Spin Lattice (T1) and Magnetization Transfer Saturation (MTsat) Imaging to Monitor Age-Related Differences in Skeletal Muscle Tissue
Abstract
:1. Introduction
2. Materials and Methods
2.1. Subjects
2.2. MRI Acquisition
2.2.1. T1 Mapping
2.2.2. Magnetization Transfer Saturation (MTsat) Imaging
2.3. Computation of T1 and MTsat Maps
2.4. Statistical Analysis
3. Results
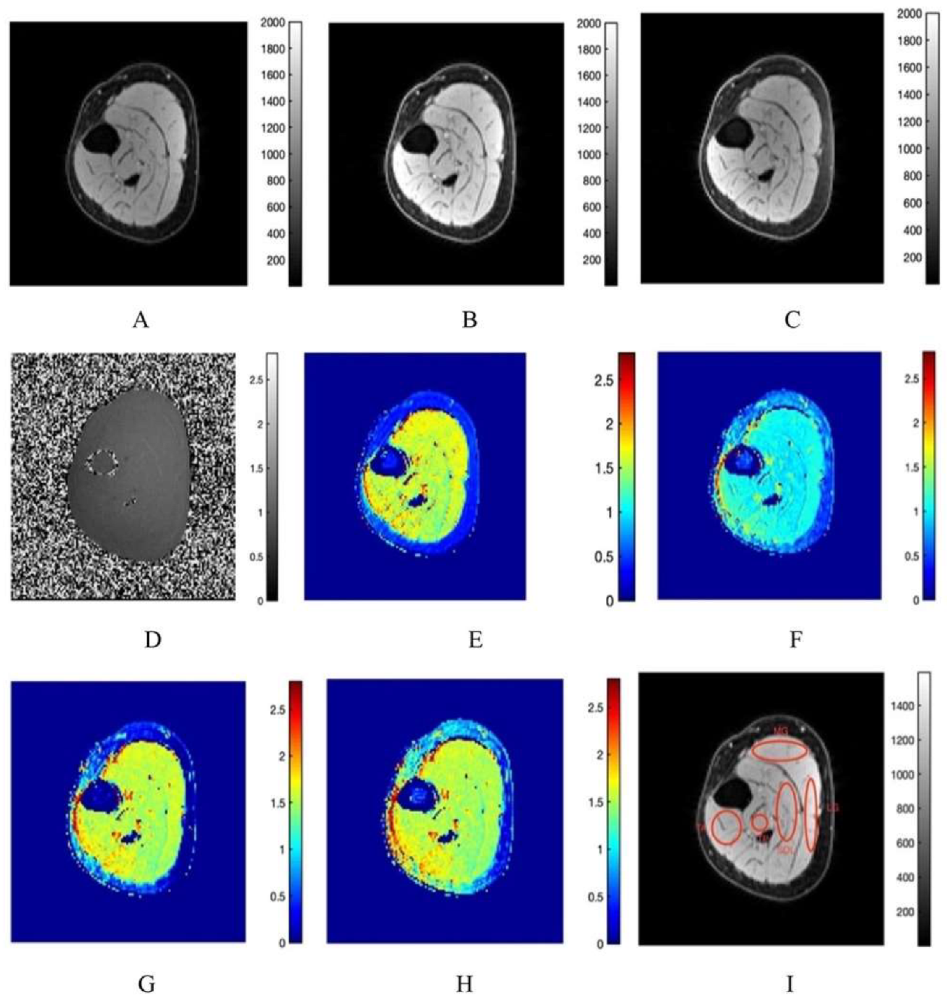
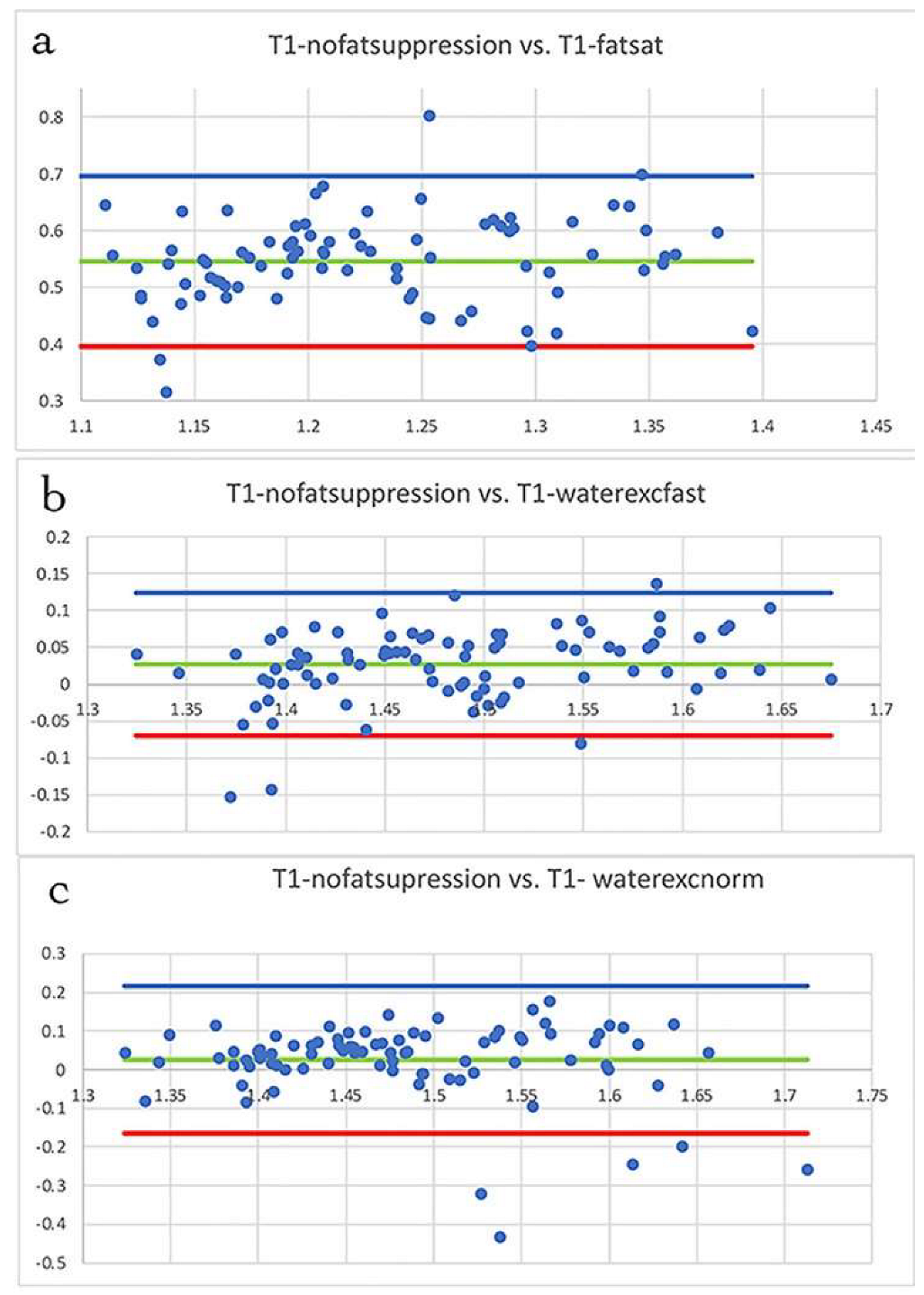
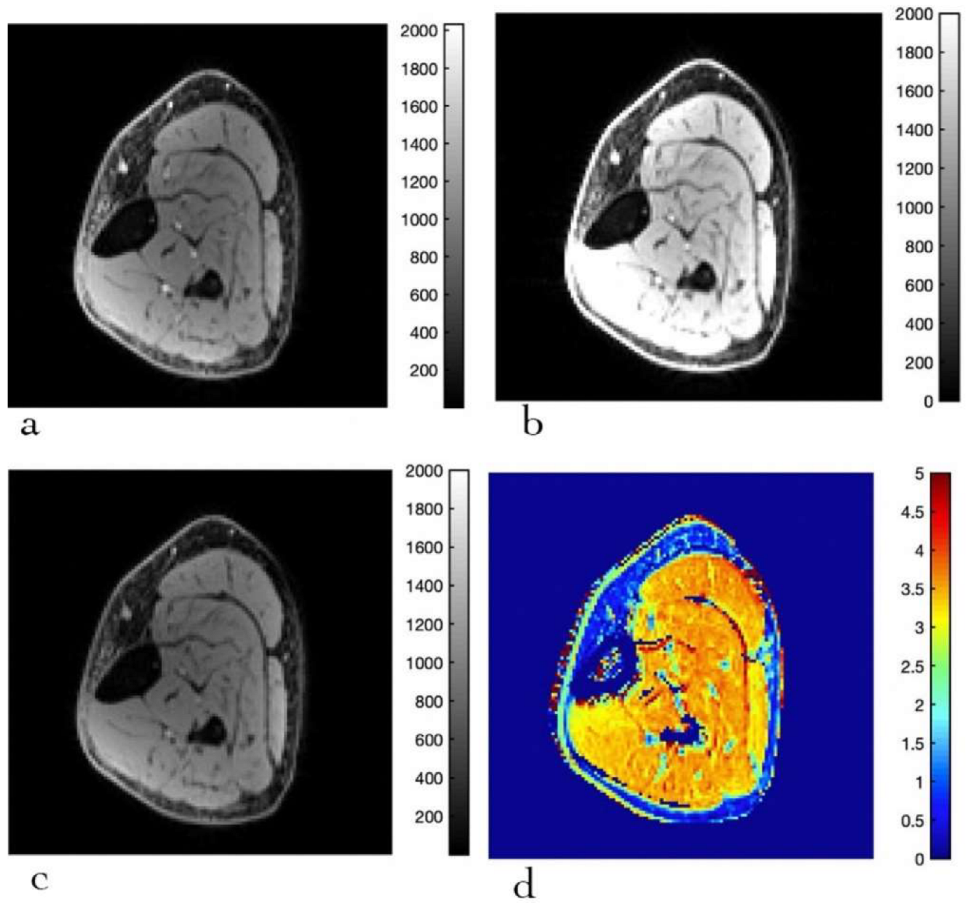
3.1. Fat Suppression
3.2. T1 Mapping
3.3. MTsat Mapping
4. Discussion
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Goodpaster, B.H.; Park, S.W.; Harris, T.B.; Kritchevsky, S.B.; Nevitt, M.; Schwartz, A.V.; Simonsick, E.M.; Tylavsky, F.A.; Visser, M.; Newman, A.B. The loss of skeletal muscle strength, mass, and quality in older adults: The health, aging and body composition study. J. Gerontol. A Biol. Sci. Med. Sci. 2006, 61, 1059–1064. [Google Scholar] [CrossRef] [PubMed]
- Matthews, G.D.K.; Huang, C.L.H.; Sun, L.; Zaidi, M. Translational musculoskeletal science: Is sarcopenia the next clinical target after osteoporosis? Ann. N. Y. Acad. Sci. 2011, 1237, 95–105. [Google Scholar] [CrossRef] [PubMed]
- Clark, B.C.; Manini, T.M. Functional consequences of sarcopenia and dynopenia in the elderly. Curr. Opin. Clin. Nutr. Metab. Care 2010, 13, 271–276. [Google Scholar] [CrossRef] [Green Version]
- Ramaswamy, K.S.; Palmer, M.L.; van der Meulen, J.H.; Renoux, A.; Kostrominova, T.Y.; Michele, D.E.; Faulkner, J.A. Lateral transmission of force is impaired in skeletal muscles of dystrophic mice and very old rats. J. Physiol. Lond. 2011, 589, 1195–1208. [Google Scholar] [CrossRef] [PubMed]
- Lacraz, G.; Rouleau, A.J.; Couture, V.; Söllrald, T.; Drouin, G.; Veillette, N.; Grandbois, M.; Grenier, G. Increased Stiffness in Aged Skeletal Muscle Impairs Muscle Progenitor Cell ProliferativeActivity. PLoS ONE 2015, 10, e0136217. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cercignani, M.; Symms, M.R.; Schmier, K.; Boulby, P.A.; Tozer, D.J.; Ron, M.; Tofts, P.S.; Barker, G.J. Three-dimensional quantitative magnetization transfer imaging of the human brain. Neuroimage 2005, 27, 436. [Google Scholar] [CrossRef] [PubMed]
- Schmierer, K.; Wheeler-Kingshott, C.A.; Tozer, D.J.; Boulby, P.A.; Parkes, H.G.; Yousry, T.A.; Scaravilli, F.; Barker, G.J.; Tofts, P.S.; Miller, D.H. Quantitative magnetic resonance of postmortem multiple sclerosis brain before and after fixation. Magn. Reson. Med. 2008, 59, 268. [Google Scholar] [CrossRef] [Green Version]
- Sled, J.G.; Pike, G.B. Quantitative interpretation of magnetization transfer in spoiled gradient echo MRI sequences. J. Magn. Reson. 2000, 145, 24–36. [Google Scholar] [CrossRef] [Green Version]
- Ramani, A.; Dalton, C.; Miller, D.H.; Tofts, P.S.; Barker, G.J. Precise estimate of fundamental in-vivo MT parameters in human brain in clinically feasible times. Magn. Reson. Imaging 2002, 20, 72. [Google Scholar] [CrossRef]
- Helms, G.; Dathe, H.; Kallenberg, K.; Dechent, P. High-resolution maps of magnetization transfer with inherent correction for RF inhomogeneity and T1 relaxation obtained from 3D FLASH MRI. Magn. Reson. Med. 2008, 60, 1396. [Google Scholar] [CrossRef]
- Zembron-Lacny, A.; Dziubek, W.; Wolny-Rokicka, E.; Dabrowska, G.; Wozniewski, M. The Relation of Inflammaging With Skeletal Muscle Properties in Elderly Men. Am. J. Mens Health 2019, 13, 1557988319841934. [Google Scholar] [CrossRef] [Green Version]
- Ponti, F.; Santoro, A.; Mercatelli, D.; Gasperini, C.; Conte, M.; Martucci, M.; Sangiorgi, L.; Franceschi, C.; Bazzocchi, A. Aging and Imaging Assessment of Body Composition: From Fat to Facts. Front. Endocrinol. 2020, 14, 861. [Google Scholar] [CrossRef] [Green Version]
- Hotamisligil, G.S. Inflammation and metabolic disorders. Nature 2006, 14, 860–867. [Google Scholar] [CrossRef]
- Petersen, A.M.; Pedersen, B.K. The anti-inflammatory effect of exercise. J. Appl. Physiol. 2005, 98, 1154–1162. [Google Scholar] [CrossRef] [Green Version]
- Zong, G.; Zhang, Z.; Yang, Q.; Wu, H.; Hu, F.B.; Sun, Q. Total and regional adiposity measured by dual-energy X-ray absorptiometry and mortality in NHANES 1999–2006. HHS Public Access 2017, 24, 2414–2421. [Google Scholar] [CrossRef] [Green Version]
- Csapo, R.; Malis, V.; Sinha, U.; Du, J.; Sinha, S. Age-associated differences in triceps surae muscle composition and strength—An MRI-based cross-sectional comparison of contractile, adipose and connective tissue. BMC Musculoskelet. Disord. 2014, 15, 209. [Google Scholar] [CrossRef] [Green Version]
- Harkins, K.D.; Xu, J.; Dula, A.N.; Li, K.; Valentine, W.M.; Gochberg, D.F.; Gore, J.C.; Does, M.D. The microstructural correlates of T1 in white matter. Magn. Reson. Med. 2016, 75, 1341–1345. [Google Scholar] [CrossRef] [Green Version]
- Damon, B.M.; Li, K.; Dortch, R.D.; Welch, E.B.; Park, J.H.; Buck, A.K.; Towse, T.F.; Does, M.D.; Gochberg, D.F.; Bryant, N.D. Quantitative Magnetic Resonance Imaging of Skeletal Muscle Disease. J. Vis. Exp. 2016, 118, 52352. [Google Scholar] [CrossRef]
- Morrow, J.M.; Sinclair, C.D.; Fischmann, A.; Reilly, M.M.; Hanna, M.G.; Yousry, T.A.; Thornton, J.S. Reproducibility, and age, body-weight and gender dependency of candidate skeletal muscle MRI outcome measures in healthy volunteers. Eur. Radiol. 2014, 24, 1610–1620. [Google Scholar] [CrossRef] [Green Version]
- Chung, S.; Kim, D.; Breton, E.; Axel, L. Rapid B1 + mapping using a pre-conditioning RF pulse with turboFLASH readout. Magn. Reson. Med. 2011, 64, 439. [Google Scholar] [CrossRef] [Green Version]
- Cabana, J.F.; Gu, Y.; Boudreau, M.; Levesque, I.R.; Atchia, Y.; Sled, J.G.; Narayanan, S.; Arnold, D.L.; Pike, G.B.; Cohen-Adad, J.; et al. Quantitative magnetization transfer imaging made easy with qMTLab: Software for data simulation, analysis, and visualization. Concepts Magn. Reson. Part A 2015, 44, 263. [Google Scholar] [CrossRef]
- Romero, I.O.; Sinha, U. Magnetization transfer saturation imaging of human calf muscle: Reproducibility and sensitivity to regional and sex differences. J. Magn. Res. Imaging 2019, 50, 1227–1237. [Google Scholar] [CrossRef] [PubMed]
- Nilwik, R.; Snijders, T.; Leenders, M.; Groen, B.B.; van Kranenburg, J.; Verdijk, L.B.; van Loon, L.J. The decline in skeletal muscle mass with aging is mainly attributed to a reduction in type II muscle fiber size. Exp. Gerontol. 2013, 48, 492. [Google Scholar] [CrossRef] [PubMed]
- Sinclair, C.D.; Samson, R.S.; Thomas, D.L.; Weiskopf, N.; Lutti, A.; Thornton, J.S.; Golay, X. Quantitative magnetization transfer in in vivo healthy human skeletal muscle at 3 T. Magn. Reson. Med. 2010, 64, 1739–1748. [Google Scholar] [CrossRef] [Green Version]
- Li, K.; Dortch, R.D.; Kroop, S.F.; Huston, J.W.; Gochberg, D.F.; Park, J.H.; Damon, B.M. A rapid approach for quantitative magnetization transfer imaging in thigh muscles using the pulsed saturation method. Magn. Reson. Imaging 2015, 33, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Gumpenberger, M.; Wessner, B.; Graf, A.; Narici, M.V.; Fink, C.; Braun, S.; Hoser, C.; Blazevich, A.J.; Csapo, R. Remodeling the Skeletal Muscle Extracellular Matrix in Older Age-Effects of Acute Exercise Stimuli on Gene Expression. Int. J. Mol. Sci. 2020, 21, 7089. [Google Scholar] [CrossRef]
- Marty, B.; Carlier, P.G. Physiological and pathological skeletal muscle T1 changes quantified using a fast inversion-recovery radial NMR imaging sequence. Sci. Rep. 2019, 9, 6852–6859. [Google Scholar] [CrossRef] [Green Version]
- Marty, B.; Coppa, B.; Carlier, P.G. Monitoring skeletal muscle chronic fatty degenerations with fast T1-mapping. Eur. Radiol. 2018, 28, 4662–4668. [Google Scholar] [CrossRef]
- Edgerton, V.R.; Smith, J.L.; Simpson, D.R. Muscle fibre type populations of human leg muscles. Histochem. J. 1975, 7, 259–266. [Google Scholar] [CrossRef]
- Johnson, M.A.; Polgar, J.; Weightman, D.; Appleton, D. Data on the distribution of fiber types in thirty-six human muscles. An autopsy study. J. Neurol. Sci. 1973, 18, 111–129. [Google Scholar] [CrossRef]
- Green, H.J.; Daub, B.; Houston, M.E.; Thomson, J.A.; Fraser, I.; Ranney, D. Human vastus lateralis and gastrocnemius muscles: A comparative histochemical analysis. J. Neurol. Sci. 1981, 52, 201–210. [Google Scholar] [CrossRef]
- Schmierer, K.; Scaravilli, F.; Altmann, D.R.; Barker, G.J.; Miller, D.H. Magnetization transfer ratio and myelin in postmortem multiple sclerosis brain. Ann. Neurol. 2004, 56, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Rottmar, M.; Haralampieva, D.; Salemi, S.; Eberhardt, C.; Wurnig, M.C.; Boss, A.; Eberli, D. Magnetization transfer MR imaging to monitor muscle tissue formation during myogenic in vivo differentiation of muscle precursor cells. Radiology 2016, 281, 436. [Google Scholar] [CrossRef] [PubMed]
| T1 in Seconds (Mean ± SD) | TA | MG | LG | SOL | TP | |
|---|---|---|---|---|---|---|
| No Fat suppression, T1 (sec) b,c,d,e | Y | 1.47 ± 0.1 | 1.47 ± 0.09 | 1.59 ± 0.08 | 1.46 ± 0.04 | 1.48 ± 0.06 |
| S | 1.53 ± 0.07 | 1.5 ± 0.11 | 1.55 ± 0.12 | 1.44 ± 0.07 | 1.46 ± 0.06 | |
| Fat Saturation, T1 (sec) a,b,c,d,e,f,g,h | Y | 1.00 ± 0.07 | 0.9 ± 0.06 | 0.98 ± 0.09 | 0.90 ± 0.04 | 0.92 ± 0.05 |
| S | 1.04 ± 0.09 | 0.96 ± 0.0.6 | 1.01 ± 0.08 | 0.94 ± 0.07 | 0.90 ± 0.02 | |
| Water Exc.(1:1), T1 (sec) a,b,c,d,e | Y | 1.46 ± 0.08 | 1.44 ± 0.08 | 1.53 ± 0.08 | 1.42 ± 0.04 | 1.44 ± 0.04 |
| S | 1.51 ± 0.06 | 1.49 ± 0.08 | 1.57 ± 0.07 | 1.44 ± 0.04 | 1.43 ± 0.04 | |
| Water Exc.(1:2:1), T1 (sec)a,b,c,e | Y | 1.45 ± 0.08 | 1.41 ± 0.08 | 1.50 ± 0.06 | 1.41 ± 0.03 | 1.43 ± 0.04 |
| S | 1.54 ± 0.12 | 1.52 ± 0.16 | 1.59 ± 0.09 | 1.48 ± 0.10 | 1.45 ± 0.13 |
| MTsat (%) a,b,c,d,e (Mean ± SD) | TA | MG | LG | SOL | TP |
|---|---|---|---|---|---|
| Young (Y) | 3.19 ± 0.11 | 3.47 ± 0.17 | 3.38 ± 0.16 | 3.53 ± 0.09 | 3.41 ± 0.12 |
| Senior (S) | 3.16 ± 0.07 | 3.31 ± 0.15 | 3.13 ± 0.16 | 3.36 ± 0.19 | 3.34 ± 0.18 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
White, J.C.; Sinha, S.; Sinha, U. Spin Lattice (T1) and Magnetization Transfer Saturation (MTsat) Imaging to Monitor Age-Related Differences in Skeletal Muscle Tissue. Diagnostics 2022, 12, 584. https://doi.org/10.3390/diagnostics12030584
White JC, Sinha S, Sinha U. Spin Lattice (T1) and Magnetization Transfer Saturation (MTsat) Imaging to Monitor Age-Related Differences in Skeletal Muscle Tissue. Diagnostics. 2022; 12(3):584. https://doi.org/10.3390/diagnostics12030584
Chicago/Turabian StyleWhite, John Cameron, Shantanu Sinha, and Usha Sinha. 2022. "Spin Lattice (T1) and Magnetization Transfer Saturation (MTsat) Imaging to Monitor Age-Related Differences in Skeletal Muscle Tissue" Diagnostics 12, no. 3: 584. https://doi.org/10.3390/diagnostics12030584
APA StyleWhite, J. C., Sinha, S., & Sinha, U. (2022). Spin Lattice (T1) and Magnetization Transfer Saturation (MTsat) Imaging to Monitor Age-Related Differences in Skeletal Muscle Tissue. Diagnostics, 12(3), 584. https://doi.org/10.3390/diagnostics12030584





