To Contrast or Not to Contrast? On the Role of Contrast Enhancement in Hand MRI Studies of Patients with Rheumatoid Arthritis
Abstract
:1. Introduction
2. Materials and Methods
2.1. Study Design and Patients
2.2. MRI Acquisition
2.3. MRI Evaluation
2.4. Statistical Analysis
3. Results
3.1. Comparison of Sum Scores and Subscores
3.2. Interrater Reliability
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Strand, V.; Singh, J.A. Improved health-related quality of life with effective disease-modifying antirheumatic drugs: Evidence from randomized controlled trials. Am. J. Manag. Care 2007, 13 (Suppl. 9), S237–S251. [Google Scholar] [PubMed]
- Wilson, R.L. Rheumatoid arthritis of the hand. Orthop. Clin. N. Am. 1986, 17, 313–343. [Google Scholar] [CrossRef]
- Zochling, J.; Braun, J. Mortality in rheumatoid arthritis and ankylosing spondylitis. Clin. Exp. Rheumatol. 2009, 27, 127–130. [Google Scholar]
- Singh, J.A.; Saag, K.G.; Bridges, S.L., Jr.; Akl, E.A.; Bannuru, R.R.; Sullivan, M.C.; Vaysbrot, E.; McNaughton, C.; Osani, M.; Shmerling, R.H.; et al. 2015 American College of Rheumatology Guideline for the Treatment of Rheumatoid Arthritis. Arthritis Rheumatol. 2016, 68, 1–26. [Google Scholar] [CrossRef] [PubMed]
- Sewerin, P.; Schleich, C.; Vordenbäumen, S.; Ostendorf, B. Update on imaging in rheumatic diseases: Cartilage. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 114), 139–144. [Google Scholar]
- Baraliakos, X.; Braun, J.; Conaghan, P.G.; Østergaard, M.; Pincus, T. Update on imaging in rheumatic diseases. Clin. Exp. Rheumatol. 2018, 36 (Suppl. 114), 2. [Google Scholar]
- Frenken, M.; Schleich, C.; Brinks, R.; Abrar, D.B.; Goertz, C.; Schneider, M.; Ostendorf, B.; Sewerin, P. The value of the simplified RAMRIS-5 in early RA patients under methotrexate therapy using high-field MRI. Arthritis Res. Ther. 2019, 21, 21. [Google Scholar] [CrossRef] [Green Version]
- Conaghan, P.G.; Østergaard, M.; Troum, O.; Bowes, M.A.; Guillard, G.; Wilkinson, B.; Xie, Z.; Andrews, J.; Stein, A.; Chapman, D.; et al. Very early MRI responses to therapy as a predictor of later radiographic progression in early rheumatoid arthritis. Arthritis Res. Ther. 2019, 21, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Østergaard, M.; Peterfy, C.G.; Bird, P.; Gandjbakhch, F.; Glinatsi, D.; Eshed, I.; Haavardsholm, E.A.; Lillegraven, S.; Bøyesen, P.; Ejbjerg, B.; et al. The OMERACT Rheumatoid Arthritis Magnetic Resonance Imaging (MRI) Scoring System: Updated Recommendations by the OMERACT MRI in Arthritis Working Group. J. Rheumatol. 2017, 44, 1706–1712. [Google Scholar] [CrossRef]
- Østergaard, M.; Conaghan, P.G.; O’Connor, P.; Szkudlarek, M.; Klarlund, M.; Emery, P.; Peterfy, C.; Genant, H.; McQUEEN, F.M.; Bird, P.; et al. Reducing Invasiveness, Duration, and Cost of Magnetic Resonance Imaging in Rheumatoid Arthritis by Omitting Intravenous Contrast Injection—Does It Change the Assessment of Inflammatory and Destructive Joint Changes by the OMERACT RAMRIS? J. Rheumatol. 2009, 36, 1806–1810. [Google Scholar] [CrossRef]
- Olchowy, C.; Cebulski, K.; Łasecki, M.; Chaber, R.; Olchowy, A.; Kałwak, K.; Zaleska-Dorobisz, U. The presence of the gadolinium-based contrast agent depositions in the brain and symptoms of gadolinium neurotoxicity—A systematic review. PLoS ONE 2017, 12, e0171704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tedeschi, E.; Caranci, F.; Giordano, F.; Angelini, V.; Cocozza, S.; Brunetti, A. Gadolinium retention in the body: What we know and what we can do. Radiol. Med. 2017, 122, 589–600. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quattrocchi, C.C.; Van Der Molen, A.J. Gadolinium Retention in the Body and Brain: Is It Time for an International Joint Research Effort? Radiology 2017, 282, 12–16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stojanov, D.; Aracki-Trenkic, A.; Benedeto-Stojanov, D. Gadolinium deposition within the dentate nucleus and globus pallidus after repeated administrations of gadolinium-based contrast agents—Current status. Neuroradiology 2016, 58, 433–441. [Google Scholar] [CrossRef] [PubMed]
- Cowling, T.; Frey, N. Macrocyclic and Linear Gadolinium Based Contrast Agents for Adults Undergoing Magnetic Resonance Imaging: A Review of Safety; Canadian Agency for Drugs and Technologies in Health: Ottawa, ON, Canada, 2019. [Google Scholar]
- Dekkers, I.A.; Roos, R.; Van Der Molen, A.J. Gadolinium retention after administration of contrast agents based on linear chelators and the recommendations of the European Medicines Agency. Eur. Radiol. 2018, 28, 1579–1584. [Google Scholar] [CrossRef] [PubMed]
- Aletaha, D.; Neogi, T.; Silman, A.J.; Funovits, J.; Felson, D.T.; Bingham, C.O., 3rd; Birnbaum, N.S.; Burmester, G.R.; Bykerk, V.P.; Cohen, M.D.; et al. 2010 Rheumatoid arthritis classification criteria: An American College of Rheumatology/European League Against Rheumatism collaborative initiative. Ann. Rheum. Dis. 2010, 69, 1580–1588. [Google Scholar] [CrossRef] [PubMed]
- Van Riel, P.L.; Renskers, L. The Disease Activity Score (DAS) and the Disease Activity Score using 28 joint counts (DAS28) in the management of rheumatoid arthritis. Clin. Exp. Rheumatol. 2016, 34, 40–44. [Google Scholar]
- Cohen, S.; Kamarck, T.; Mermelstein, R. A global measure of perceived stress. J. Health Soc. Behav. 1983, 24, 385–396. [Google Scholar] [CrossRef]
- Landis, J.R.; Koch, G.G. The Measurement of Observer Agreement for Categorical Data. Biometrics 1977, 33, 159–174. [Google Scholar] [CrossRef] [Green Version]
- Haavardsholm, E.A.; Østergaard, M.; Ejbjerg, B.J.; Kvan, N.P.; Kvien, T.K. Introduction of a novel magnetic resonance imaging tenosynovitis score for rheumatoid arthritis: Reliability in a multireader longitudinal study. Ann. Rheum. Dis. 2007, 66, 1216–1220. [Google Scholar] [CrossRef] [Green Version]
- Di Matteo, A.; Mankia, K.; Azukizawa, M.; Wakefield, R.J. The Role of Musculoskeletal Ultrasound in the Rheumatoid Arthritis Continuum. Curr. Rheumatol. Rep. 2020, 22, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Docking, S.I.; Ooi, C.C.; Connell, D. Tendinopathy: Is Imaging Telling Us the Entire Story? J. Orthop. Sports Phys. Ther. 2015, 45, 842–852. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sudoł-Szopińska, I.; Jans, L.; Teh, J. Rheumatoid arthritis: What do MRI and ultrasound show. J. Ultrason. 2017, 17, 5–16. [Google Scholar] [CrossRef] [PubMed]
- Sudoł-Szopińska, I.; Jurik, A.G.; Eshed, I.; Lennart, J.; Grainger, A.; Østergaard, M.; Klauser, A.; Cotten, A.; Wick, M.C.; Maas, M.; et al. Recommendations of the ESSR Arthritis Subcommittee for the Use of Magnetic Resonance Imaging in Musculoskeletal Rheumatic Diseases. Semin. Musculoskelet. Radiol. 2015, 19, 396–411. [Google Scholar] [CrossRef] [Green Version]
- McQueen, F.M. The MRI view of synovitis and tenosynovitis in inflammatory arthritis: Implications for diagnosis and management. Ann. N. Y. Acad. Sci. 2009, 1154, 21–34. [Google Scholar] [CrossRef]
- De Vries, B.A.; Breda, S.J.; Sveinsson, B.; McWalter, E.J.; Meuffels, D.E.; Krestin, G.P.; Hargreaves, B.A.; Gold, G.E.; Oei, E.H.G. Detection of knee synovitis using non-contrast-enhanced qDESS compared with contrast-enhanced MRI. Arthritis Res. Ther. 2021, 23, 1–9. [Google Scholar] [CrossRef]
- Gupta, R.K.; Nath, K.; Prasad, A.; Prasad, K.; Husain, M.; Rathore, R.; Husain, N.; Srivastava, C.; Khetan, P.; Trivedi, R.; et al. In Vivo Demonstration of Neuroinflammatory Molecule Expression in Brain Abscess with Diffusion Tensor Imaging. Am. J. Neuroradiol. 2008, 29, 326–332. [Google Scholar] [CrossRef] [Green Version]
- Gupta, R.K.; Hasan, K.M.; Mishra, A.M.; Jha, D.; Husain, M.; Prasad, K.N.; Narayana, P.A. High Fractional Anisotropy in Brain Abscesses versus Other Cystic Intracranial Lesions. Am. J. Neuroradiol. 2005, 26, 1107–1114. [Google Scholar]
- Agarwal, V.; Kumar, M.; Singh, J.K.; Rathore, R.K.S.; Misra, R.; Gupta, R.K. Diffusion tensor anisotropy magnetic resonance imaging: A new tool to assess synovial inflammation. Rheumatology 2009, 48, 378–382. [Google Scholar] [CrossRef] [Green Version]
- Sandford, H.J.C.; MacKay, J.W.; Watkins, L.E.; Gold, G.E.; Kogan, F.; Mazzoli, V. Gadolinium-free assessment of synovitis using diffusion tensor imaging. NMR Biomed. 2021, 35, e4614. [Google Scholar] [CrossRef]
- König, H.; Sieper, J.; Wolf, K.J. Rheumatoid arthritis: Evaluation of hypervascular and fibrous pannus with dynamic MR imaging enhanced with Gd-DTPA. Radiology 1990, 176, 473–477. [Google Scholar] [CrossRef] [PubMed]
- Stomp, W.; Krabben, A.; Van Der Heijde, D.; Huizinga, T.W.J.; Bloem, J.L.; Østergaard, M.; van der Helm-van Mil, A.H.; Reijnierse, M. Aiming for a simpler early arthritis MRI protocol: Can Gd contrast administration be eliminated? Eur. Radiol. 2015, 25, 1520–1527. [Google Scholar] [CrossRef] [PubMed]
- Damman, W.; Liu, R.; Reijnierse, M.; Rosendaal, F.R.; Bloem, J.L.; Kloppenburg, M. Effusion attenuates the effect of synovitis on radiographic progression in patients with hand osteoarthritis: A longitudinal magnetic resonance imaging study. Clin. Rheumatol. 2021, 40, 315–319. [Google Scholar] [CrossRef] [PubMed]
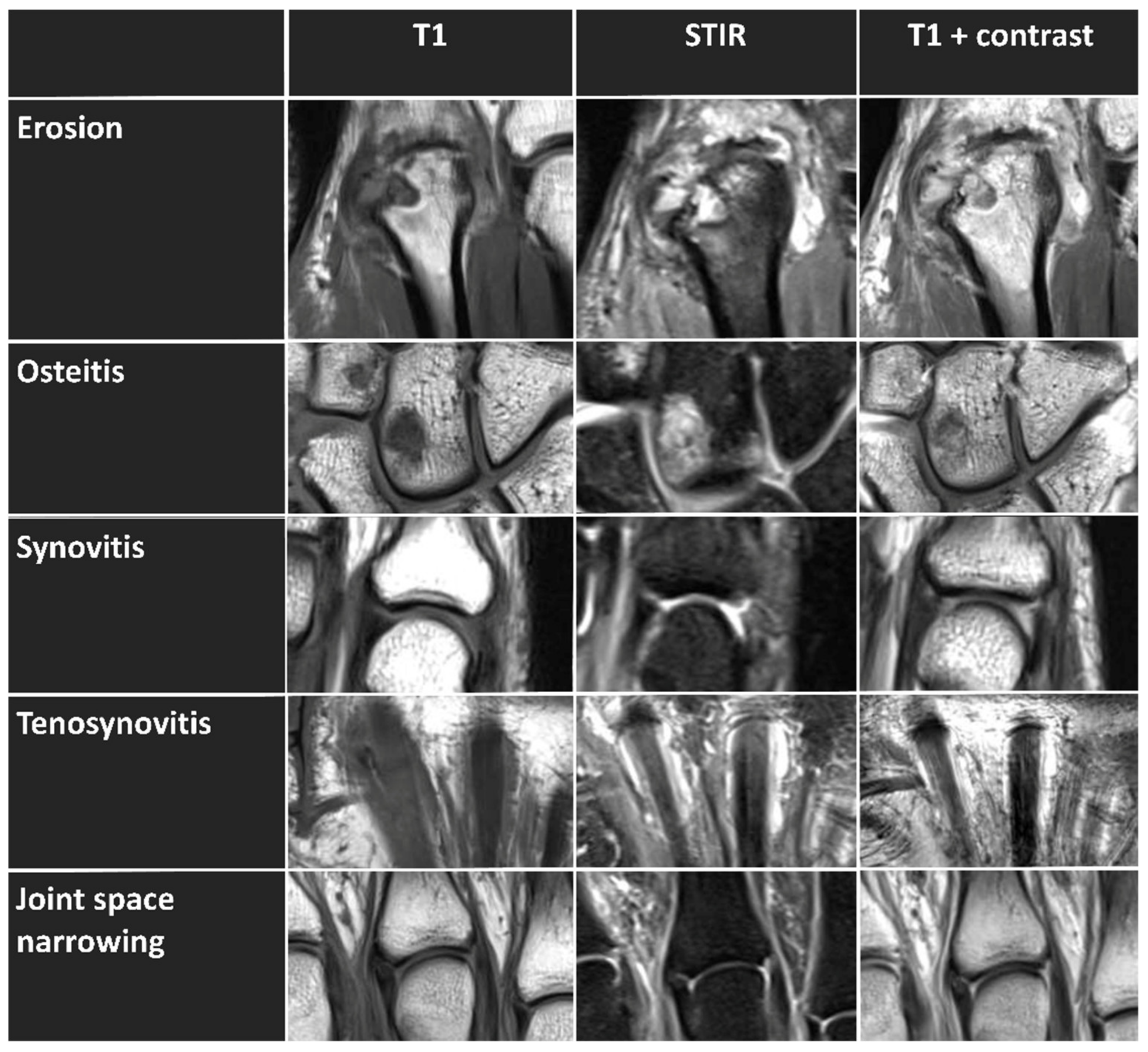
| Imaging Feature | Anatomic Location | MRI Sequence: | |
|---|---|---|---|
| RAMRIS-SAFE (without Contrast Enhancement) | RAMRIS (with Contrast Enhancement) | ||
| Erosion | Distal radius and distal ulna | coronal T1 w/o contrast | coronal T1 w/o contrast |
| All carpal bones and metacarpal bases | |||
| MCP joints (II-V) | |||
| Osteitis | Distal radius and distal ulna | coronal STIR w/o contrast | coronal STIR w/o contrast |
| All carpal bones and metacarpal bases MCP joints (II-V) | |||
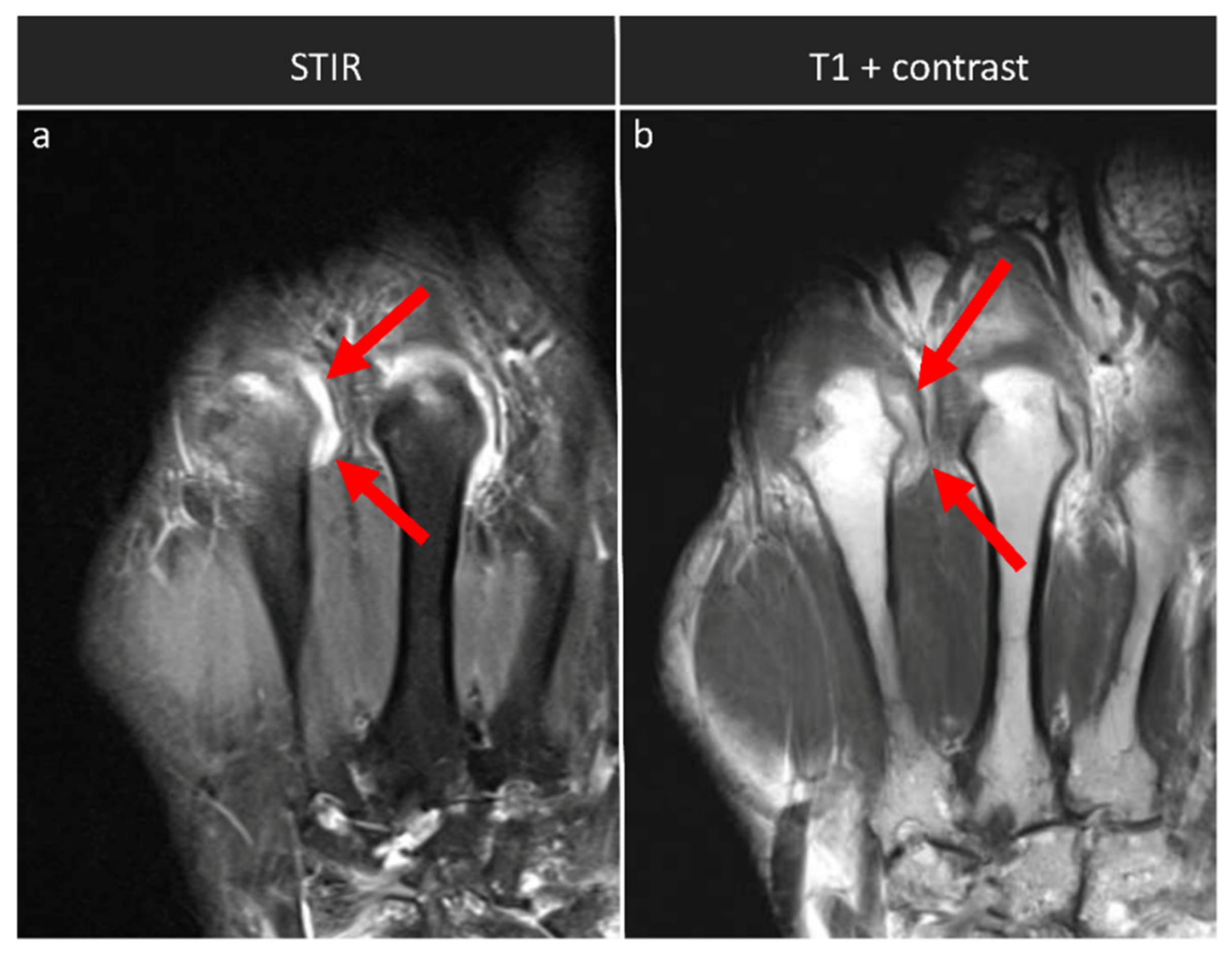
| Synovitis | Distal radio-ulnar joint Radiocarpal joint Intercarpal and carpometacarpal joints MCP (II-V) joints | coronal STIR, axial T1 w/o contrast | coronal and axial T1 + contrast |
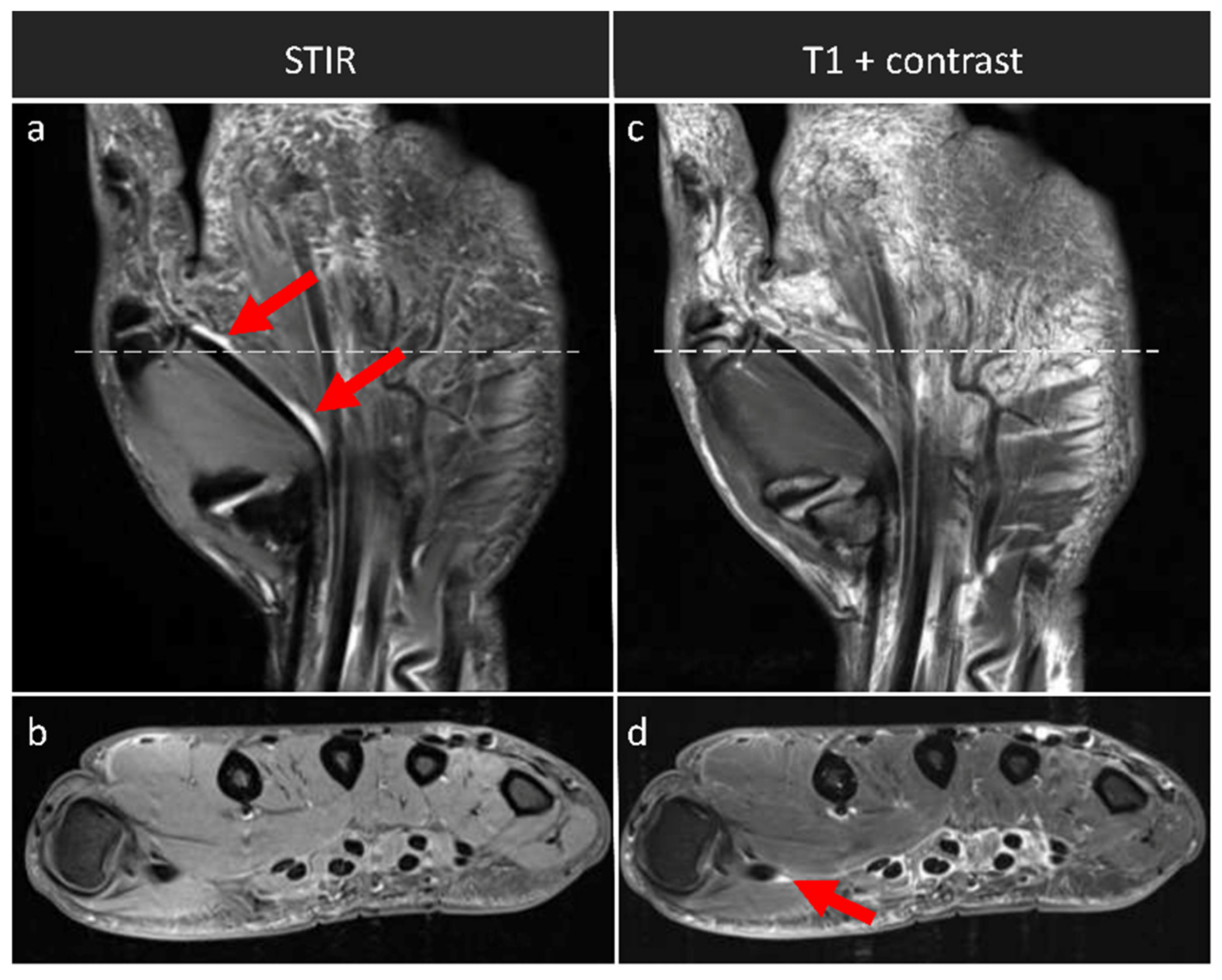
| Tenosynovitis | Flexor tendons at the level of MCP joints | coronal STIR, axial T1 w/o contrast | coronal and axial T1 + contrast |
| Joint space narrowing | All radiocarpal, intercarpal, carpometacarpal, and MCP joints (n = 17) | coronal T1 w/o contrast | coronal T1 w/o contrast |
| T1 (TSE) | STIR | T1 (SE) | T1 (TSE) + Contrast | T1 (SE) + Contrast | |
|---|---|---|---|---|---|
| Orientation | coronal | coronal | axial | coronal | axial |
| GBCA | no | no | no | yes | yes |
| Spectral fat suppression | no | no | yes | no | yes |
| TE [ms] | 27 | 31 | 16 | 27 | 16 |
| TR [ms] | 862 | 5560 | 702 | 862 | 702 |
| Slice thickness [mm] | 2.5 | 2.5 | 2.5 | 2.5 | 2.5 |
| FoV [mm2] | 140 × 140 | 140 × 140 | 120 × 120 | 140 × 140 | 120 × 120 |
| Inversion time [ms] | - | 210 | - | - | - |
| Matrix size [pixels2] | 512 × 512 | 448 × 312 | 384 × 288 | 512 × 512 | 384 × 288 |
| Voxel size [mm3] | 0.3 × 0.3 × 2.5 | 0.3 × 0.3 × 2.5 | 0.3 × 0.3 × 2.5 | 0.3 × 0.3 × 2.5 | 0.3 × 0.3 × 2.5 |
| Slice number [n] | 17 | 17 | 20 | 17 | 20 |
| Acquisition time [min:sec] | 4:57 | 4:23 | 4:56 | 4:57 | 3:42 |
| Mean | SD | 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1. DAS-28 | 4.13 | 1.41 | ||||||||||
| 2. CRP | 1.55 | 2.15 | 0.499 ** | |||||||||
| 3. Synovitis-contrast | 8.39 | 6.27 | 0.469 ** | 0.239 | ||||||||
| 4. Synovitis-SAFE | 8.52 | 5.59 | 0.463 ** | 0.305 | 0.937 ** | |||||||
| 5. Osteitis | 4.06 | 7.77 | 0.418 ** | 0.104 | 0.684 ** | 0.671 ** | ||||||
| 6. Erosion | 10.39 | 12.95 | 0.458 ** | 0.056 | 0.651 ** | 0.670 ** | 0.805 ** | |||||
| 7. JSN | 8.03 | 12.47 | 0.541 ** | 0.208 | 0.564 ** | 0.578 ** | 0.712 ** | 0.676 ** | ||||
| 8. Tenosynovitis-contrast | 2.68 | 2.57 | 0.023 ** | −0.068 | 0.579 ** | 0.515 ** | 0.196 ** | 0.279 ** | 0.130 ** | |||
| 9. Tenosynovitis-SAFE | 2.26 | 2.42 | 0.085 ** | 0.052 | 0.390 ** | 0.381 ** | 0.304 ** | 0.229 * | 0.212 ** | 0.380 ** | ||
| 10. RAMRIS | 33.55 | 34.94 | 0.480 ** | 0.131 | 0.867 ** | 0.846 ** | 0.852 ** | 0.854 ** | 0.787 ** | 0.481 ** | 0.374 * | |
| 11. RAMRIS-SAFE | 33.26 | 34.36 | 0.491 ** | 0.166 | 0.839 ** | 0.859 ** | 0.863 ** | 0.873 ** | 0.812 ** | 0.378 ** | 0.401 * | 0.976 ** |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frenken, M.; Rübsam, G.; Mewes, A.; Radke, K.L.; Li, L.; Wilms, L.M.; Nebelung, S.; Abrar, D.B.; Sewerin, P. To Contrast or Not to Contrast? On the Role of Contrast Enhancement in Hand MRI Studies of Patients with Rheumatoid Arthritis. Diagnostics 2022, 12, 465. https://doi.org/10.3390/diagnostics12020465
Frenken M, Rübsam G, Mewes A, Radke KL, Li L, Wilms LM, Nebelung S, Abrar DB, Sewerin P. To Contrast or Not to Contrast? On the Role of Contrast Enhancement in Hand MRI Studies of Patients with Rheumatoid Arthritis. Diagnostics. 2022; 12(2):465. https://doi.org/10.3390/diagnostics12020465
Chicago/Turabian StyleFrenken, Miriam, Gesa Rübsam, Alexander Mewes, Karl Ludger Radke, Lien Li, Lena M. Wilms, Sven Nebelung, Daniel B. Abrar, and Philipp Sewerin. 2022. "To Contrast or Not to Contrast? On the Role of Contrast Enhancement in Hand MRI Studies of Patients with Rheumatoid Arthritis" Diagnostics 12, no. 2: 465. https://doi.org/10.3390/diagnostics12020465
APA StyleFrenken, M., Rübsam, G., Mewes, A., Radke, K. L., Li, L., Wilms, L. M., Nebelung, S., Abrar, D. B., & Sewerin, P. (2022). To Contrast or Not to Contrast? On the Role of Contrast Enhancement in Hand MRI Studies of Patients with Rheumatoid Arthritis. Diagnostics, 12(2), 465. https://doi.org/10.3390/diagnostics12020465








