Clear Cell Renal Cell Carcinoma Metastasized to the Ampulla of Vater 16 Years after Nephrectomy—A Rare Case
Abstract
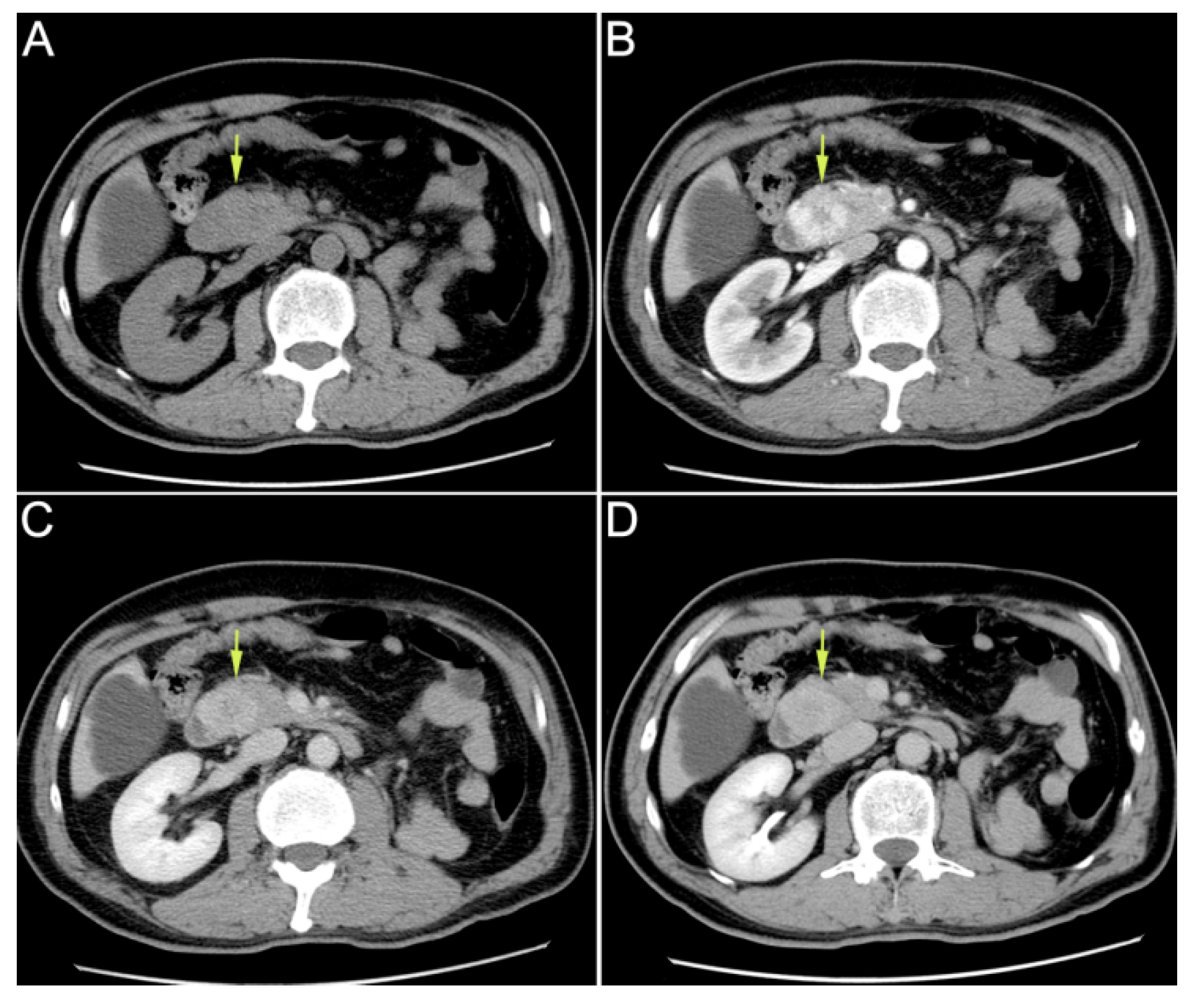
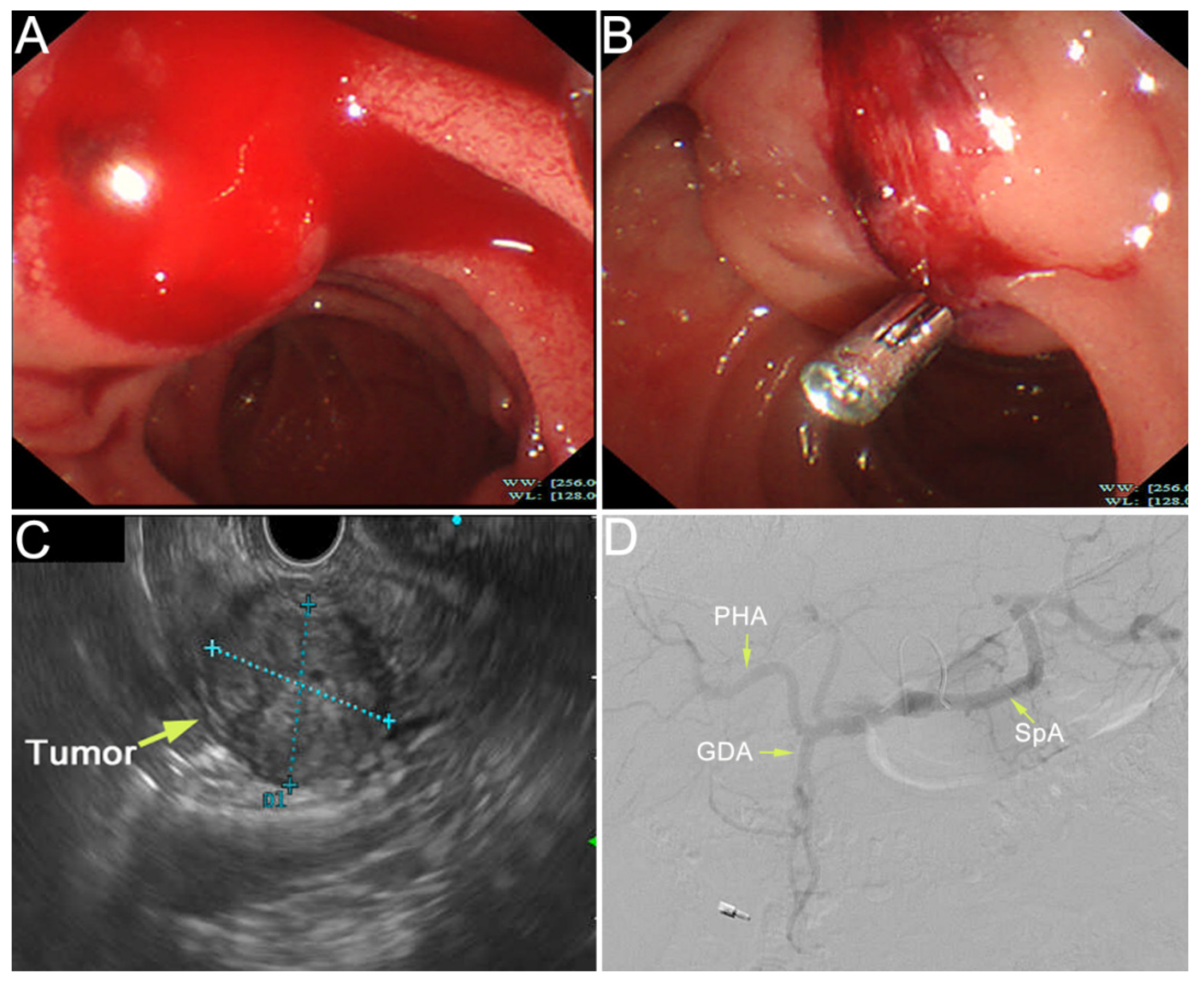
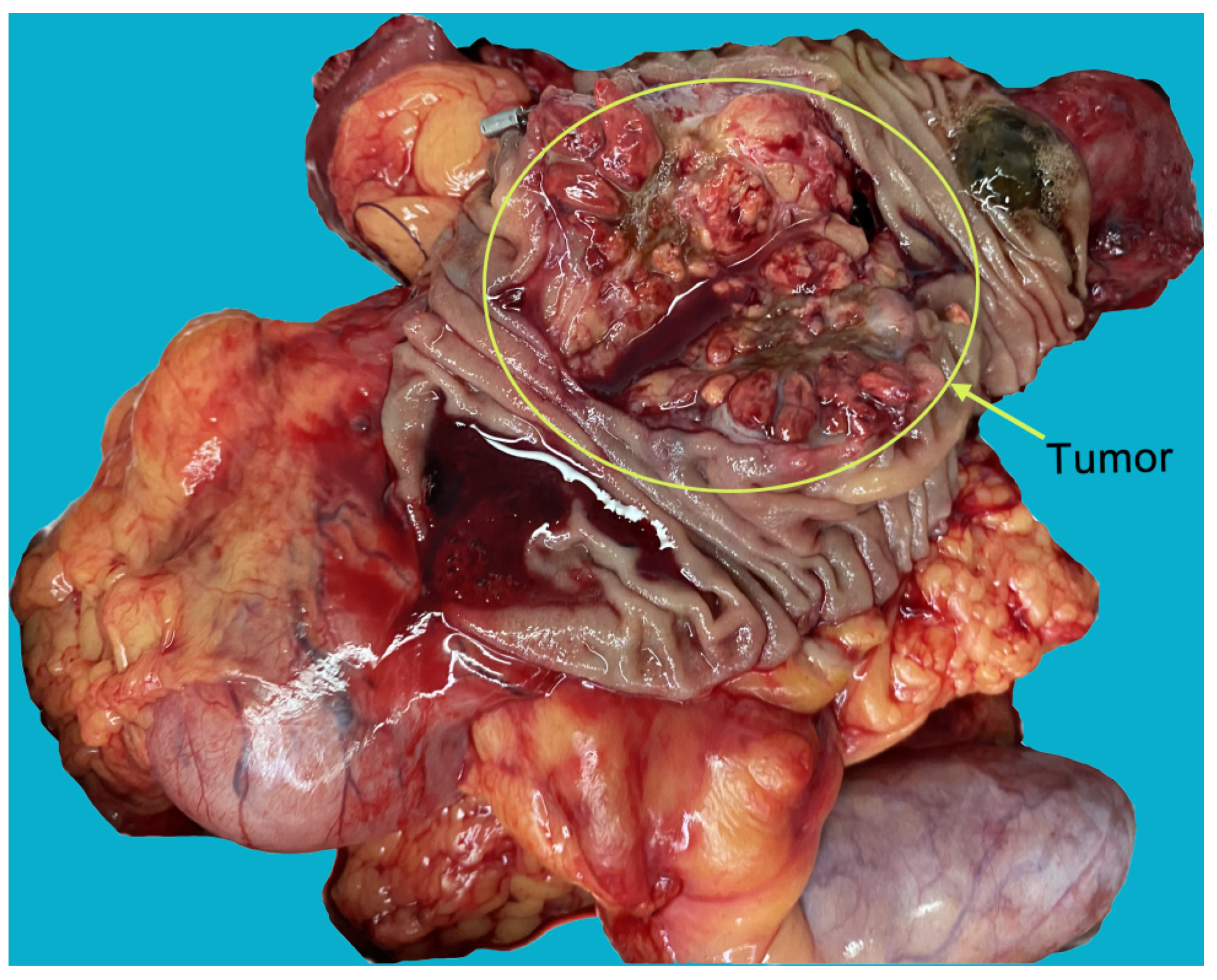
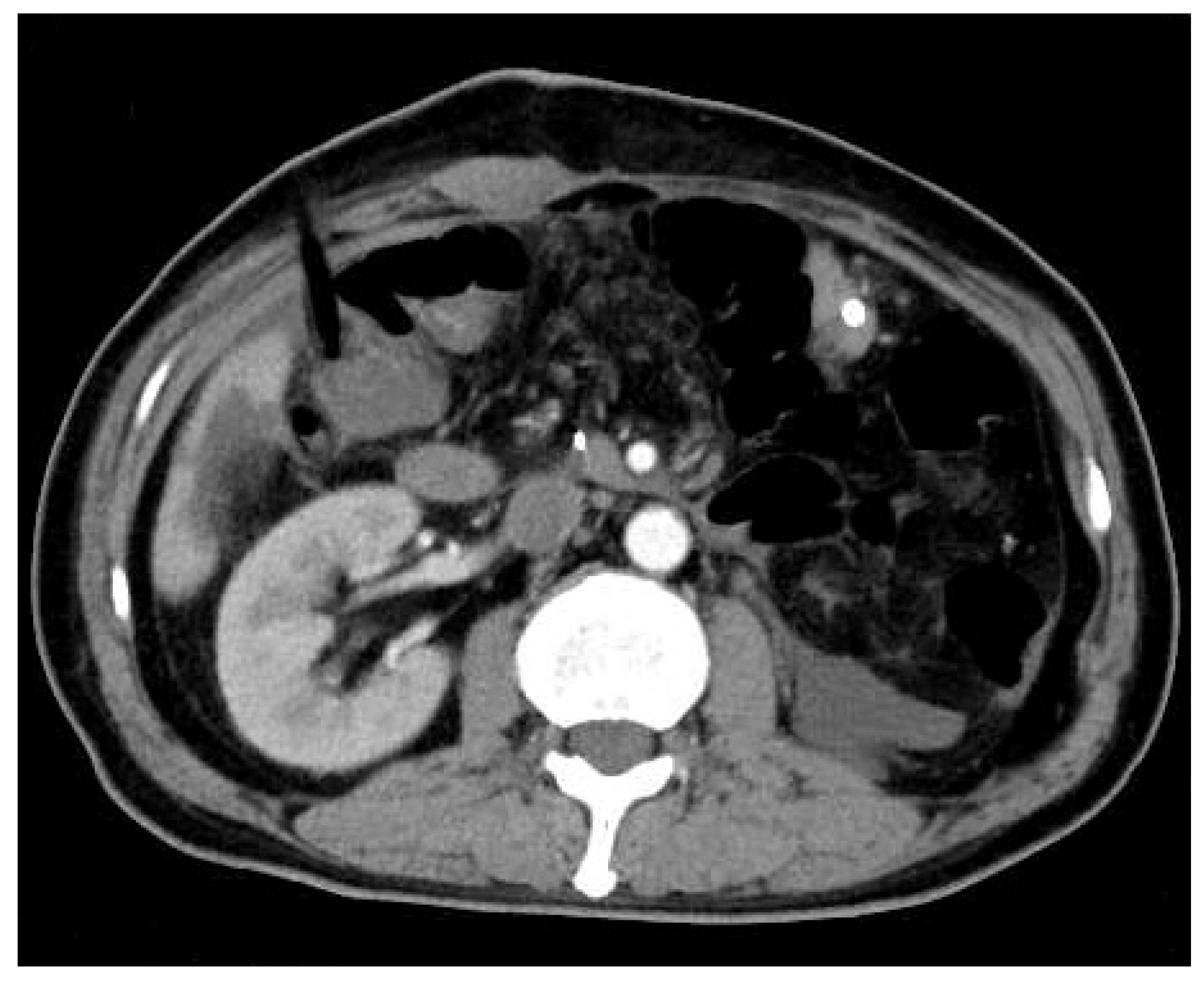
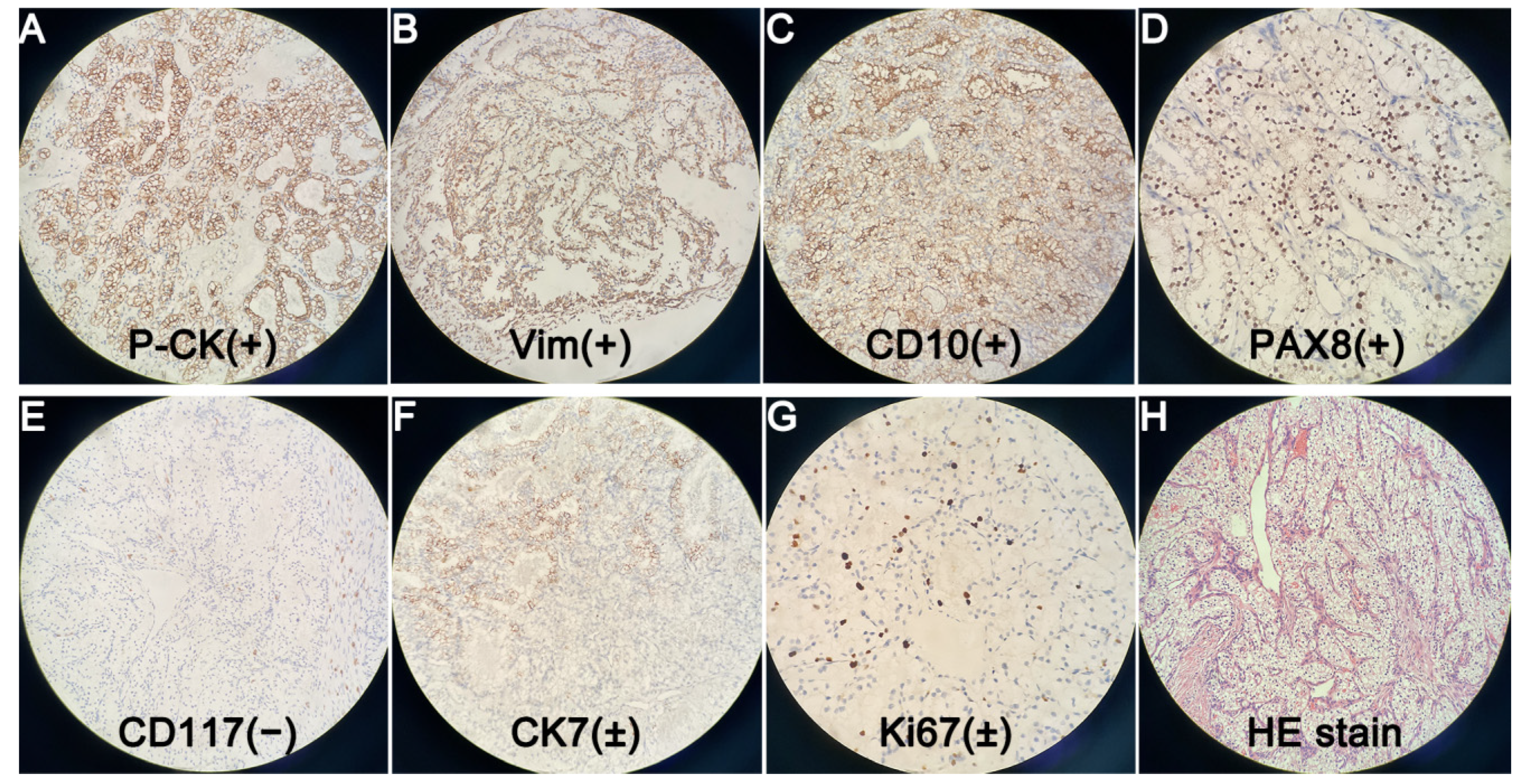
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gralnek, I.M.; Stanley, A.J.; Morris, A.J.; Camus, M.; Lau, J.; Lanas, A.; Laursen, S.B.; Radaelli, F.; Papanikolaou, I.S.; Cúrdia Gonçalves, T.; et al. Endoscopic diagnosis and management of nonvariceal upper gastrointestinal hemorrhage (NVUGIH): European Society of Gastrointestinal Endoscopy (ESGE) Guideline—Update 2021. Endoscopy 2021, 53, 300–332. [Google Scholar] [CrossRef] [PubMed]
- Chan, H.F.; Lai, K.W.; Yung, A.W.T.; Luk, W.H.; Cheng, L.F.; Ma, J.K.F. Transcatheter arterial embolisation can be the standard treatment for non-variceal upper gastrointestinal bleeding refractory to endoscopy. Hong Kong Med. J. 2019, 25, 164–166. [Google Scholar] [CrossRef] [PubMed]
- Driedger, M.R.; Puig, C.A.; Thiels, C.A.; Bergquist, J.R.; Ubl, D.S.; Habermann, E.B.; Grotz, T.E.; Smoot, R.L.; Nagorney, D.M.; Cleary, S.P.; et al. Emergent pancreatectomy for neoplastic disease: Outcomes analysis of 534 ACS-NSQIP patients. BMC Surg. 2020, 20, 169. [Google Scholar] [CrossRef] [PubMed]
- Morita, Y.; Kashima, K.; Suzuki, M.; Kinosada, H.; Teramoto, A.; Matsumiya, Y.; Uzawa, N. Differential Diagnosis between Oral Metastasis of Renal Cell Carcinoma and Salivary Gland Cancer. Diagnostics 2021, 11, 506. [Google Scholar] [CrossRef] [PubMed]
- Cheong, D.; Rho, S.Y.; Kim, J.H.; Kang, C.M.; Lee, W.J. Laparoscopic pancreaticoduodenectomy for renal cell carcinoma metastasized to ampulla of Vater: A case report and literature review. Ann. Hepatobiliary Pancreat Surg. 2018, 22, 83–89. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Ignatavicius, P.; Lizdenis, P.; Pranys, D.; Gulbinas, A.; Pundzius, J.; Barauskas, G. Long-term Survival of Patient with Ampulla of Vater Metastasis of Renal Cell Carcinoma. Prague Med. Rep. 2018, 119, 165–169. [Google Scholar] [CrossRef] [PubMed]
- Sarocchi, F.; Gilg, M.M.; Schreiber, F.; Langner, C. Secondary tumours of the ampulla of Vater: Case report and review of the literature. Mol. Clin. Oncol. 2018, 8, 274–280. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, T.; Oe, N.; Matsumori, T. A Hypervascular Mass at the Papilla of Vater. Gastroenterology 2019, 156, e7–e9. [Google Scholar] [CrossRef] [PubMed]
- Escudier, B.; Porta, C.; Schmidinger, M.; Rioux-Leclercq, N.; Bex, A.; Khoo, V.; Grünwald, V.; Gillessen, S.; Horwich, A. Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann. Oncol. 2019, 30, 706–720. [Google Scholar] [CrossRef] [PubMed]
- Dabestani, S.; Marconi, L.; Hofmann, F.; Stewart, F.; Lam, T.B.; Canfield, S.E.; Staehler, M.; Powles, T.; Ljungberg, B.; Bex, A. Local treatments for metastases of renal cell carcinoma: A systematic review. Lancet Oncol. 2014, 15, e549–e561. [Google Scholar] [CrossRef]
- Powles, T.; Albiges, L.; Bex, A.; Grünwald, V.; Porta, C.; Procopio, G.; Schmidinger, M.; Suárez, C.; de Velasco, G.; ESMO Guidelines Committee. ESMO Clinical Practice Guideline update on the use of immunotherapy in early stage and advanced renal cell carcinoma. Ann. Oncol. 2021, 32, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lu, J.; Zhou, W.; Wei, X.; Wang, K.; Zhou, L.; Xu, X. Clear Cell Renal Cell Carcinoma Metastasized to the Ampulla of Vater 16 Years after Nephrectomy—A Rare Case. Diagnostics 2022, 12, 571. https://doi.org/10.3390/diagnostics12030571
Lu J, Zhou W, Wei X, Wang K, Zhou L, Xu X. Clear Cell Renal Cell Carcinoma Metastasized to the Ampulla of Vater 16 Years after Nephrectomy—A Rare Case. Diagnostics. 2022; 12(3):571. https://doi.org/10.3390/diagnostics12030571
Chicago/Turabian StyleLu, Jun, Weijiang Zhou, Xuyong Wei, Kai Wang, Lixin Zhou, and Xiao Xu. 2022. "Clear Cell Renal Cell Carcinoma Metastasized to the Ampulla of Vater 16 Years after Nephrectomy—A Rare Case" Diagnostics 12, no. 3: 571. https://doi.org/10.3390/diagnostics12030571
APA StyleLu, J., Zhou, W., Wei, X., Wang, K., Zhou, L., & Xu, X. (2022). Clear Cell Renal Cell Carcinoma Metastasized to the Ampulla of Vater 16 Years after Nephrectomy—A Rare Case. Diagnostics, 12(3), 571. https://doi.org/10.3390/diagnostics12030571





