Diagnostic Framework of Pelvic Massive Necrosis with Peritonitis following Chemoradiation for Locally Advanced Cervical Cancer: When Is the Surgery Not Demandable? A Case Report and Literature Review
Abstract
:1. Introduction
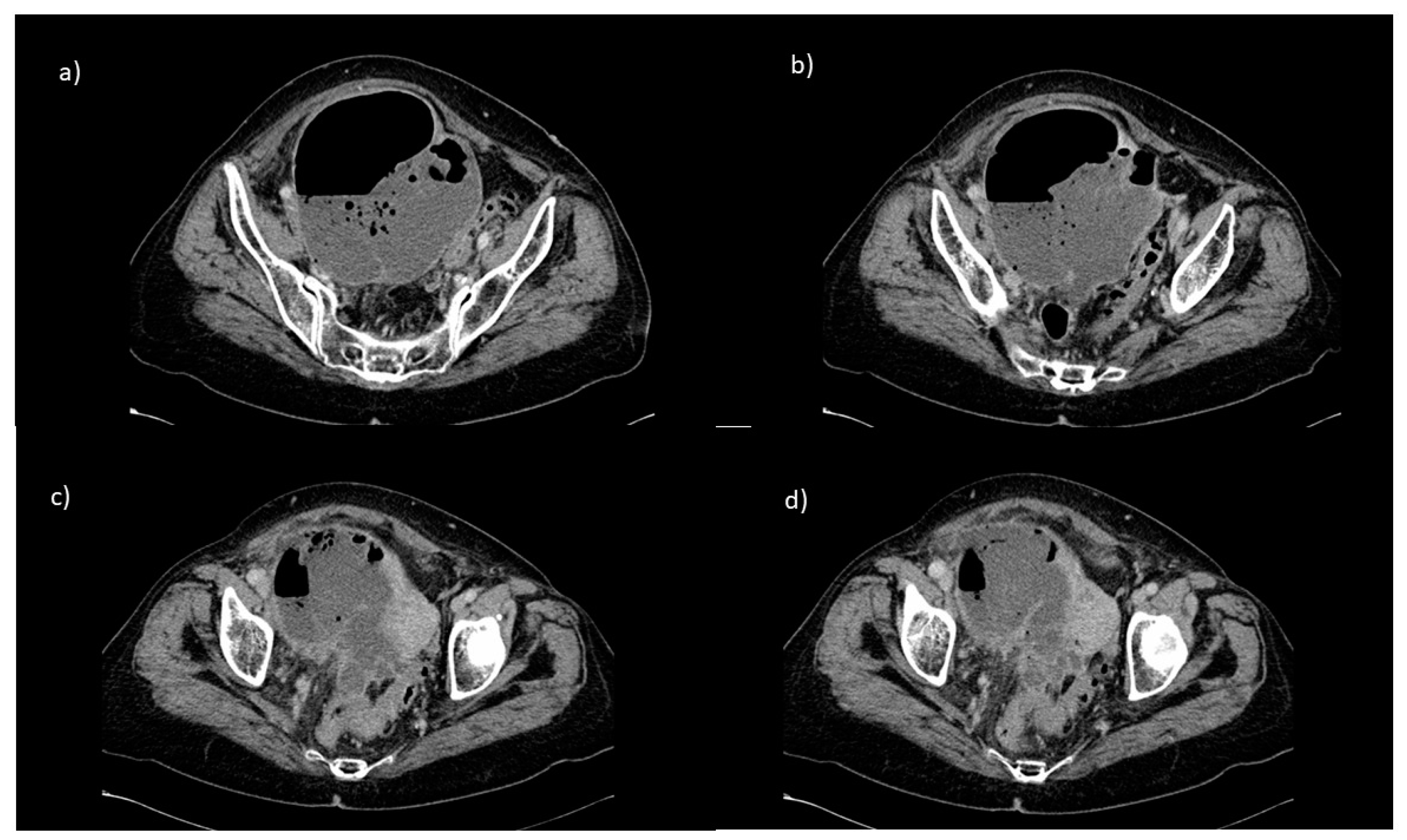
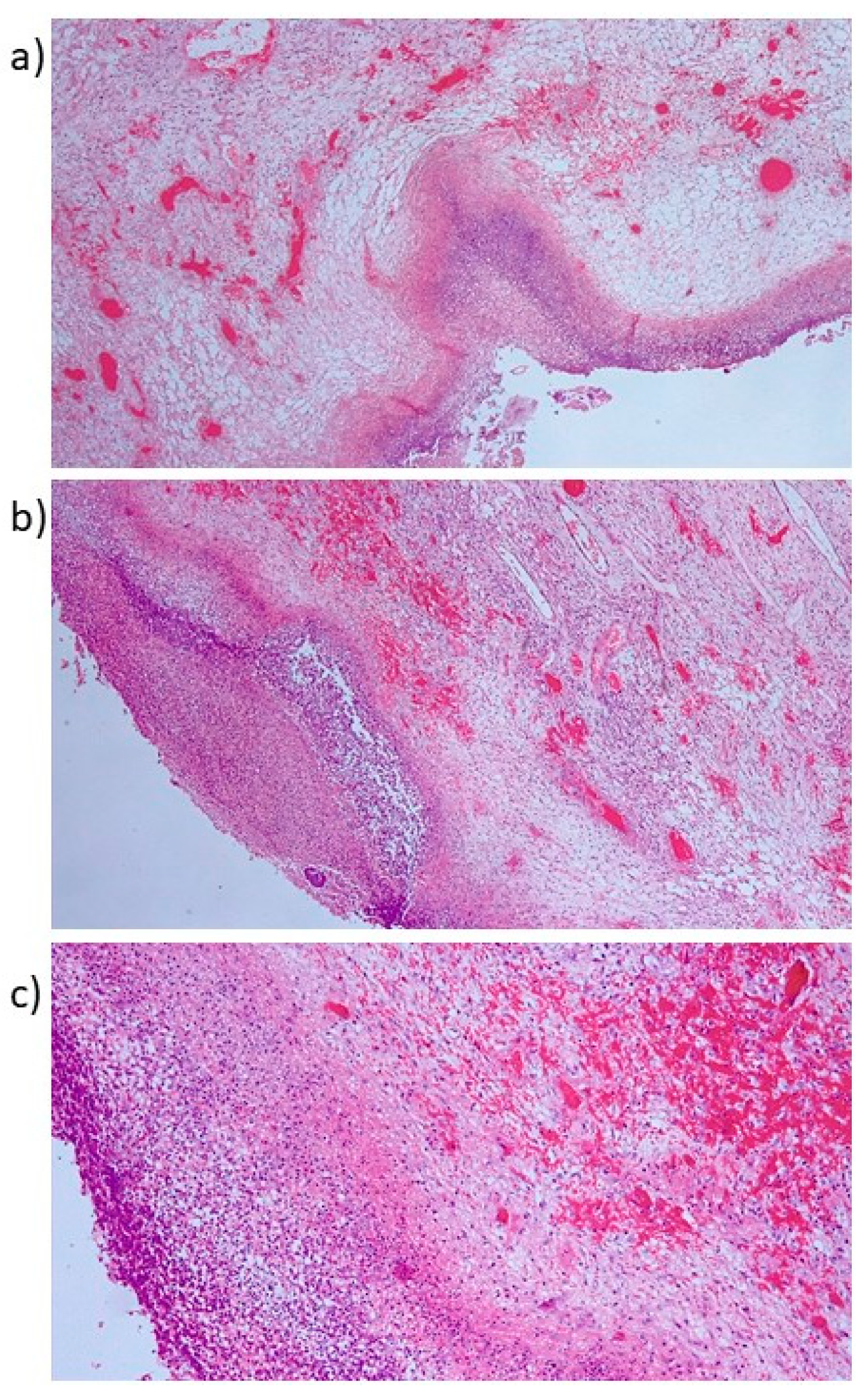
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Datta, N.R.; Stutz, E.; Liu, M.; Rogers, S.; Klingbiel, D.; Siebenhüner, A.; Singh, S.; Bodis, S. Concurrent chemoradiotherapy vs. radiotherapy alone in locally advanced cervix cancer: A systematic review and meta-analysis. Gynecol. Oncol. 2017, 145, 374–385. [Google Scholar] [CrossRef]
- Eifel, P.J. Concurrent chemotherapy and radiation therapy as the standard of care for cervical cancer. Nat. Clin. Pract. Oncol. 2006, 3, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Kirwan, J.M.; Symonds, P.; Green, J.A.; Tierney, J.; Collingwood, M.; Williams, C.J. A systematic review of acute and late toxicity of concomitant chemoradiation for cervical cancer. Radiother. Oncol. 2003, 68, 217–226. [Google Scholar] [CrossRef]
- Kizilay, F.; Simsir, A.; Cureklibatir, I.; Cal, C. Long-Term Outcomes of Patients Who Underwent Ureterocutaneostomy. Bull. Urooncol. 2018, 17, 54–58. [Google Scholar] [CrossRef]
- Korkes, F.; Fernandes, E.; Gushiken, F.A.; Glina, F.P.A.; Baccaglini, W.; Timóteo, F.; Glina, S. Bricker ileal conduit vs. Cutaneous ureterostomy after radical cystectomy for bladder cancer: A systematic review. Int. Braz. J. Urol. 2022, 48, 18–30. [Google Scholar] [CrossRef]
- Green, J.A.; Kirwan, J.M.; Tierney, J.F.; Symonds, P.; Fresco, L.; Collingwood, M.; Williams, C.J. Survival and recurrence after concomitant chemotherapy and radiotherapy for cancer of the uterine cervix: A systematic review and meta-analysis. Lancet. 2001, 358, 781–786. [Google Scholar] [CrossRef]
- Chemoradiotherapy for Cervical Cancer Meta-Analysis Collaboration. Reducing uncertainties about the effects of chemoradiotherapy for cervical cancer: A systematic review and meta-analysis of individual patient data from 18 randomized trials. J. Clin. Oncol. 2008, 26, 5802–5812. [Google Scholar] [CrossRef] [Green Version]
- Güth, U.; Ella, W.A.; Olaitan, A.; Hadwin, R.J.; Arora, R.; McCormack, M. Total vaginal necrosis: A representative example of underreporting severe late toxic reaction after concomitant chemoradiation for cervical cancer. Int. J. Gynecol. Cancer 2010, 20, 54–60. [Google Scholar] [CrossRef]
- Nakano, T.; Kato, S.; Ohno, T.; Tsujii, H.; Sato, S.; Fukuhisa, K.; Arai, T. Long-term results of high-dose rate intracavitary brachytherapy for squamous cell carcinoma of the uterine cervix. Cancer 2005, 103, 92–101. [Google Scholar] [CrossRef]
- Yamada, T.; Ishihara, S.; Kawai, M.; Itoh, Y.; Naganawa, S.; Ikeda, M. Analysis of late adverse events and their chronological changes after radiation therapy for cervical cancer. Nagoya J. Med. Sci. 2018, 80, 487–496. [Google Scholar]
- Bentzen, S.M. Preventing or reducing late side effects of radiation therapy: Radiobiology meets molecular pathology. Nat. Rev. Cancer 2006, 6, 702–713. [Google Scholar] [CrossRef] [PubMed]
- Matthews, K.S.; Rocconi, R.P.; Straughn, J.M. Complete uterine necrosis following chemoradiation for advanced cervical cancer: A case report. Gynecol. Oncol. 2007, 106, 265–267. [Google Scholar] [CrossRef] [PubMed]
- Fokdal, L.; Tanderup, K.; Pötter, R.; Sturdza, A.; Kirchheiner, K.; Chargari, C.; Jürgenliemk-Schulz, I.M.; Segedin, B.; Tan, L.T.; Hoskin, P.; et al. Risk factors for ureteral stricture after radi-ochemotherapy including image guided adaptive brachytherapy in cervical cancer: Results from the EMBRACE studies. Int. J. Radiat. Oncol. Biol. Phys. 2019, 103, 887–894. [Google Scholar] [CrossRef] [PubMed]
- Spampinato, S.; Fokdal, L.U.; Pötter, R.; Haie-Meder, C.; Lindegaard, J.C.; Schmid, M.P.; Sturdza, A.; Jürgenliemk-Schulz, I.M.; Mahantshetty, U.; Segedin, B.; et al. Risk factors and dose-effects for bladder fistula, bleeding and cystitis after radiotherapy with imaged-guided adaptive brachytherapy for cervical cancer: An EM-BRACE analysis. Radiother. Oncol. 2021, 158, 312–320. [Google Scholar] [CrossRef]
- Zelga, P.; Tchórzewski, M.; Zelga, M.; Sobotkowski, J.; Dziki, A. Radiation-induced rectovaginal fistulas in locally advanced gynaecological malignancies-new patients, old problem? Langenbecks Arch. Surg. 2017, 402, 1079–1088. [Google Scholar] [CrossRef] [Green Version]
- Sun, R.; Koubaa, I.; Limkin, E.J.; Dumas, I.; Bentivegna, E.; Castanon, E.; Gouy, S.; Baratiny, C.; Monnot, F.; Maroun, P.; et al. Locally advanced cervical cancer with bladder invasion: Clinical outcomes and predictive factors for vesicovaginal fistulae. Oncotarget. 2018, 9, 9299–9310. [Google Scholar] [CrossRef]
- Papadopoulou, I.; Stewart, V.; Barwick, T.D.; Park, W.H.; Soneji, N.; Rockall, A.G.; Bharwani, N. Post-Radiation Therapy Imaging Appearances in Cervical Carcinoma. Radiographics 2016, 36, 538–553. [Google Scholar] [CrossRef]
- Nakao, Y.; Hashiguchi, M.; Nishiyama, S.; Aihara, S.; Iwasaka, T.; Yokoyama, M. Preoperative Chemoradiotherapy in Locally Advanced Bulky Squamous Cell Carcinoma of the Uterine Cervix. Int. J. Gynecol. Cancer 2017, 27, 1943–1948. [Google Scholar] [CrossRef]
- Jia, A.Y.; Viswanathan, A.N. Vaginal necrosis: A rare late toxicity after radiation therapy. Gynecol. Oncol. 2021, 160, 602–609. [Google Scholar] [CrossRef]
- DeMuylder, X.; Corman, J.; Giroux, L.; Methot, Y.; Poljicak, M.; Péloquin, A.; Audet-Lapointe, P.; Smeesters, C.; Beland, G.; Falardeau, M. Complications of the treatment of cervix neoplasms by radiotherapy. Can. J. Surg. 1986, 29, 267–272. [Google Scholar]
- Marnitz, S.; Köhler, C.; Füller, J.; Hinkelbein, W.; Schneider, A. Uterus necrosis after radiochemotherapy in two patients with advanced cervical cancer. Strahlenther. Onkol. 2006, 182, 45–51. [Google Scholar] [CrossRef] [PubMed]
- Micha, J.P.; Goldstein, B.H.; Rettenmaier, M.A.; Caillouette, J.T.; Fee, M.J.; Brown, J.V. Pelvic radiation necrosis and osteomyelitis following chemoradiation for advanced stage vulvar and cervical carcinoma. Gynecol. Oncol. 2006, 101, 349–352. [Google Scholar] [CrossRef] [PubMed]
- Fawaz, Z.S.; Barkati, M.; Beauchemin, M.C.; Sauthier, P.; Gauthier, P.; Nguyen, T.V. Cervical necrosis after chemoradiation for cervical cancer: Case series and literature review. Radiat. Oncol. 2013, 8, 220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gosset, M.; Chargari, C.; Bentivegna, E.; Leary, A.; Genestie, C.; Maulard, A.; Morice, P.; Gouy, S. Should We Cease to Perform Salvage Hysterectomy After Chemoradiation and Brachytherapy in Locally Advanced Cervical Cancer? Anticancer Res. 2019, 39, 2919–2926. [Google Scholar] [CrossRef] [Green Version]
- Shim, S.H.; Kim, S.N.; Chae, S.H.; Kim, J.E.; Lee, S.J. Impact of adjuvant hysterectomy on prognosis in patients with locally advanced cervical cancer treated with concurrent chemoradiotherapy: A meta-analysis. J. Gynecol. Oncol. 2018, 29, e25. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Decker, M.A.; Burke, J.J., II; Gallup, D.G.; Silverio, R.W.; Weems, D.; Duttenhaver, J.; Purcell, D. Completion hysterectomy after radiation therapy for bulky cervical cancer stages IB, IIA, and IIB: Complications and survival rates. Am. J. Obstet. Gynecol. 2004, 191, 654–658. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sanna, E.; Chiappe, G.; Lavra, F.; Nemolato, S.; Oppi, S.; Macciò, A.; Madeddu, C. Diagnostic Framework of Pelvic Massive Necrosis with Peritonitis following Chemoradiation for Locally Advanced Cervical Cancer: When Is the Surgery Not Demandable? A Case Report and Literature Review. Diagnostics 2022, 12, 440. https://doi.org/10.3390/diagnostics12020440
Sanna E, Chiappe G, Lavra F, Nemolato S, Oppi S, Macciò A, Madeddu C. Diagnostic Framework of Pelvic Massive Necrosis with Peritonitis following Chemoradiation for Locally Advanced Cervical Cancer: When Is the Surgery Not Demandable? A Case Report and Literature Review. Diagnostics. 2022; 12(2):440. https://doi.org/10.3390/diagnostics12020440
Chicago/Turabian StyleSanna, Elisabetta, Giacomo Chiappe, Fabrizio Lavra, Sonia Nemolato, Sara Oppi, Antonio Macciò, and Clelia Madeddu. 2022. "Diagnostic Framework of Pelvic Massive Necrosis with Peritonitis following Chemoradiation for Locally Advanced Cervical Cancer: When Is the Surgery Not Demandable? A Case Report and Literature Review" Diagnostics 12, no. 2: 440. https://doi.org/10.3390/diagnostics12020440
APA StyleSanna, E., Chiappe, G., Lavra, F., Nemolato, S., Oppi, S., Macciò, A., & Madeddu, C. (2022). Diagnostic Framework of Pelvic Massive Necrosis with Peritonitis following Chemoradiation for Locally Advanced Cervical Cancer: When Is the Surgery Not Demandable? A Case Report and Literature Review. Diagnostics, 12(2), 440. https://doi.org/10.3390/diagnostics12020440







