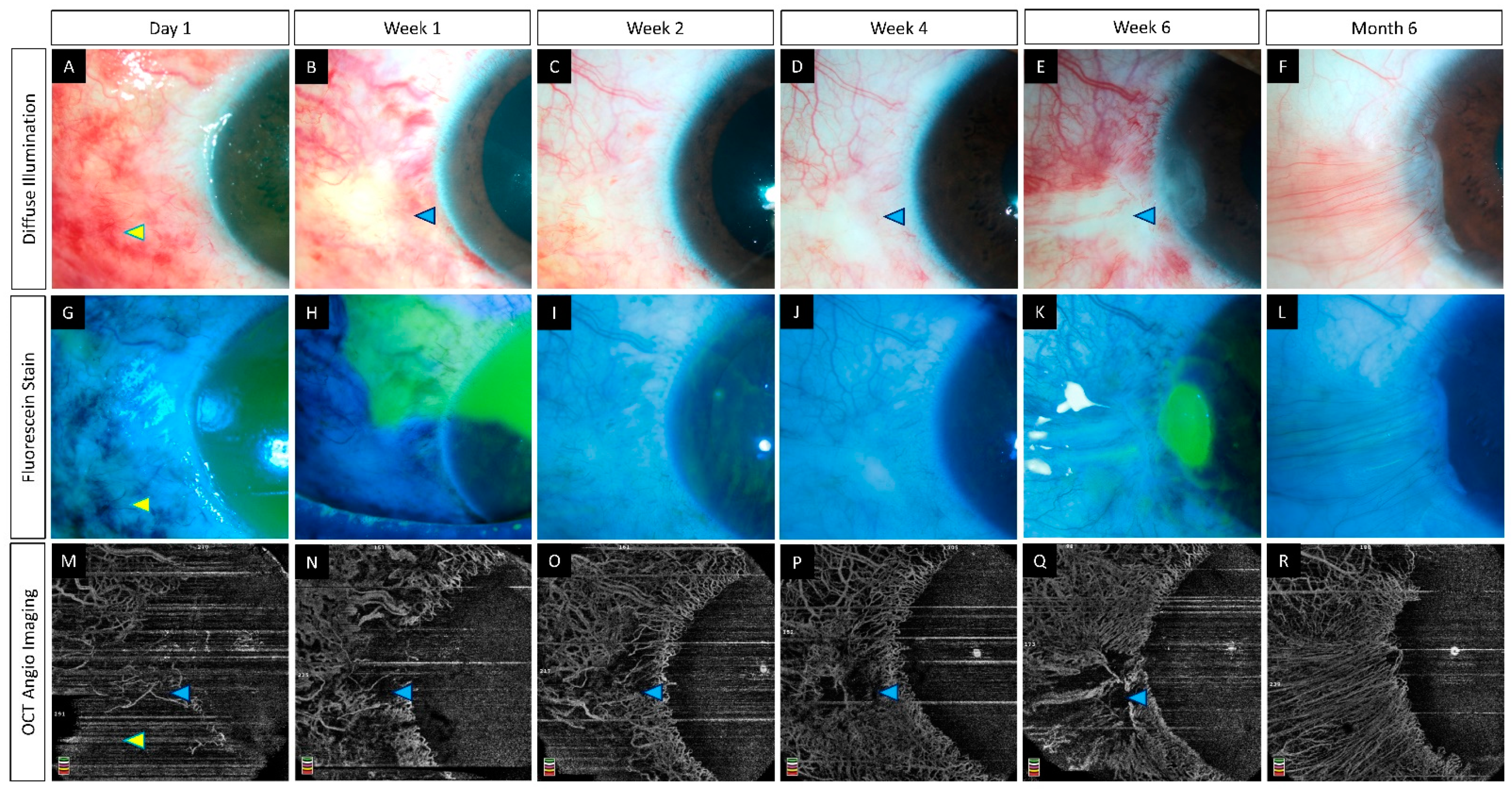
6.3. Clinical Studies
7. Future Directions
8. Summary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Haagdorens, M.; Van Acker, S.I.; Van Gerwen, V.; Ní Dhubhghaill, S.; Koppen, C.; Tassignon, M.-J.; Zakaria, N. Limbal Stem Cell Deficiency: Current Treatment Options and Emerging Therapies. Stem Cells Int. 2016, 2016, 9798374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kam, K.W.; Patel, C.N.; Nikpoor, N.; Yu, M.; Basu, S. Limbal Ischemia: Reliability of Clinical Assessment and Implications in the Management of Ocular Burns. Indian J. Ophthalmol. 2019, 67, 32–36. [Google Scholar] [CrossRef] [PubMed]
- Ayres, M.; Smallwood, R.; Brooks, A.M.; Chan, E.; Fagan, X. Anterior Segment Optical Coherence Tomography Angiography. J. Vis. Commun. Med. 2019, 42, 153–157. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.D.; Devarajan, K.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. Optical Coherence Tomography Angiography for the Anterior Segment. Eye Vis. 2019, 6, 4. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cozzi, M.; Staurenghi, G.; Invernizzi, A. Anterior Segment and Ocular Adnexa OCT Angiography. Ophthalmology 2020, 127, 220. [Google Scholar] [CrossRef]
- Patel, C.N.; Antony, A.K.; Kommula, H.; Shah, S.; Singh, V.; Basu, S. Optical Coherence Tomography Angiography of Perilimbal Vasculature: Validation of a Standardised Imaging Algorithm. Br. J. Ophthalmol. 2020, 104, 404–409. [Google Scholar] [CrossRef]
- Fujimoto, J.G.; Pitris, C.; Boppart, S.A.; Brezinski, M.E. Optical Coherence Tomography: An Emerging Technology for Biomedical Imaging and Optical Biopsy. Neoplasia 2000, 2, 9–25. [Google Scholar] [CrossRef] [Green Version]
- Borrelli, E.; Parravano, M.; Sacconi, R.; Costanzo, E.; Querques, L.; Vella, G.; Bandello, F.; Querques, G. Guidelines on Optical Coherence Tomography Angiography Imaging: 2020 Focused Update. Ophthalmol. Ther. 2020, 9, 697–707. [Google Scholar] [CrossRef]
- Munk, M.R.; Giannakaki-Zimmermann, H.; Berger, L.; Huf, W.; Ebneter, A.; Wolf, S.; Zinkernagel, M.S. OCT-Angiography: A Qualitative and Quantitative Comparison of 4 OCT-A Devices. PLoS ONE 2017, 12, e0177059. [Google Scholar] [CrossRef]
- Onishi, A.C.; Fawzi, A.A. An Overview of Optical Coherence Tomography Angiography and the Posterior Pole. Ther. Adv. Ophthalmol. 2019, 11, 2515841419840249. [Google Scholar] [CrossRef] [Green Version]
- Jia, Y.; Tan, O.; Tokayer, J.; Potsaid, B.; Wang, Y.; Liu, J.J.; Kraus, M.F.; Subhash, H.; Fujimoto, J.G.; Hornegger, J.; et al. Split-Spectrum Amplitude-Decorrelation Angiography with Optical Coherence Tomography. Opt. Express 2012, 20, 4710–4725. [Google Scholar] [CrossRef] [Green Version]
- An, L.; Wang, R.K. In Vivo Volumetric Imaging of Vascular Perfusion within Human Retina and Choroids with Optical Micro-Angiography. Opt. Express 2008, 16, 11438–11452. [Google Scholar] [CrossRef]
- Wang, R.K.; An, L.; Francis, P.; Wilson, D.J. Depth-Resolved Imaging of Capillary Networks in Retina and Choroid Using Ultrahigh Sensitive Optical Microangiography. Opt. Lett 2010, 35, 1467–1469. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stanga, P.E.; Tsamis, E.; Papayannis, A.; Stringa, F.; Cole, T.; Jalil, A. Swept-Source Optical Coherence Tomography AngioTM (Topcon Corp, Japan): Technology Review. Dev. Ophthalmol. 2016, 56, 13–17. [Google Scholar] [CrossRef] [PubMed]
- Ang, M.; Cai, Y.; Tan, A.C.S. Swept Source Optical Coherence Tomography Angiography for Contact Lens-Related Corneal Vascularization. J. Ophthalmol. 2016, 2016, 9685297. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devarajan, K.; Di Lee, W.; Ong, H.S.; Lwin, N.C.; Chua, J.; Schmetterer, L.; Mehta, J.S.; Ang, M. Vessel Density and En-Face Segmentation of Optical Coherence Tomography Angiography to Analyse Corneal Vascularisation in an Animal Model. Eye Vis. 2019, 6, 2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, M.; Cai, Y.; MacPhee, B.; Sim, D.A.; Keane, P.A.; Sng, C.C.A.; Egan, C.A.; Tufail, A.; Larkin, D.F.; Wilkins, M.R. Optical Coherence Tomography Angiography and Indocyanine Green Angiography for Corneal Vascularisation. Br. J. Ophthalmol. 2016, 100, 1557–1563. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.; Zhu, W.; Zhuang, H.; Xu, J.; Sun, X.; Le, Q.; Li, G.; Wang, Y. In Vivo Confocal Microscopy of Conjunctival Goblet Cells in Patients with Sjogren’s Syndrome Dry Eye. Br. J. Ophthalmol. 2010, 94, 1454–1458. [Google Scholar] [CrossRef]
- Stanzel, T.P.; Devarajan, K.; Lwin, N.C.; Yam, G.H.; Schmetterer, L.; Mehta, J.S.; Ang, M. Comparison of Optical Coherence Tomography Angiography to Indocyanine Green Angiography and Slit Lamp Photography for Corneal Vascularization in an Animal Model. Sci. Rep. 2018, 8, 11493. [Google Scholar] [CrossRef] [Green Version]
- Ang, M.; Devarajan, K.; Das, S.; Stanzel, T.; Tan, A.; Girard, M.; Schmetterer, L.; Mehta, J.S. Comparison of Anterior Segment Optical Coherence Tomography Angiography Systems for Corneal Vascularisation. Br. J. Ophthalmol. 2018, 102, 873–877. [Google Scholar] [CrossRef]
- Kuckelkorn, R.; Remky, A.; Wolf, S.; Reim, M.; Redbrake, C. Video Fluorescein Angiography of the Anterior Eye Segment in Severe Eye Burns. Acta Ophthalmol. Scand. 1997, 75, 675–680. [Google Scholar] [CrossRef] [PubMed]
- Cai, Y.; Alio Del Barrio, J.L.; Wilkins, M.R.; Ang, M. Serial Optical Coherence Tomography Angiography for Corneal Vascularization. Graefes Arch. Clin. Exp. Ophthalmol. 2017, 255, 135–139. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Tzankova, V.; Li, C.; Sharma, S. Intravenous Fluorescein Angiography-Associated Adverse Reactions. Can. J. Ophthalmol. 2016, 51, 321–325. [Google Scholar] [CrossRef] [PubMed]
- Anijeet, D.R.; Zheng, Y.; Tey, A.; Hodson, M.; Sueke, H.; Kaye, S.B. Imaging and Evaluation of Corneal Vascularization Using Fluorescein and Indocyanine Green Angiography. Investig. Ophthalmol. Vis. Sci. 2012, 53, 650–658. [Google Scholar] [CrossRef] [Green Version]
- Ang, M.; Tan, A.C.S.; Cheung, C.M.G.; Keane, P.A.; Dolz-Marco, R.; Sng, C.C.A.; Schmetterer, L. Optical Coherence Tomography Angiography: A Review of Current and Future Clinical Applications. Graefes Arch. Clin. Exp. Ophthalmol. 2018, 256, 237–245. [Google Scholar] [CrossRef]
- Fung, S.S.M.; Stewart, R.M.K.; Dhallu, S.K.; Sim, D.A.; Keane, P.A.; Wilkins, M.R.; Tuft, S.J. Anterior Segment Optical Coherence Tomographic Angiography Assessment of Acute Chemical Injury. Am. J. Ophthalmol. 2019, 205, 165–174. [Google Scholar] [CrossRef]
- Shu, X.; Beckmann, L.; Zhang, H.F. Visible-Light Optical Coherence Tomography: A Review. J. Biomed. Opt. 2017, 22, 121707. [Google Scholar] [CrossRef]
- Akagi, T.; Uji, A.; Huang, A.S.; Weinreb, R.N.; Yamada, T.; Miyata, M.; Kameda, T.; Ikeda, H.O.; Tsujikawa, A. Conjunctival and Intrascleral Vasculatures Assessed Using Anterior Segment Optical Coherence Tomography Angiography in Normal Eyes. Am. J. Ophthalmol. 2018, 196, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Giarratano, Y.; Bianchi, E.; Gray, C.; Morris, A.; MacGillivray, T.; Dhillon, B.; Bernabeu, M.O. Automated Segmentation of Optical Coherence Tomography Angiography Images: Benchmark Data and Clinically Relevant Metrics. Transl. Vis. Sci. Technol. 2020, 9, 5. [Google Scholar] [CrossRef]
- Siddiqui, Y.; Yin, J. Anterior Segment Applications of Optical Coherence Tomography Angiography. Semin. Ophthalmol. 2019, 34, 264–269. [Google Scholar] [CrossRef]
- Luo, M.; Li, Y.; Zhuo, Y. Advances and Current Clinical Applications of Anterior Segment Optical Coherence Tomography Angiography. Front. Med. 2021, 8, 721442. [Google Scholar] [CrossRef] [PubMed]
- Meyer, P.A. The Circulation of the Human Limbus. Eye 1989, 3, 121–127. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Chodosh, J. Angiography of the Limbus and Cornea. Int. Ophthalmol. Clin. 2019, 59, 19–29. [Google Scholar] [CrossRef] [PubMed]
- Brunner, M.; Romano, V.; Steger, B.; Vinciguerra, R.; Lawman, S.; Williams, B.; Hicks, N.; Czanner, G.; Zheng, Y.; Willoughby, C.E.; et al. Imaging of Corneal Neovascularization: Optical Coherence Tomography Angiography and Fluorescence Angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 1263–1269. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ang, M.; Foo, V.; Ke, M.; Tan, B.; Tong, L.; Schmetterer, L.; Mehta, J.S. Role of Anterior Segment Optical Coherence Tomography Angiography in Assessing Limbal Vasculature in Acute Chemical Injury of the Eye. Br. J. Ophthalmol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Li, X.-X.; Wu, W.; Zhou, H.; Deng, J.-J.; Zhao, M.-Y.; Qian, T.-W.; Yan, C.; Xu, X.; Yu, S.-Q. A Quantitative Comparison of Five Optical Coherence Tomography Angiography Systems in Clinical Performance. Int. J. Ophthalmol. 2018, 11, 1784–1795. [Google Scholar] [CrossRef]
- Pichi, F.; Roberts, P.; Neri, P. The Broad Spectrum of Application of Optical Coherence Tomography Angiography to the Anterior Segment of the Eye in Inflammatory Conditions: A Review of the Literature. J. Ophthalmic. Inflamm. Infect. 2019, 9, 18. [Google Scholar] [CrossRef] [Green Version]
- Ang, M.; Baskaran, M.; Werkmeister, R.M.; Chua, J.; Schmidl, D.; Aranha dos Santos, V.; Garhöfer, G.; Mehta, J.S.; Schmetterer, L. Anterior Segment Optical Coherence Tomography. Prog. Retin. Eye Res. 2018, 66, 132–156. [Google Scholar] [CrossRef]
- Varma, S.; Shanbhag, S.S.; Donthineni, P.R.; Mishra, D.K.; Singh, V.; Basu, S. High-Resolution Optical Coherence Tomography Angiography Characteristics of Limbal Stem Cell Deficiency. Diagnostics 2021, 11, 1130. [Google Scholar] [CrossRef]
- Durbin, M.K.; An, L.; Shemonski, N.D.; Soares, M.; Santos, T.; Lopes, M.; Neves, C.; Cunha-Vaz, J. Quantification of Retinal Microvascular Density in Optical Coherence Tomographic Angiography Images in Diabetic Retinopathy. JAMA Ophthalmol. 2017, 135, 370–376. [Google Scholar] [CrossRef]
- Bostanci Ceran, B.; Ozates, S.; Arifoglu, H.B.; Tasindi, E. Changes in Limbal Optical Coherence Tomography Angiography Outcomes in Patients With Overnight Contact Lens Wear. Eye Contact Lens 2021, 47, 552–554. [Google Scholar] [CrossRef] [PubMed]
- Huang, P.-W.; Lee, C.-H. Automatic Classification for Pathological Prostate Images Based on Fractal Analysis. IEEE Trans. Med. Imaging 2009, 28, 1037–1050. [Google Scholar] [CrossRef]
- Kim, A.Y.; Chu, Z.; Shahidzadeh, A.; Wang, R.K.; Puliafito, C.A.; Kashani, A.H. Quantifying Microvascular Density and Morphology in Diabetic Retinopathy Using Spectral-Domain Optical Coherence Tomography Angiography. Investig. Ophthalmol. Vis. Sci. 2016, 57, OCT362–OCT370. [Google Scholar] [CrossRef] [PubMed]
- Reif, R.; Qin, J.; An, L.; Zhi, Z.; Dziennis, S.; Wang, R. Quantifying Optical Microangiography Images Obtained from a Spectral Domain Optical Coherence Tomography System. Int. J. Biomed. Imaging 2012, 2012, 509783. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Q.; Zheng, F.; Motulsky, E.H.; Gregori, G.; Chu, Z.; Chen, C.-L.; Li, C.; de Sisternes, L.; Durbin, M.; Rosenfeld, P.J.; et al. A Novel Strategy for Quantifying Choriocapillaris Flow Voids Using Swept-Source OCT Angiography. Investig. Ophthalmol. Vis. Sci. 2018, 59, 203–211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hagag, A.M.; Gao, S.S.; Jia, Y.; Huang, D. Optical Coherence Tomography Angiography: Technical Principles and Clinical Applications in Ophthalmology. Taiwan J. Ophthalmol. 2017, 7, 115–129. [Google Scholar] [CrossRef] [PubMed]
- Yao, X.; Alam, M.N.; Le, D.; Toslak, D. Quantitative Optical Coherence Tomography Angiography: A Review. Exp. Biol. Med. 2020, 245, 301–312. [Google Scholar] [CrossRef]
- Chu, Z.; Lin, J.; Gao, C.; Xin, C.; Zhang, Q.; Chen, C.-L.; Roisman, L.; Gregori, G.; Rosenfeld, P.J.; Wang, R.K. Quantitative Assessment of the Retinal Microvasculature Using Optical Coherence Tomography Angiography. J. Biomed. Opt. 2016, 21, 066008. [Google Scholar] [CrossRef] [Green Version]
- Tabatabaei, S.A.; Soleimani, M.; Mirshahi, R.; Zandian, M.; Ghasemi, H.; Hashemian, M.N.; Ghomi, Z. Selective Localized Tenonplasty for Corneal Burns Based on the Findings of Ocular Surface Fluorescein Angiography. Cornea 2017, 36, 1014–1017. [Google Scholar] [CrossRef]
- Kuckelkorn, R.; Redbrake, C.; Reim, M. Tenonplasty: A New Surgical Approach for the Treatment of Severe Eye Burns. Ophthalmic. Surg. Lasers 1997, 28, 105–110. [Google Scholar] [CrossRef]
- Sharma, N.; Kaur, M.; Agarwal, T.; Sangwan, V.S.; Vajpayee, R.B. Treatment of Acute Ocular Chemical Burns. Surv. Ophthalmol. 2018, 63, 214–235. [Google Scholar] [CrossRef]
- Bizrah, M.; Yusuf, A.; Ahmad, S. An Update on Chemical Eye Burns. Eye 2019, 33, 1362–1377. [Google Scholar] [CrossRef] [PubMed]
- Sangwan, V.S. Limbal Stem Cells in Health and Disease. Biosci. Rep. 2001, 21, 385–405. [Google Scholar] [CrossRef] [Green Version]
- Le, Q.; Xu, J.; Deng, S.X. The Diagnosis of Limbal Stem Cell Deficiency. Ocul. Surf. 2018, 16, 58–69. [Google Scholar] [CrossRef]
- Tey, K.Y.; Gan, J.; Foo, V.; Tan, B.; Ke, M.Y.; Schmetterer, L.; Mehta, J.S.; Ang, M. Role of Anterior Segment Optical Coherence Tomography Angiography in the Assessment of Acute Chemical Ocular Injury: A Pilot Animal Model Study. Sci. Rep. 2021, 11, 16625. [Google Scholar] [CrossRef] [PubMed]
- Luisi, J.; Kraft, E.R.; Giannos, S.A.; Patel, K.; Schmitz-Brown, M.E.; Reffatto, V.; Merkley, K.H.; Gupta, P.K. Longitudinal Assessment of Alkali Injury on Mouse Cornea Using Anterior Segment Optical Coherence Tomography. Transl. Vis. Sci. Technol. 2021, 10, 6. [Google Scholar] [CrossRef] [PubMed]
- Rocha de Lossada, C.; Pagano, L.; Gadhvi, K.A.; Borroni, D.; Figueiredo, G.; Kaye, S.; Romano, V. Persistent Loss of Marginal Corneal Arcades after Chemical Injury. Indian J. Ophthalmol. 2020, 68, 2543–2544. [Google Scholar] [CrossRef]
- Fard, A.; Wang, Y.; Kost, J.; Sha, P.; Mitra, R.; Leahy, C. Ultra-Widefield Anterior Segment OCT Angiography for Visualization of Iris, Limbus and Sclera. Investig. Ophthalmol. Vis. Sci. 2020, 61, PB0028. [Google Scholar]
- Mazlin, V.; Xiao, P.; Scholler, J.; Irsch, K.; Grieve, K.; Fink, M.; Boccara, A.C. Real-Time Non-Contact Cellular Imaging and Angiography of Human Cornea and Limbus with Common-Path Full-Field/SD OCT. Nat. Commun. 2020, 11, 1868. [Google Scholar] [CrossRef]
| Indices | Method of Calculation [14,15] | Range [28,41] | |
|---|---|---|---|
| Superficial layer | Deep layer | ||
| Vessel density (%) | Area occupied by the vessels/ Total area of the background | 24–31 | 21–29 |
| Vessel length density (%) | Skeletonized vessel pixel area/ total pixel count | 4–5 | 3.5–4.5 |
| Vessel diameter index | Total vessel area in binarized image/ Total vessel area in the skeletonized image | 10–17 | 10–18 |
| Fractal dimension | Box counting method | 1.3–1.5 | 1.2–1.5 |
| Author (Year) | Study Population (Eyes) | Device Used | Duration of Follow up (Months) | Outcome |
|---|---|---|---|---|
| Fung et al., (2019) [26] | Humans (15) | AngioVue | 1 | (i) Clinically determined area of ischemia is underestimated when compared to estimated ischemia on OCTA. (ii) Area of ischemia on OCTA correlates with visual acuity at 3 months. |
| Ang et al., (2021) [35] | Humans (10) | (i) AngioVue (ii) Plex Elite | 3 | (i) Good agreement between the two devices for the measurement of VD. (ii) OCTA is more reliable than clinical examination for the assessment of limbal ischemia. (iii) Area of ischemia may predict the future development of LSCD. |
| Tey et al., (2021) [55] | Rabbits (12) | Spectralis-domain OCT (Nidek Co., Ltd., Gamagori, Aichi, Japan) | 1 | (i) Decrease in VD correlated with the severity of the induced injury. (ii) Proposed classification system which incorporates limbal ischemia as estimated by OCTA. |
| Luisi et al., (2021) [56] | Mice (12) | Envisu R2200 (Bioptigen, Durham, NC) | 0.5 | (i) Both OCTA and FA angiography modalities yielded similar results. (ii) Detection of neovascularization in the limbal area as early as 4 days following the injury. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kate, A.; Basu, S. Role of Anterior Segment-Optical Coherence Tomography Angiography in Acute Ocular Burns. Diagnostics 2022, 12, 607. https://doi.org/10.3390/diagnostics12030607
Kate A, Basu S. Role of Anterior Segment-Optical Coherence Tomography Angiography in Acute Ocular Burns. Diagnostics. 2022; 12(3):607. https://doi.org/10.3390/diagnostics12030607
Chicago/Turabian StyleKate, Anahita, and Sayan Basu. 2022. "Role of Anterior Segment-Optical Coherence Tomography Angiography in Acute Ocular Burns" Diagnostics 12, no. 3: 607. https://doi.org/10.3390/diagnostics12030607
APA StyleKate, A., & Basu, S. (2022). Role of Anterior Segment-Optical Coherence Tomography Angiography in Acute Ocular Burns. Diagnostics, 12(3), 607. https://doi.org/10.3390/diagnostics12030607







