The Prognostic Value of Anemia in Patients with Preserved, Mildly Reduced and Recovered Ejection Fraction
Abstract
1. Introduction
2. Methods
2.1. Patient Population
2.1.1. HFpEF-HFmrEF Cohort
2.1.2. HFrecEF Cohort
2.2. Baseline Evaluation, Endpoint and Follow-Up
2.3. Statistics
3. Results
3.1. Baseline Clinical Characteristics of the Total Patient Cohort
3.2. Baseline Clinical Characteristics by Ejection Fraction
3.3. Baseline Clinical Characteristics by the Presence of Anemia
3.4. Outcome of the HFpEF-HFmrEF Patient Cohort
3.5. Baseline Clinical Characteristics of the Selected HFpEF-HFmrEF and HFrecEF Cohort by Propensity Score Matching
3.6. Outcome of the HFpEF-HFmrEF vs. HFrecEF Patient Cohort
4. Discussion
- Our HFpEF-HFmrEF cohort is a vulnerable group with a high frequency of comorbidities;
- Although the proportion of HFmrEF patients was low in our patient cohort, the characteristics of these patients differed from those with HFpEF: in terms of the ischemic etiology, the proportions of men and serum NT-proBNP levels were higher;
- More than one-third of our patients suffered from anemia, and exhibited advanced heart failure symptoms, laboratory- and echocardiographic parameters;
- In the total patient cohort, in addition to furosemide therapy, anemia was an independent predictor of all-cause mortality, and the risk of death was almost three times higher than in those with normal hemoglobin levels;
- The presence of anemia was associated with a significantly higher risk of all-cause mortality in HFpEF-HFmrEF and those with >40% of LVEF from HFrecEF patients compared with those without anemia;
- By propensity score matching, the outcomes of HFpEF-HFmrEF and HFrecEF patients with anemia were poor and did not differ significantly.
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ponikowski, P.; Voors, A.A.; Anker, S.D.; Bueno, H.; Cleland, J.G.F.; Coats, A.J.S.; Falk, V.; González-Juanatey, J.R.; Harjola, V.P.; Jankowska, E.A.; et al. 2016 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure: The Task Force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC)Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur. Heart J. 2016, 37, 2129–2200. [Google Scholar] [PubMed]
- Braunstein, J.B.; Anderson, G.F.; Gerstenblith, G.; Weller, W.; Niefeld, M.; Herbert, R.; Wu, A.W. Noncardiac comorbidity increases preventable hospitalizations and mortality among Medicare beneficiaries with chronic heart failure. J. Am. Coll. Cardiol. 2003, 42, 1226–1233. [Google Scholar] [CrossRef]
- van Deursen, V.M.; Damman, K.; van der Meer, P.; Wijkstra, P.J.; Luijckx, G.J.; van Beek, A.; van Veldhuisen, D.J.; Voors, A.A. Co-morbidities in heart failure. Heart Fail. Rev. 2014, 19, 163–172. [Google Scholar] [CrossRef] [PubMed]
- Chamberlain, A.M.; St Sauver, J.L.; Gerber, Y.; Manemann, S.M.; Boyd, C.M.; Dunlay, S.M.; Rocca, W.A.; Finney Rutten, L.J.; Jiang, R.; Weston, S.A.; et al. Multimorbidity in heart failure: A community perspective. Am. J. Med. 2015, 128, 38–45. [Google Scholar] [CrossRef] [PubMed]
- Baldi, I.; Azzolina, D.; Berchialla, P.; Gregori, D.; Scotti, L.; Corrao, G. Comorbidity-adjusted relative survival in newly hospitalized heart failure patients: A population-based study. Int. J. Cardiol. 2017, 243, 385–388. [Google Scholar] [CrossRef]
- Triposkiadis, F.; Giamouzis, G.; Parissis, J.; Starling, R.C.; Boudoulas, H.; Skoularigis, J.; Butler, J.; Filippatos, G. Reframing the association and significance of co-morbidities in heart failure. Eur. J. Heart Fail. 2016, 18, 744–758. [Google Scholar] [CrossRef]
- Mentz, R.J.; Kelly, J.P.; von Lueder, T.G.; Voors, A.A.; Lam, C.S.; Cowie, M.R.; Kjeldsen, K.; Jankowska, E.A.; Atar, D.; Butler, J.; et al. Noncardiac comorbidities in heart failure with reduced versus preserved ejection fraction. J. Am. Coll. Cardiol. 2014, 64, 2281–2293. [Google Scholar] [CrossRef]
- Anand, I.S. Anemia and chronic heart failure implications and treatment options. J. Am. Coll. Cardiol. 2008, 52, 501–511. [Google Scholar] [CrossRef]
- Duchnowski, P.; Hryniewiecki, T.; Stokłosa, P.; Kuśmierczyk, M.; Szymański, P. Number of erythrocytes as a prognostic marker in patients undergoing heart valve surgery. Kardiol. Pol. 2018, 76, 791–793. [Google Scholar] [CrossRef]
- Muzzarelli, S.; Pfisterer, M. Anemia as independent predictor of major events in elderly patients with chronic angina. Am. Heart J. 2006, 152, 991–996. [Google Scholar] [CrossRef]
- Duchnowski, P.; Hryniewiecki, T.; Zatorska, K.; Żebrowska, A.; Kuśmierczyk, M.; Szymański, P. High-sensitivity troponin T as a prognostic marker in patients undergoing aortic valve replacement. Pol. Arch. Intern. Med. 2017, 127, 628–630. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Latini, R.; Masson, S.; Anand, I.S.; Missov, E.; Carlson, M.; Vago, T.; Angelici, L.; Barlera, S.; Parrinello, G.; Maggioni, A.P.; et al. Prognostic value of very low plasma concentrations of troponin T in patients with stable chronic heart failure. Circulation 2007, 116, 1242–1249. [Google Scholar] [CrossRef] [PubMed]
- Berry, C.; Poppe, K.K.; Gamble, G.D.; Earle, N.J.; Ezekowitz, J.A.; Squire, I.B.; McMurray, J.J.V.; McAlister, F.A.; Komajda, M.; Swedberg, K.; et al. Prognostic significance of anaemia in patients with heart failure with preserved and reduced ejection fraction: Results from the MAGGIC individual patient data meta-analysis. QJM 2016, 109, 377–382. [Google Scholar] [CrossRef] [PubMed]
- Groenveld, H.F.; Januzzi, J.L.; Damman, K.; van Wijngaarden, J.; Hillege, H.L.; van Veldhuisen, D.J.; van der Meer, P. Anemia and mortality in heart failure patients a systematic review and meta-analysis. J. Am. Coll. Cardiol. 2008, 52, 818–827. [Google Scholar] [CrossRef]
- Savarese, G.; Jonsson, Å.; Hallberg, A.C.; Dahlström, U.; Edner, M.; Lund, L.H. Prevalence of, associations with, and prognostic role of anemia in heart failure across the ejection fraction spectrum. Int. J. Cardiol. 2020, 298, 59–65. [Google Scholar] [CrossRef]
- Bozkurt, B.; Coats, A.J.S.; Tsutsui, H.; Abdelhamid, C.M.; Adamopoulos, S.; Albert, N.; Anker, S.D.; Atherton, J.; Böhm, M.; Butler, J.; et al. Universal Definition and Classification of Heart Failure: A Report of the Heart Failure Society of America, Heart Failure Association of the European Society of Cardiology, Japanese Heart Failure Society and Writing Committee of the Universal Definition of Heart Failure. J. Card Fail. 2021, 23, 352–380. [Google Scholar]
- McDonagh, T.A.; Metra, M.; Adamo, M.; Gardner, R.S.; Baumbach, A.; Böhm, M.; Burri, H.; Butler, J.; Čelutkienė, J.; Chioncel, O.; et al. 2021 ESC Guidelines for the diagnosis and treatment of acute and chronic heart failure. Eur. Heart J. 2021, 42, 3599–3726. [Google Scholar] [CrossRef]
- Kosztin, A.; Vamos, M.; Aradi, D.; Schwertner, W.R.; Kovacs, A.; Nagy, K.V.; Zima, E.; Geller, L.; Duray, G.Z.; Kutyifa, V.; et al. De novo implantation vs. upgrade cardiac resynchronization therapy: A systematic review and meta-analysis. Heart Fail. Rev. 2018, 23, 15–26. [Google Scholar] [CrossRef]
- Tokodi, M.; Schwertner, W.R.; Kovács, A.; Tősér, Z.; Staub, L.; Sárkány, A.; Lakatos, B.K.; Behon, A.; Boros, A.M.; Perge, P.; et al. Machine learning-based mortality prediction of patients undergoing cardiac resynchronization therapy: The SEMMELWEIS-CRT score. Eur. Heart J. 2020, 41, 1747–1756. [Google Scholar] [CrossRef]
- Behon, A.; Schwertner, W.R.; Merkel, E.D.; Kovács, A.; Lakatos, B.K.; Zima, E.; Gellér, L.; Kutyifa, V.; Kosztin, A.; Merkely, B. Lateral left ventricular lead position is superior to posterior position in long-term outcome of patients who underwent cardiac resynchronization therapy. ESC Heart Fail. 2020, 7, 3374–3382. [Google Scholar] [CrossRef]
- Schwertner, W.R.; Behon, A.; Merkel, E.D.; Tokodi, M.; Kovács, A.; Zima, E.; Osztheimer, I.; Molnár, L.; Király, Á.; Papp, R.; et al. Long-term survival following upgrade compared with de novo cardiac resynchronization therapy implantation: A single-centre, high-volume experience. Europace 2021, 23, 1310–1318. [Google Scholar] [CrossRef] [PubMed]
- Oktay, A.A.; Rich, J.D.; Shah, S.J. The emerging epidemic of heart failure with preserved ejection fraction. Curr. Heart Fail. Rep. 2013, 10, 401–410. [Google Scholar] [CrossRef] [PubMed]
- Okuno, K.; Naito, Y.; Asakura, M.; Sugahara, M.; Ando, T.; Yasumura, S.; Nagai, T.; Saito, Y.; Yoshikawa, T.; Masuyama, T.; et al. Effective blood hemoglobin level to predict prognosis in heart failure with preserved left ventricular ejection fraction: Results of the Japanese heart failure syndrome with preserved ejection fraction registry. Heart Vessel. 2019, 34, 1168–1177. [Google Scholar] [CrossRef] [PubMed]
- Iorio, A.; Senni, M.; Barbati, G.; Greene, S.J.; Poli, S.; Zambon, E.; Di Nora, C.; Cioffi, G.; Tarantini, L.; Gavazzi, A.; et al. Prevalence and prognostic impact of non-cardiac co-morbidities in heart failure outpatients with preserved and reduced ejection fraction: A community-based study. Eur. J. Heart Fail. 2018, 20, 1257–1266. [Google Scholar] [CrossRef] [PubMed]
- Brouwers, F.P.; de Boer, R.A.; van der Harst, P.; Voors, A.A.; Gansevoort, R.T.; Bakker, S.J.; Hillege, H.L.; van Veldhuisen, D.J.; van Gilst, W.H. Incidence and epidemiology of new onset heart failure with preserved vs. reduced ejection fraction in a community-based cohort: 11-year follow-up of PREVEND. Eur. Heart J. 2013, 34, 1424–1431. [Google Scholar] [CrossRef]
- Vos, T.; Abajobir, A.A.; Abbafati, C.; Abbas, K.M.; Abate, K.H.; Abd-Allah, F.; Abdulle, A.M.; Abebo, T.A.; Abera, S.F.; Aboyans, V.; et al. Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: A systematic analysis for the Global Burden of Disease Study 2017. Lancet 2018, 392, 1789–1858. [Google Scholar]
- Roger, V.L. Epidemiology of heart failure. Circ. Res. 2013, 113, 646–659. [Google Scholar] [CrossRef]
- van Riet, E.E.; Hoes, A.W.; Limburg, A.; Landman, M.A.; van der Hoeven, H.; Rutten, F.H. Prevalence of unrecognized heart failure in older persons with shortness of breath on exertion. Eur. J. Heart Fail. 2014, 16, 772–777. [Google Scholar] [CrossRef]
- Chioncel, O.; Lainscak, M.; Seferovic, P.M.; Anker, S.D.; Crespo-Leiro, M.G.; Harjola, V.P.; Parissis, J.; Laroche, C.; Piepoli, M.F.; Fonseca, C.; et al. Epidemiology and one-year outcomes in patients with chronic heart failure and preserved, mid-range and reduced ejection fraction: An analysis of the ESC Heart Failure Long-Term Registry. Eur. J. Heart Fail. 2017, 19, 1574–1585. [Google Scholar] [CrossRef]
- Anker, S.D.; Butler, J.; Filippatos, G.; Ferreira, J.P.; Bocchi, E.; Böhm, M.; Brunner-La Rocca, H.P.; Choi, D.J.; Chopra, V.; Chuquiure-Valenzuela, E.; et al. Empagliflozin in Heart Failure with a Preserved Ejection Fraction. N. Engl. J. Med. 2021, 385, 1451–1461. [Google Scholar] [CrossRef]
- Dunlay, S.M.; Roger, V.L.; Redfield, M.M. Epidemiology of heart failure with preserved ejection fraction. Nat. Rev. Cardiol. 2017, 14, 591–602. [Google Scholar] [CrossRef] [PubMed]
- Fonarow, G.C.; Stough, W.G.; Abraham, W.T.; Albert, N.M.; Gheorghiade, M.; Greenberg, B.H.; O’Connor, C.M.; Sun, J.L.; Yancy, C.W.; Young, J.B. Characteristics, treatments, and outcomes of patients with preserved systolic function hospitalized for heart failure: A report from the OPTIMIZE-HF Registry. J. Am. Coll. Cardiol. 2007, 50, 768–777. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, M.A.; Swedberg, K.; Granger, C.B.; Held, P.; McMurray, J.J.; Michelson, E.L.; Olofsson, B.; Ostergren, J.; Yusuf, S.; Pocock, S. Effects of candesartan on mortality and morbidity in patients with chronic heart failure: The CHARM-Overall programme. Lancet 2003, 362, 759–766. [Google Scholar] [CrossRef]
- Solomon, S.D.; McMurray, J.J.V.; Anand, I.S.; Ge, J.; Lam, C.S.P.; Maggioni, A.P.; Martinez, F.; Packer, M.; Pfeffer, M.A.; Pieske, B.; et al. Angiotensin-Neprilysin Inhibition in Heart Failure with Preserved Ejection Fraction. N. Engl. J. Med. 2019, 381, 1609–1620. [Google Scholar] [CrossRef]
- Lund, L.H.; Donal, E.; Oger, E.; Hage, C.; Persson, H.; Haugen-Löfman, I.; Ennezat, P.V.; Sportouch-Dukhan, C.; Drouet, E.; Daubert, J.C.; et al. Association between cardiovascular vs. non-cardiovascular co-morbidities and outcomes in heart failure with preserved ejection fraction. Eur. J. Heart Fail. 2014, 16, 992–1001. [Google Scholar] [CrossRef]
- Anand, I.S.; Gupta, P. Anemia and Iron Deficiency in Heart Failure: Current Concepts and Emerging Therapies. Circulation 2018, 138, 80–98. [Google Scholar] [CrossRef]
- Anker, S.D.; Comin Colet, J.; Filippatos, G.; Willenheimer, R.; Dickstein, K.; Drexler, H.; Lüscher, T.F.; Bart, B.; Banasiak, W.; Niegowska, J.; et al. Ferric carboxymaltose in patients with heart failure and iron deficiency. N. Engl. J. Med. 2009, 361, 2436–2448. [Google Scholar] [CrossRef]
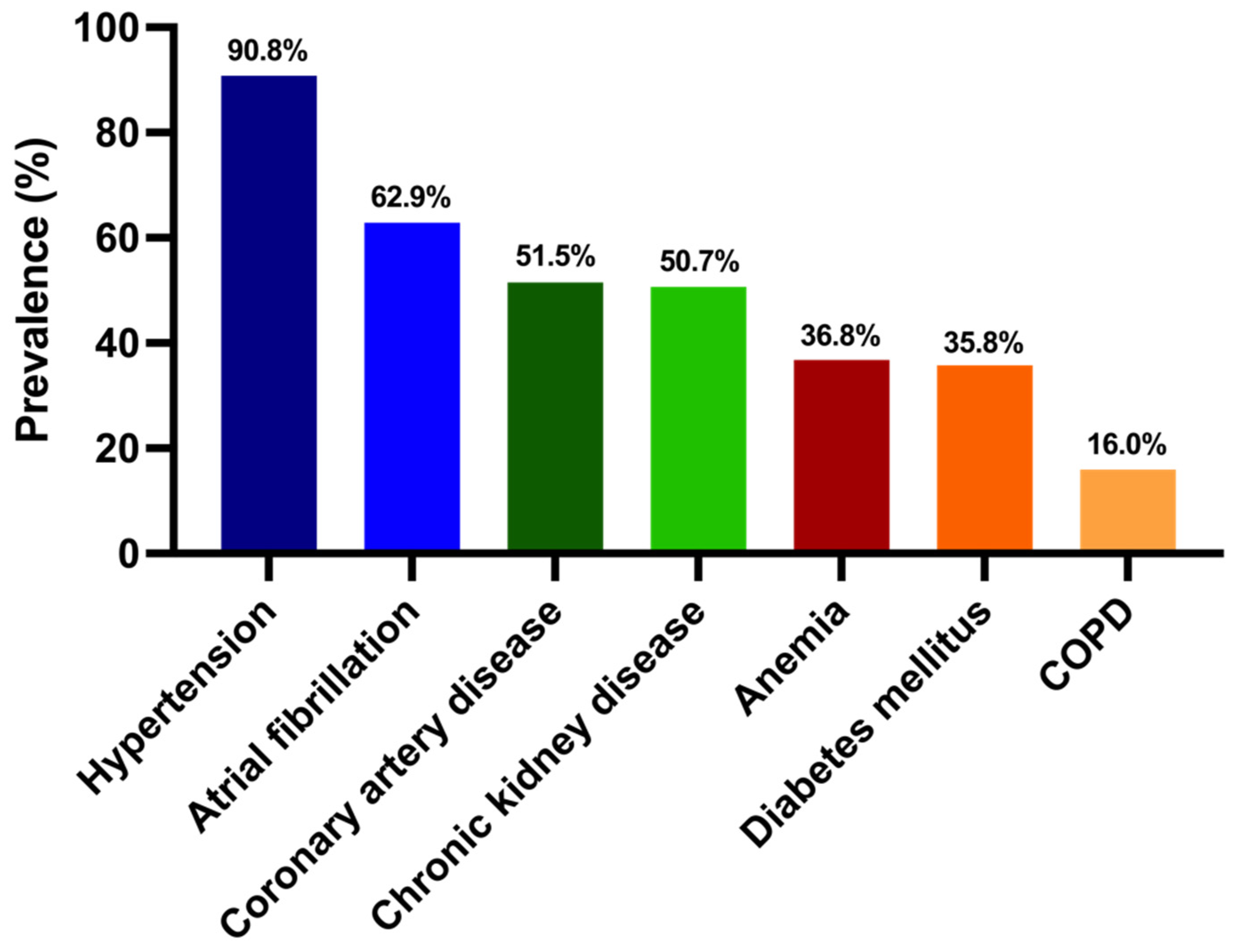


| Baseline Variables | All Patients (n = 375) | HFpEF (n = 326) | HFmrEF (n = 49) | p Value |
|---|---|---|---|---|
| Age (years, median/IQR) | 75 (69–82) | 76 (69–82) | 74 (68–80) | 0.21 |
| Gender (female, n, %) | 196 (52) | 180 (55) | 16 (33) | <0.01 *** |
| NYHA III/IV (n, %) | 129 (38) | 109 (37) | 20 (42) | 0.64 |
| Ischemic etiology (n, %) | 212 (57) | 176 (55) | 36 (74) | 0.01 ** |
| Systolic BP (mmHg, median/IQR) | 135 (123–151) | 136 (125–152) | 131 (118–140) | 0.06 |
| Diastolic BP (mmHg, mean ± SD) | 79 ± 13 | 79 ± 13 | 80 ± 10 | 0.86 |
| BMI (kg/m2, median/IQR) | 30.1 (25.9–34.1) | 30.1 (26.0–33.9) | 30.0 (25.4–36.7) | 0.80 |
| LBBB morphology (n, %) | 25 (7) | 22 (7) | 3 (7) | 1.0 |
| Medical history | ||||
| Hypertension (n, %) | 334 (91) | 293 (91) | 41 (87) | 0.42 |
| Atrial fibrillation (n, %) | 236 (63) | 206 (63) | 30 (61) | 0.87 |
| CAD (n, %) | 193 (52) | 160 (49) | 33 (67) | 0.02 * |
| CKD (n, %) | 190 (51) | 169 (52) | 21 (43) | 0.28 |
| T2DM (n, %) | 133 (36) | 116 (36) | 17 (35) | 1.00 |
| Prior MI (n, %) | 118 (32) | 93 (29) | 25 (51) | <0.01 *** |
| Prior PCI (n, %) | 123 (33) | 103 (32) | 20 (41) | 0.25 |
| Prior CABG (n, %) | 35 (9) | 27 (8) | 8 (17) | 0.11 |
| COPD (n, %) | 60 (16) | 52 (16) | 8 (16) | 1.00 |
| Laboratory parameters | ||||
| Creatinine (µmol/L, median/IQR) | 92 (76–117) | 91 (76–117) | 93 (81–116) | 0.83 |
| Cholesterol (mmol/L, median/IQR) | 4.2 (3.4–5.0) | 4.2 (3.4–5.0) | 3.8 (3.1–4.6) | 0.12 |
| eGFR (ml/min/1.73 m2, median/IQR) | 58 (46–78) | 58 (45–77) | 67 (50–81) | 0.07 |
| Urea (mmol/L, median/IQR) | 7.2 (5.7–9.0) | 7.2 (5.7–9.0) | 7.2 (5.0–9.0) | 0.26 |
| NT-proBNP (pg/mL, median/IQR) | 1085 (621–1973) | 1070 (603–1887) | 1543 (684–2816) | 0.02 * |
| Echocardiographic parameters | ||||
| LVEF (%, median/IQR) | 55 (50–60) | 56 (55–60) | 45 (42–47) | NA |
| LVEDD (mm, median/IQR) | 47 (43–51) | 46 (42–51) | 50 (46–53) | <0.01 *** |
| LVESD (mm, mean/IQR) | 32 (28–37) | 31 (27–36) | 37 (34–43) | <0.01 *** |
| Medical treatment | ||||
| Beta blocker (n, %) | 321 (87) | 276 (85) | 45 (94) | 0.17 |
| ACEi/ARB (n, %) | 315 (85) | 279 (86) | 36 (74) | 0.03 * |
| MRA (n, %) | 105 (28) | 88 (27) | 17 (35) | 0.31 |
| Furosemide (n, %) | 231 (62) | 194 (60) | 37 (75) | 0.04 * |
| OAC (n, %) | 217 (59) | 191 (60) | 26 (53) | 0.44 |
| Baseline Variables | All Patients (n = 375) | With Anemia (n = 138) | Without Anemia (n = 237) | p Value |
|---|---|---|---|---|
| Age (years; median/IQR) | 75 (69–82) | 77 (70–82) | 75 (69–81) | 0.08 |
| Gender (female; n, %) | 196 (52) | 60 (44) | 136 (57) | 0.01 ** |
| NYHA III/IV (n, %) | 129 (38) | 70 (53) | 59 (28) | <0.01 *** |
| Ischemic etiology (n, %) | 212 (57) | 83 (61) | 129 (55) | 0.31 |
| Systolic BP (mmHg, median/IQR) | 135 (123–151) | 133 (124–154) | 137 (122–150) | 0.94 |
| Diastolic BP (mmHg, mean ± SD) | 79 ± 13 | 75 ± 13 | 82 ± 12 | <0.01 *** |
| BMI (kg/m2, median/IQR) | 30.1 (25.9–34.1) | 29.7 (25.4–33.6) | 30.2 (26.2–34.5) | 0.37 |
| LBBB morphology (n, %) | 25 (7) | 11 (8) | 15 (7) | 0.67 |
| Medical history | ||||
| Hypertension (n, %) | 334 (91) | 125 (92) | 209 (90) | 0.71 |
| Atrial fibrillation (n, %) | 236 (63) | 81 (59) | 155 (65) | 0.22 |
| CAD (n, %) | 193 (52) | 75 (54) | 118 (50) | 0.45 |
| CKD (n, %) | 190 (51) | 87 (63) | 103 (44) | <0.01 *** |
| T2DM (n, %) | 133 (36) | 59 (43) | 74 (32) | 0.03 * |
| Prior MI (n, %) | 118 (32) | 48 (35) | 70 (30) | 0.30 |
| Prior PCI (n, %) | 123 (33) | 48 (35) | 75 (32) | 0.57 |
| Prior CABG (n, %) | 35 (9) | 21 (15) | 14 (6) | <0.01 *** |
| COPD (n, %) | 60 (16) | 26 (19) | 34 (14) | 0.31 |
| Laboratory parameters | ||||
| Creatinine (µmol/L, median/IQR) | 92 (76–117) | 105 (79–136) | 87 (75–105) | <0.01 *** |
| Cholesterol (mmol/L, median/IQR) | 4.2 (3.4–5.0) | 3.8 (3.1–4.5) | 4.4 (3.7–5.3) | <0.01 *** |
| eGFR (ml/min/1.73 m2, median/IQR) | 58 (46–78) | 52 (38–67) | 61 (50–79) | <0.01 *** |
| Urea (mmol/L, median/IQR) | 7.2 (5.7–9.0) | 8.2 (6.3–10.8) | 6.7 (5.4–8.4) | <0.01 *** |
| NT-proBNP (pg/mL, median/IQR) | 1085 (621–1973) | 1584 (761–3088) | 912 (558–1647) | <0.01 *** |
| Echocardiographic parameters | ||||
| LVEF (%, median/IQR) | 55 (50–60) | 55 (52–60) | 55 (50–60) | 0.81 |
| LVEDD (mm, median/IQR) | 47 (43–51) | 47 (44–51) | 46 (41–51) | 0.04 * |
| LVESD (mm, mean/IQR) | 32 (28–37) | 33 (29–38) | 31 (27–36) | 0.02 * |
| Medical treatment | ||||
| Beta blocker (n, %) | 321 (87) | 109 (81) | 212 (90) | 0.02 * |
| ACEi/ARB (n, %) | 315 (85) | 113 (83) | 202 (85) | 0.56 |
| MRA (n, %) | 105 (28) | 38 (28) | 67 (28) | 1.00 |
| Furosemide (n, %) | 231 (62) | 98 (72) | 133 (56) | <0.01 *** |
| OAC (n, %) | 217 (59) | 71 (53) | 146 (62) | 0.10 |
| Baseline Variables | HFpEF-HFmrEF Patients (n = 75) | HFrecEF Patients (n = 75) | p Value |
|---|---|---|---|
| Age (years, median/IQR) | 71.5 (63.7–79.4) | 70.2 (62.8–74.4) | 0.09 |
| Gender (female, n, %) | 25 (33) | 27 (36) | 0.73 |
| Ischemic etiology (n, %) | 40 (53) | 36 (48) | 0.51 |
| Nonischemic etiology (n, %) | 35 (47) | 39 (52) | 0.51 |
| Systolic BP (mmHg, median/IQR) | 132 (124–145) | 128 (114–145) | 0.32 |
| Heart rate (1/min, median/IQR) | 76 (65–85) | 75 (75–75) | 0.90 |
| Weight (kg, median/IQR) | 85 (80–103) | 80 (73–96) | 0.07 |
| Height (cm, median/IQR) | 172 (162–178) | 170 (162–176) | 0.84 |
| BMI (kg/m2, median/IQR) | 31 (27–35) | 28 (26–34) | 0.06 |
| Medical history | |||
| Hypertension (n, %) | 65 (87) | 56 (75) | 0.06 |
| CKD (n, %) | 32 (43) | 36 (48) | 0.51 |
| T2DM (n, %) | 29 (39) | 28 (37) | 0.82 |
| Prior MI (n, %) | 31 (41) | 27 (36) | 0.50 |
| Prior PCI (n, %) | 47 (63) | 49 (66) | 0.65 |
| Anemia (n, %) | 22 (29) | 24 (32) | 0.72 |
| Nicotinismus (n, %) | 2 (3) | 2 (3) | 0.99 |
| COPD (n, %) | 15 (19) | 8 (11) | 0.1 |
| Laboratory parameters | |||
| Creatinine (µmol/L, median/IQR) | 93 (77–117) | 96 (73–122) | 0.83 |
| Hemoglobin (g/dL, median/IQR) | 13.5 (12.1–14.4) | 13.1 (12–14.6) | 0.72 |
| eGFR (ml/min/1.73 m2, median/IQR) | 62 (48–83) | 60 (47–83) | 0.50 |
| Echocardiographic parameter | |||
| LVEF (%, median/IQR)) | 48 (45–50) | 45 (41–50) | 0.06 |
| All-Cause Mortality | HR | 95% CI | p Value |
|---|---|---|---|
| HFpEF-HFmrEF patients with anemia vs. HFpEF-HFmrEF patients with normal serum hemoglobin levels | 4.36 | 0.93/20.50 | 0.03 * |
| HFrecEF patients with anemia vs. HFrecEF patients with normal serum hemoglobin levels | 9.64 | 3.44/27.00 | <0.01 *** |
| HFpEF-HFmrEF patients with anemia vs. HFrecEF patients with anemia | 1.42 | 0.47/4.35 | 0.45 |
| HFpEF-HFmrEF patients without anemia vs. HFrecEF patients without anemia | 1.55 | 0.33/7.24 | 0.48 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pintér, A.; Behon, A.; Veres, B.; Merkel, E.D.; Schwertner, W.R.; Kuthi, L.K.; Masszi, R.; Lakatos, B.K.; Kovács, A.; Becker, D.; et al. The Prognostic Value of Anemia in Patients with Preserved, Mildly Reduced and Recovered Ejection Fraction. Diagnostics 2022, 12, 517. https://doi.org/10.3390/diagnostics12020517
Pintér A, Behon A, Veres B, Merkel ED, Schwertner WR, Kuthi LK, Masszi R, Lakatos BK, Kovács A, Becker D, et al. The Prognostic Value of Anemia in Patients with Preserved, Mildly Reduced and Recovered Ejection Fraction. Diagnostics. 2022; 12(2):517. https://doi.org/10.3390/diagnostics12020517
Chicago/Turabian StylePintér, Anita, Anett Behon, Boglárka Veres, Eperke Dóra Merkel, Walter Richard Schwertner, Luca Katalin Kuthi, Richard Masszi, Bálint Károly Lakatos, Attila Kovács, Dávid Becker, and et al. 2022. "The Prognostic Value of Anemia in Patients with Preserved, Mildly Reduced and Recovered Ejection Fraction" Diagnostics 12, no. 2: 517. https://doi.org/10.3390/diagnostics12020517
APA StylePintér, A., Behon, A., Veres, B., Merkel, E. D., Schwertner, W. R., Kuthi, L. K., Masszi, R., Lakatos, B. K., Kovács, A., Becker, D., Merkely, B., & Kosztin, A. (2022). The Prognostic Value of Anemia in Patients with Preserved, Mildly Reduced and Recovered Ejection Fraction. Diagnostics, 12(2), 517. https://doi.org/10.3390/diagnostics12020517







