Biliary Atresia: A Complex Hepatobiliary Disease with Variable Gene Involvement, Diagnostic Procedures, and Prognosis
Abstract
1. Introduction
2. Materials and Methods
3. Histology of the Developing Fetal Liver
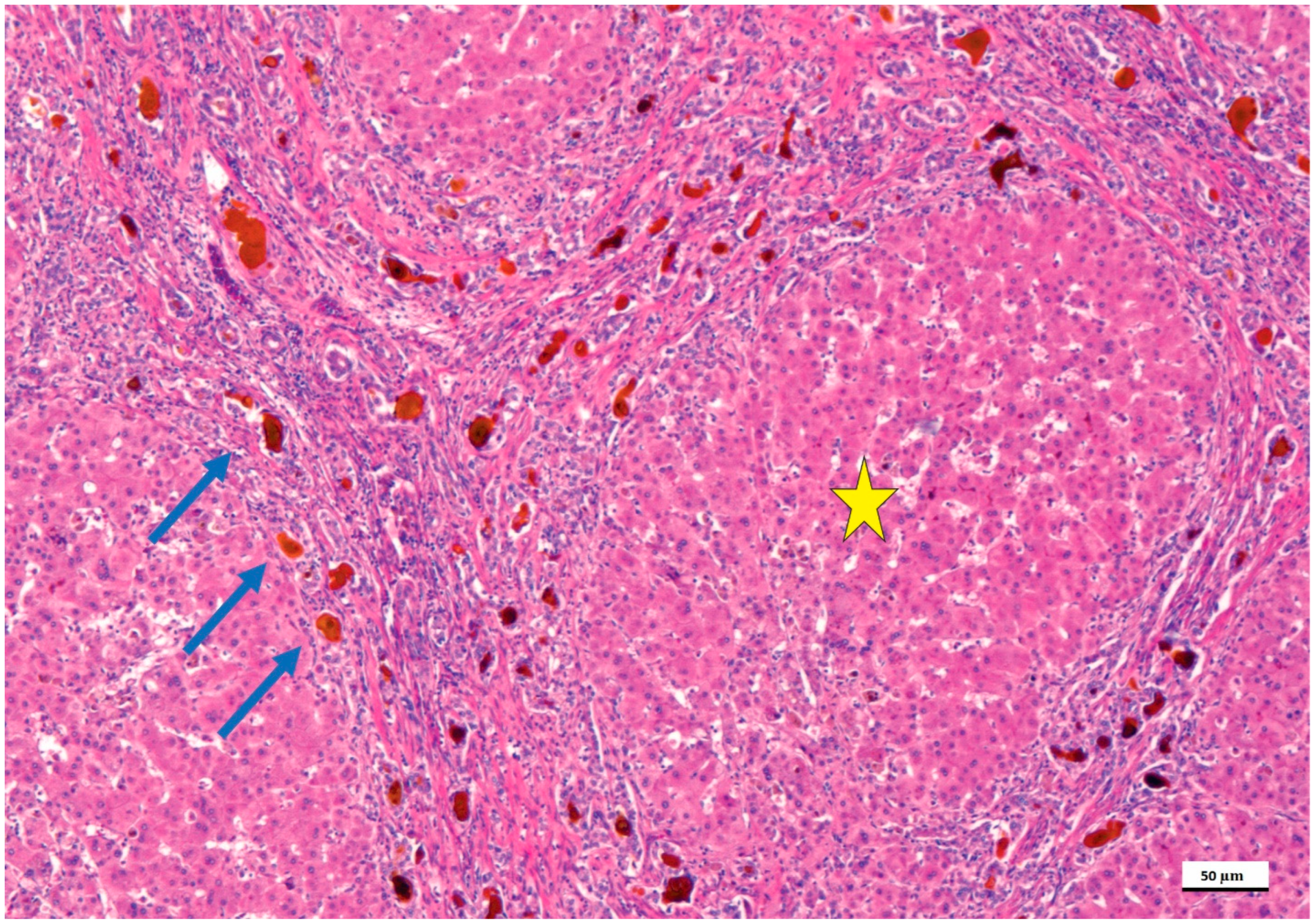
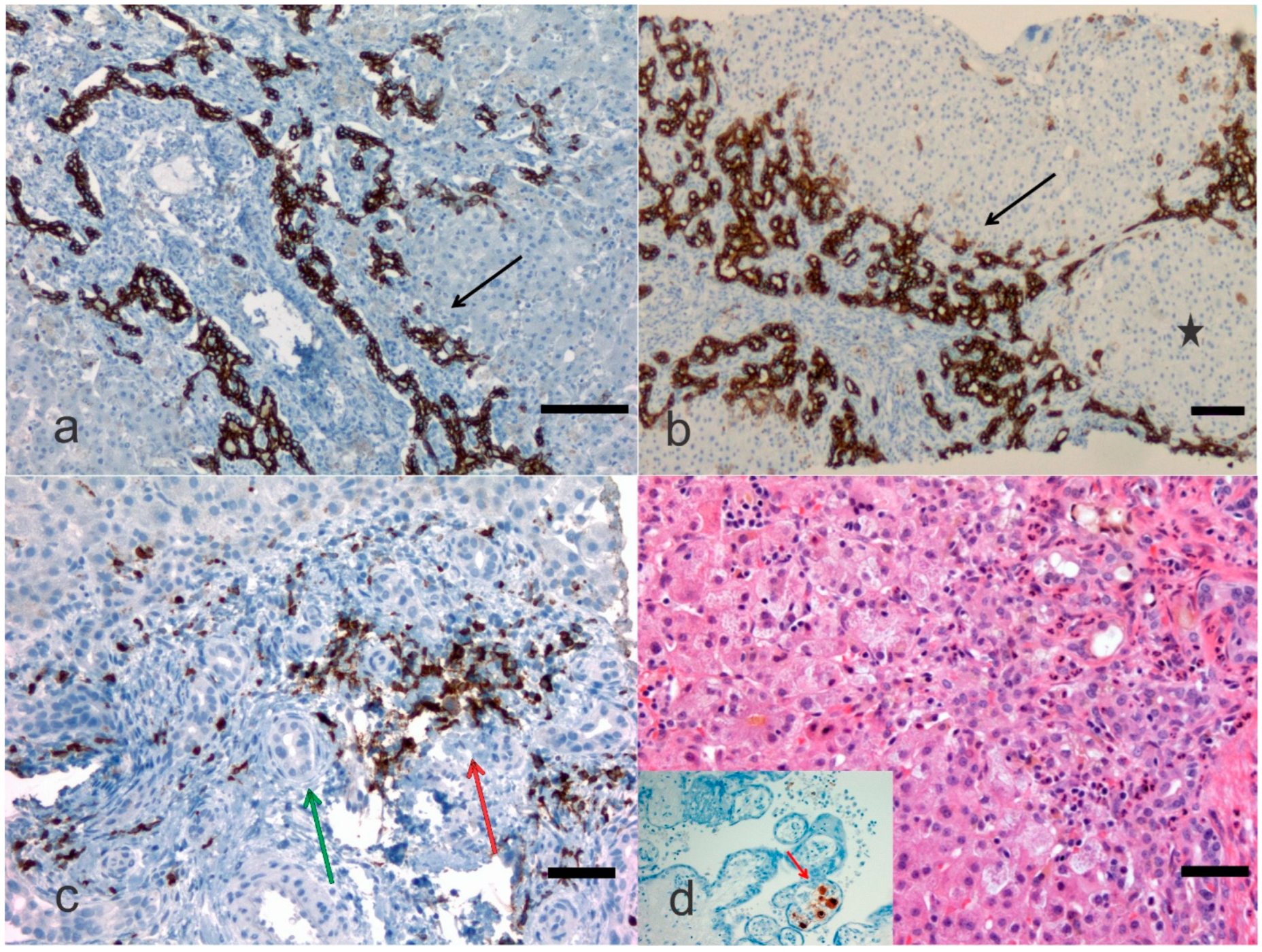
4. Pathological Anatomy of the Biliary Atresia
5. Genetic Complexities and Perspectives
6. Non-Genetic Ambiguities
7. Treatment
8. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ananth, R. Neonatal Cholestasis: A Primer of Selected Etiologies. Pediatr. Ann. 2018, 47, e433–e439. [Google Scholar] [CrossRef]
- Pang, W.B.; Zhang, T.C.; Chen, Y.J.; Peng, C.H.; Wang, Z.M.; Wu, D.Y.; Wang, K. Ten-Year Experience in the Prevention of Post-Kasai Cholangitis. Surg. Infect. 2019, 20, 231–235. [Google Scholar] [CrossRef] [PubMed]
- Chan, K.W.E.; Lee, K.H.; Wong, H.Y.V.; Tsui, S.Y.B.; Mou, J.W.C.; Tam, Y.H.P. Ten-Year Native Liver Survival Rate After Laparoscopic and Open Kasai Portoenterostomy for Biliary Atresia. J. Laparoendosc. Adv. Surg. Tech. A 2019, 29, 121–125. [Google Scholar] [CrossRef]
- Wong, Z.H.; Davenport, M. What Happens after Kasai for Biliary Atresia? A European Multicenter Survey. Eur. J. Pediatr. Surg. 2019, 29, 1–6. [Google Scholar] [CrossRef] [PubMed]
- Garcia, A.V.; Cowles, R.A.; Kato, T.; Hardy, M.A. Morio Kasai: A remarkable impact beyond the Kasai procedure. J. Pediatr. Surg. 2012, 47, 1023–1027. [Google Scholar] [CrossRef]
- Sergi, C.M. Genetics of Biliary Atresia: A Work in Progress for a Disease with an Unavoidable Sequela into Liver Cirrhosis following Failure of Hepatic Portoenterostomy. In Liver Cirrhosis—Debates and Current Challenges; Tsoulfas, G., Ed.; IntechOpen: London, UK, 2019. [Google Scholar]
- Sergi, C.; Benstz, J.; Feist, D.; Nutzenadel, W.; Otto, H.F.; Hofmann, W.J. Bile duct to portal space ratio and ductal plate remnants in liver disease of infants aged less than 1 year. Pathology 2008, 40, 260–267. [Google Scholar] [CrossRef]
- Obayashi, J.; Tanaka, K.; Ohyama, K.; Manabe, S.; Nagae, H.; Shima, H.; Sato, H.; Furuta, S.; Wakisaka, M.; Koike, J.; et al. Relation between amount of bile ducts in portal canal and outcomes in biliary atresia. Pediatr. Surg. Int. 2016, 32, 833–838. [Google Scholar] [CrossRef]
- Chen, G.; Xue, P.; Zheng, S.; Chen, L.; Ma, Y. A pathological scoring system in the diagnosis and judgment of prognosis of biliary atresia. J. Pediatr. Surg. 2015, 50, 2119–2123. [Google Scholar] [CrossRef]
- Czubkowski, P.; Cielecka-Kuszyk, J.; Rurarz, M.; Kaminska, D.; Markiewicz-Kijewska, M.; Pawlowska, J. The limited prognostic value of liver histology in children with biliary atresia. Ann. Hepatol. 2015, 14, 902–909. [Google Scholar] [CrossRef]
- Safwan, M.; Ramachandran, P.; Vij, M.; Shanmugam, N.; Rela, M. Impact of ductal plate malformation on survival with native liver in children with biliary atresia. Pediatr. Surg. Int. 2015, 31, 837–843. [Google Scholar] [CrossRef]
- Vukovic, J.; Grizelj, R.; Bojanic, K.; Coric, M.; Luetic, T.; Batinica, S.; Kujundzic-Tiljak, M.; Schroeder, D.R.; Sprung, J. Ductal plate malformation in patients with biliary atresia. Eur. J. Pediatr. 2012, 171, 1799–1804. [Google Scholar] [CrossRef]
- Yamaguti, D.C.; Patricio, F.R. Morphometrical and immunohistochemical study of intrahepatic bile ducts in biliary atresia. Eur. J. Gastroenterol. Hepatol. 2011, 23, 759–765. [Google Scholar] [CrossRef]
- Dorn, L.; Menezes, L.F.; Mikuz, G.; Otto, H.F.; Onuchic, L.F.; Sergi, C. Immunohistochemical detection of polyductin and co-localization with liver progenitor cell markers during normal and abnormal development of the intrahepatic biliary system and in adult hepatobiliary carcinomas. J. Cell. Mol. Med. 2009, 13, 1279–1290. [Google Scholar] [CrossRef][Green Version]
- Pacheco, M.C.; Campbell, K.M.; Bove, K.E. Ductal plate malformation-like arrays in early explants after a Kasai procedure are independent of splenic malformation complex (heterotaxy). Pediatr. Dev. Pathol. 2009, 12, 355–360. [Google Scholar] [CrossRef]
- Shimadera, S.; Iwai, N.; Deguchi, E.; Kimura, O.; Ono, S.; Fumino, S.; Higuchi, K. Significance of ductal plate malformation in the postoperative clinical course of biliary atresia. J. Pediatr. Surg. 2008, 43, 304–307. [Google Scholar] [CrossRef]
- Beiler, H.A.; Sergi, C.; Wagner, G.; Zachariou, Z. Accessory liver in an infant with congenital diaphragmatic hernia. J. Pediatr. Surg. 2001, 36, 1–3. [Google Scholar] [CrossRef]
- Sergi, C.M. Pathology of Childhood and Adolescence. An Illustrated Guide; Springer: Berlin/Heidelberg, Germany, 2019. [Google Scholar]
- Sergi, C.; Schulze, B.R.; Hager, H.D.; Beedgen, B.; Zilow, E.; Linderkamp, O.; Otto, H.F.; Tariverdian, G. Wolf-Hirschhorn syndrome: Case report and review of the chromosomal aberrations associated with diaphragmatic defects. Pathologica 1998, 90, 285–293. [Google Scholar]
- Tremblay, K.D.; Zaret, K.S. Distinct populations of endoderm cells converge to generate the embryonic liver bud and ventral foregut tissues. Dev. Biol. 2005, 280, 87–99. [Google Scholar] [CrossRef]
- Amella, C.; Cappello, F.; Kahl, P.; Fritsch, H.; Lozanoff, S.; Sergi, C. Spatial and temporal dynamics of innervation during the development of fetal human pancreas. Neuroscience 2008, 154, 1477–1487. [Google Scholar] [CrossRef]
- Sergi, C.; Adam, S.; Kahl, P.; Otto, H.F. The remodeling of the primitive human biliary system. Early Hum. Dev. 2000, 58, 167–178. [Google Scholar] [CrossRef]
- Lemaigre, F.P. Development of the biliary tract. Mech. Dev. 2003, 120, 81–87. [Google Scholar] [CrossRef]
- Johnson, C.A.; Gissen, P.; Sergi, C. Molecular pathology and genetics of congenital hepatorenal fibrocystic syndromes. J. Med. Genet. 2003, 40, 311–319. [Google Scholar] [CrossRef]
- Sergi, C.; Kahl, P.; Otto, H.F. Contribution of apoptosis and apoptosis-related proteins to the malformation of the primitive intrahepatic biliary system in Meckel syndrome. Am. J. Pathol. 2000, 156, 1589–1598. [Google Scholar] [CrossRef]
- Carvalho, N.M.N.; Torres, S.M.; Cavalcante, J.C.B.; Ximenes, A.C.M.; Landim Junior, J.A.; Moreira, S. Hepatoportoenterostomy Surgery Technique. J. Pediatr. Surg. 2018, 54, 1715–1718. [Google Scholar] [CrossRef]
- Sergi, C.; Adam, S.; Kahl, P.; Otto, H.F. Study of the malformation of ductal plate of the liver in Meckel syndrome and review of other syndromes presenting with this anomaly. Pediatr. Dev. Pathol. 2000, 3, 568–583. [Google Scholar] [CrossRef]
- Russo, P.; Magee, J.C.; Anders, R.A.; Bove, K.E.; Chung, C.; Cummings, O.W.; Finegold, M.J.; Finn, L.S.; Kim, G.E.; Lovell, M.A.; et al. Key Histopathologic Features of Liver Biopsies That Distinguish Biliary Atresia From Other Causes of Infantile Cholestasis and Their Correlation With Outcome: A Multicenter Study. Am. J. Surg. Pathol. 2016, 40, 1601–1615. [Google Scholar] [CrossRef]
- Wang, Z.; Xie, X.; Zhao, J.; Fu, M.; Li, Y.; Zhong, W.; Xia, H.; Zhang, Y.; Zhang, R.Z. The intragenic epistatic association of ADD3 with biliary atresia in Southern Han Chinese population. Biosci. Rep. 2018, 38, BSR20171688. [Google Scholar] [CrossRef]
- Ke, J.; Zeng, S.; Mao, J.; Wang, J.; Lou, J.; Li, J.; Chen, X.; Liu, C.; Huang, L.M.; Wang, B.; et al. Common genetic variants of GPC1 gene reduce risk of biliary atresia in a Chinese population. J. Pediatr. Surg. 2016, 51, 1661–1664. [Google Scholar] [CrossRef]
- Smith, K. Biliary tract: GPC1 genetic risk further links Hedgehog signalling with pathogenesis of biliary atresia. Nat. Rev. Gastroenterol. Hepatol. 2013, 10, 127. [Google Scholar] [CrossRef]
- Filmus, J.; Capurro, M.; Rast, J. Glypicans. Genome Biol. 2008, 9, 224. [Google Scholar] [CrossRef]
- Tian, L.; Ye, Z.; Kafka, K.; Stewart, D.; Anders, R.; Schwarz, K.B.; Jang, Y.Y. Biliary Atresia Relevant Human Induced Pluripotent Stem Cells Recapitulate Key Disease Features in a Dish. J. Pediatr. Gastroenterol. Nutr. 2019, 68, 56–63. [Google Scholar] [CrossRef]
- Zeng, S.; Sun, P.; Chen, Z.; Mao, J.; Wang, J.; Wang, B.; Liu, L. Association between single nucleotide polymorphisms in the ADD3 gene and susceptibility to biliary atresia. PLoS ONE 2014, 9, e107977. [Google Scholar] [CrossRef]
- Li, J.; Gao, W.; Zuo, W.; Liu, X. Association between rs17095355 polymorphism on 10q24 and susceptibility to biliary atresia: A meta-analysis. J. Matern. Fetal Neonatal Med. 2017, 30, 1882–1886. [Google Scholar] [CrossRef]
- Mezina, A.; Karpen, S.J. Genetic contributors and modifiers of biliary atresia. Dig. Dis. 2015, 33, 408–414. [Google Scholar] [CrossRef]
- Choi, W.T.; Kakar, S. Immunohistochemistry in the Diagnosis of Hepatocellular Carcinoma. Gastroenterol. Clin. N. Am. 2017, 46, 311–325. [Google Scholar] [CrossRef]
- Sangkhathat, S.; Laochareonsuk, W.; Maneechay, W.; Kayasut, K.; Chiengkriwate, P. Variants Associated with Infantile Cholestatic Syndromes Detected in Extrahepatic Biliary Atresia by Whole Exome Studies: A 20-Case Series from Thailand. J. Pediatr. Genet. 2018, 7, 67–73. [Google Scholar] [CrossRef]
- Schon, P.; Tsuchiya, K.; Lenoir, D.; Mochizuki, T.; Guichard, C.; Takai, S.; Maiti, A.K.; Nihei, H.; Weil, J.; Yokoyama, T.; et al. Identification, genomic organization, chromosomal mapping and mutation analysis of the human INV gene, the ortholog of a murine gene implicated in left-right axis development and biliary atresia. Hum. Genet. 2002, 110, 157–165. [Google Scholar] [CrossRef]
- Tumgor, G.; Cogulu, O.; Onay, H.; Ekmekci, A.Y.; Aydogdu, S.; Durmaz, B.; Kilic, M.; Ozkinay, F. Unusual presentation of biliary atresia splenic malformation syndrome with autosomal dominant hypospadias. Genet. Couns. 2011, 22, 347–351. [Google Scholar]
- Santos, J.L.; Carvalho, E.; Bezerra, J.A. Advances in biliary atresia: From patient care to research. Braz. J. Med. Biol. Res. 2010, 43, 522–527. [Google Scholar] [CrossRef]
- Davit-Spraul, A.; Baussan, C.; Hermeziu, B.; Bernard, O.; Jacquemin, E. CFC1 gene involvement in biliary atresia with polysplenia syndrome. J. Pediatr. Gastroenterol. Nutr. 2008, 46, 111–112. [Google Scholar] [CrossRef]
- Eminoglu, T.F.; Polat, E.; Gokce, S.; Ezgu, F.S.; Senel, S.; Apaydin, S. Cystic fibrosis presenting with neonatal cholestasis simulating biliary atresia in a patient with a novel mutation. Indian J. Pediatr. 2013, 80, 502–504. [Google Scholar] [CrossRef]
- Demeilliers, C.; Jacquemin, E.; Barbu, V.; Mergey, M.; Paye, F.; Fouassier, L.; Chignard, N.; Housset, C.; Lomri, N.E. Altered hepatobiliary gene expressions in PFIC1: ATP8B1 gene defect is associated with CFTR downregulation. Hepatology 2006, 43, 1125–1134. [Google Scholar] [CrossRef]
- Mao, Y.; Tang, S.; Yang, L.; Li, K. Inhibition of the Notch Signaling Pathway Reduces the Differentiation of Hepatic Progenitor Cells into Cholangiocytes in Biliary Atresia. Cell. Physiol. Biochem. 2018, 49, 1074–1082. [Google Scholar] [CrossRef]
- Liu, Y.; Wang, H.; Dong, C.; Feng, J.X.; Huang, Z.H. Clinical Features and Genetic Analysis of Pediatric Patients with Alagille Syndrome Presenting Initially with Liver Function Abnormalities. Curr. Med. Sci. 2018, 38, 304–309. [Google Scholar] [CrossRef]
- Ohashi, K.; Togawa, T.; Sugiura, T.; Ito, K.; Endo, T.; Aoyama, K.; Negishi, Y.; Kudo, T.; Ito, R.; Saitoh, S. Combined genetic analyses can achieve efficient diagnostic yields for subjects with Alagille syndrome and incomplete Alagille syndrome. Acta Paediatr. 2017, 106, 1817–1824. [Google Scholar] [CrossRef]
- Zagory, J.A.; Dietz, W.; Park, A.; Fenlon, M.; Xu, J.; Utley, S.; Mavila, N.; Wang, K.S. Notch signaling promotes ductular reactions in biliary atresia. J. Surg. Res. 2017, 215, 250–256. [Google Scholar] [CrossRef]
- Kohsaka, T.; Yuan, Z.R.; Guo, S.X.; Tagawa, M.; Nakamura, A.; Nakano, M.; Kawasasaki, H.; Inomata, Y.; Tanaka, K.; Miyauchi, J. The significance of human jagged 1 mutations detected in severe cases of extrahepatic biliary atresia. Hepatology 2002, 36, 904–912. [Google Scholar] [CrossRef]
- Yang, Y.; Jin, Z.; Dong, R.; Zheng, C.; Huang, Y.; Zheng, Y.; Shen, Z.; Chen, G.; Luo, X.; Zheng, S. MicroRNA-29b/142-5p contribute to the pathogenesis of biliary atresia by regulating the IFN-γ gene. Cell. Death Dis. 2018, 9, 545. [Google Scholar] [CrossRef]
- Hsu, Y.A.; Lin, C.H.; Lin, H.J.; Huang, C.C.; Lin, H.C.; Chen, Y.C.; Chang, C.Y.; Huang, S.H.; Lin, J.M.; Lee, K.R.; et al. Effect of microRNA-155 on the interferon-γ signaling pathway in biliary atresia. Chin. J. Physiol. 2016, 59, 315–322. [Google Scholar] [CrossRef]
- Shimadera, S.; Iwai, N.; Deguchi, E.; Kimura, O.; Fumino, S.; Yokoyama, T. The inv mouse as an experimental model of biliary atresia. J. Pediatr. Surg. 2007, 42, 1555–1560. [Google Scholar] [CrossRef]
- Sadek, K.H.; Ezzat, S.; Abdel-Aziz, S.A.; Alaraby, H.; Mosbeh, A.; Abdel-Rahman, M.H. Macrophage Migration Inhibitory Factor (MIF) Gene Promotor Polymorphism Is Associated with Increased Fibrosis in Biliary Atresia Patients, but Not with Disease Susceptibility. Ann. Hum. Genet. 2017, 81, 177–183. [Google Scholar] [CrossRef]
- Arikan, C.; Berdeli, A.; Ozgenc, F.; Tumgor, G.; Yagci, R.V.; Aydogdu, S. Positive association of macrophage migration inhibitory factor gene-173G/C polymorphism with biliary atresia. J. Pediatr. Gastroenterol. Nutr. 2006, 42, 77–82. [Google Scholar] [CrossRef]
- Liu, F.; Zeng, J.; Zhu, D.; Zhang, R.; Xu, X.; Wang, M.; Zhang, Y.; Xia, H.; Feng, Z. Association of polymorphism in the VEGFA gene 3′-UTR + 936T/C with susceptibility to biliary atresia in a Southern Chinese Han population. J. Clin. Lab. Anal. 2018, 32, e22342. [Google Scholar] [CrossRef]
- Liu, B.; Wei, J.; Li, M.; Jiang, J.; Zhang, H.; Yang, L.; Wu, H.; Zhou, Q. Association of common genetic variants in VEGFA with biliary atresia susceptibility in Northwestern Han Chinese. Gene 2017, 628, 87–92. [Google Scholar] [CrossRef]
- Edom, P.T.; Meurer, L.; da Silveira, T.R.; Matte, U.; dos Santos, J.L. Immunolocalization of VEGF A and its receptors, VEGFR1 and VEGFR2, in the liver from patients with biliary atresia. Appl. Immunohistochem. Mol. Morphol. 2011, 19, 360–368. [Google Scholar] [CrossRef]
- Higashiyama, H.; Ozawa, A.; Sumitomo, H.; Uemura, M.; Fujino, K.; Igarashi, H.; Imaimatsu, K.; Tsunekawa, N.; Hirate, Y.; Kurohmaru, M.; et al. Embryonic cholecystitis and defective gallbladder contraction in the Sox17-haploinsufficient mouse model of biliary atresia. Development 2017, 144, 1906–1917. [Google Scholar] [CrossRef]
- Waisbourd-Zinman, O.; Koh, H.; Tsai, S.; Lavrut, P.M.; Dang, C.; Zhao, X.; Pack, M.; Cave, J.; Hawes, M.; Koo, K.A.; et al. The toxin biliatresone causes mouse extrahepatic cholangiocyte damage and fibrosis through decreased glutathione and SOX17. Hepatology 2016, 64, 880–893. [Google Scholar] [CrossRef]
- Uemura, M.; Ozawa, A.; Nagata, T.; Kurasawa, K.; Tsunekawa, N.; Nobuhisa, I.; Taga, T.; Hara, K.; Kudo, A.; Kawakami, H.; et al. Sox17 haploinsufficiency results in perinatal biliary atresia and hepatitis in C57BL/6 background mice. Development 2013, 140, 639–648. [Google Scholar] [CrossRef]
- Leung, A.K.C.; Leung, A.A.M.; Wong, A.H.C.; Sergi, C.M.; Kamsites, J.K.M. Giardiasis: An overview. Recent Pat. Inflamm. Allergy Drug Discov. 2019, 13, 134–143. [Google Scholar] [CrossRef]
- Kong, T.; Feulefack, J.; Ruether, K.; Shen, F.; Zheng, W.; Chen, X.Z.; Sergi, C. Ethnic Differences in Genetic Ion Channelopathies Associated with Sudden Cardiac Death: A Systematic Review and Meta-Analysis. Ann. Clin. Lab. Sci. 2017, 47, 481–490. [Google Scholar]
- Kawazoe, A.; Shitara, K. Next-generation sequencing and biomarkers for gastric cancer: What is the future? Ther. Adv. Med. Oncol. 2019, 11, 1758835919848189. [Google Scholar] [CrossRef]
- You, Z.; Wen, J.; Cheng, L.; Ye, H.; Li, B. Screening of targeted genes in extrahepatic bile ducts of mice with experimental biliary atresia. Mol. Med. Rep. 2015, 12, 4326–4331. [Google Scholar] [CrossRef]
- Zerfaoui, M.; Naura, A.S.; Errami, Y.; Hans, C.P.; Rezk, B.M.; Park, J.; Elsegeiny, W.; Kim, H.; Lord, K.; Kim, J.G.; et al. Effects of PARP-1 deficiency on airway inflammatory cell recruitment in response to LPS or TNF: Differential effects on CXCR2 ligands and Duffy Antigen Receptor for Chemokines. J. Leukoc. Biol. 2009, 86, 1385–1392. [Google Scholar] [CrossRef]
- Hirano, Y.; Hirano, F.; Fujii, H.; Makino, I. Fibrates suppress chenodeoxycholic acid-induced RANTES expression through inhibition of NF-kappaB activation. Eur. J. Pharmacol. 2002, 448, 19–26. [Google Scholar] [CrossRef]
- Schukfeh, N.; Al-Gamrah, A.; Petersen, C.; Kuebler, J.F. Detection of hepatotropic viruses has no impact on the prognosis after Kasai procedure. J. Pediatr. Surg. 2012, 47, 1828–1832. [Google Scholar] [CrossRef]
- Rauschenfels, S.; Krassmann, M.; Al-Masri, A.N.; Verhagen, W.; Leonhardt, J.; Kuebler, J.F.; Petersen, C. Incidence of hepatotropic viruses in biliary atresia. Eur. J. Pediatr. 2009, 168, 469–476. [Google Scholar] [CrossRef]
- Landing, B.H. Considerations of the pathogenesis of neonatal hepatitis, biliary atresia and choledochal cyst—The concept of infantile obstructive cholangiopathy. Prog. Pediatr. Surg. 1974, 6, 113–139. [Google Scholar]
- Zhang, S.; Goswami, S.; Ma, J.; Meng, L.; Wang, Y.; Zhu, F.; Zhang, D.; Zheng, S.; Dong, R.; Xiao, X.; et al. CD4+T Cell Subset Profiling in Biliary Atresia Reveals ICOS− Regulatory T Cells as a Favorable Prognostic Factor. Front. Pediatr. 2019, 7, 279. [Google Scholar] [CrossRef]
- Ye, J.; Lai, D.; Cao, D.; Tan, L.; Hu, L.; Zha, H.; Yang, J.; Shu, Q. Altered T-Cell Receptor β-Chain and Lactate Dehydrogenase Are Associated With the Immune Pathogenesis of Biliary Atresia. Front Med. 2021, 8, 778500. [Google Scholar] [CrossRef]
- Zhao, X.; Lorent, K.; Escobar-Zarate, D.; Rajagopalan, R.; Loomes, K.M.; Gillespie, K.; Mesaros, C.; Estrada, M.A.; Blair, I.A.; Winkler, J.D.; et al. Impaired Redox and Protein Homeostasis as Risk Factors and Therapeutic Targets in Toxin-Induced Biliary Atresia. Gastroenterology 2020, 159, 1068–1084.e2. [Google Scholar] [CrossRef]
- Yang, Y.; Wang, J.; Zhan, Y.; Chen, G.; Shen, Z.; Zheng, S.; Dong, R. The synthetic toxin biliatresone causes biliary atresia in mice. Lab. Investig. 2020, 100, 1425–1435. [Google Scholar] [CrossRef]
- Bezerra, J.A.; Wells, R.G.; Mack, C.L.; Karpen, S.J.; Hoofnagle, J.H.; Doo, E.; Sokol, R.J. Biliary Atresia: Clinical and Research Challenges for the 21(st) Century. Hepatology 2018, 68, 1163–1173. [Google Scholar] [CrossRef]
- Davenport, M. Biliary atresia: From Australia to the zebrafish. J. Pediatr. Surg. 2016, 51, 200–205. [Google Scholar] [CrossRef]
- Ningappa, M.; So, J.; Glessner, J.; Ashokkumar, C.; Ranganathan, S.; Min, J.; Higgs, B.W.; Sun, Q.; Haberman, K.; Schmitt, L.; et al. The Role of ARF6 in Biliary Atresia. PLoS ONE 2015, 10, e0138381. [Google Scholar] [CrossRef]
- Huang, Y.H.; Shih, H.H.; Tiao, M.M.; Huang, C.C.; Kuo, K.C.; Huang, F.C.; Yang, Y.L.; Chuang, J.H. Toll-like receptor 7 agonist induces hypoplasia of the biliary system in a neonatal mouse model. J. Microbiol. Immunol. Infect. 2018, 51, 166–173. [Google Scholar] [CrossRef]
- Fu, M.; Lin, Z.; Lin, H.; Tong, Y.; Wang, H.; Chen, H.; Chen, Y.; Zhang, R. A Silver Nanoparticle Method for Ameliorating Biliary Atresia Syndrome in Mice. J. Vis. Exp. 2018, 140, e58158. [Google Scholar] [CrossRef]
- Lakshminarayanan, B.; Davenport, M. Biliary atresia: A comprehensive review. J. Autoimmun. 2016, 73, 1–9. [Google Scholar] [CrossRef]
- Ihn, K.; Na, Y.; Ho, I.G.; Lee, D.; Koh, H.; Han, S.J. A periodic comparison of the survival and prognostic factors of biliary atresia after Kasai portoenterostomy: A single-center study in Korea. Pediatr. Surg. Int. 2019, 35, 285–292. [Google Scholar] [CrossRef]
- Parolini, F.; Boroni, G.; Milianti, S.; Tonegatti, L.; Armellini, A.; Garcia Magne, M.; Pedersini, P.; Torri, F.; Orizio, P.; Benvenuti, S.; et al. Biliary atresia: 20–40-year follow-up with native liver in an Italian centre. J. Pediatr. Surg. 2018, 54, 1440–1444. [Google Scholar] [CrossRef]
- Hasan, M.S.; Karim, A.B.; Rukunuzzaman, M.; Haque, A.; Akhter, M.A.; Shoma, U.K.; Yasmin, F.; Rahman, M.A. Role of Liver Biopsy in the Diagnosis of Neonatal Cholestasis due to Biliary Atresia. Mymensingh Med. J. 2018, 27, 826–833. [Google Scholar]
- Madadi-Sanjani, O.; Kuebler, J.F.; Dippel, S.; Gigina, A.; Falk, C.S.; Vieten, G.; Petersen, C.; Klemann, C. Long-term outcome and necessity of liver transplantation in infants with biliary atresia are independent of cytokine milieu in native liver and serum. Cytokine 2018, 111, 382–388. [Google Scholar] [CrossRef]
- Li, S.; Ma, N.; Meng, X.; Zhang, W.; Sun, C.; Dong, C.; Wang, K.; Wu, B.; Gao, W. The effects of Kasai procedure on living donor liver transplantation for children with biliary atresia. J. Pediatr. Surg. 2018, 54, 1436–1439. [Google Scholar] [CrossRef]
- Witt, M.; van Wessel, D.B.E.; de Kleine, R.H.J.; Bruggink, J.L.M.; Hulscher, J.B.F.; Verkade, H.J.; Netherlands Study Group on Biliary Atresia Registry. Prognosis of Biliary Atresia After 2-year Survival With Native Liver: A Nationwide Cohort Analysis. J. Pediatr. Gastroenterol. Nutr. 2018, 67, 689–694. [Google Scholar] [CrossRef]
- Arafa, R.S.; Abdel Haie, O.M.; El-Azab, D.S.; Abdel-Rahman, A.M.; Sira, M.M. Significant hepatic expression of IL-2 and IL-8 in biliary atresia compared with other neonatal cholestatic disorders. Cytokine 2016, 79, 59–65. [Google Scholar] [CrossRef]
- Santos, J.L.; Kieling, C.O.; Meurer, L.; Vieira, S.; Ferreira, C.T.; Lorentz, A.; Silveira, T.R. The extent of biliary proliferation in liver biopsies from patients with biliary atresia at portoenterostomy is associated with the postoperative prognosis. J. Pediatr. Surg. 2009, 44, 695–701. [Google Scholar] [CrossRef]
- Obayashi, J.; Kawaguchi, K.; Manabe, S.; Nagae, H.; Wakisaka, M.; Koike, J.; Takagi, M.; Kitagawa, H. Prognostic factors indicating survival with native liver after Kasai procedure for biliary atresia. Pediatr. Surg. Int. 2017, 33, 1047–1052. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sergi, C.M.; Gilmour, S. Biliary Atresia: A Complex Hepatobiliary Disease with Variable Gene Involvement, Diagnostic Procedures, and Prognosis. Diagnostics 2022, 12, 330. https://doi.org/10.3390/diagnostics12020330
Sergi CM, Gilmour S. Biliary Atresia: A Complex Hepatobiliary Disease with Variable Gene Involvement, Diagnostic Procedures, and Prognosis. Diagnostics. 2022; 12(2):330. https://doi.org/10.3390/diagnostics12020330
Chicago/Turabian StyleSergi, Consolato M., and Susan Gilmour. 2022. "Biliary Atresia: A Complex Hepatobiliary Disease with Variable Gene Involvement, Diagnostic Procedures, and Prognosis" Diagnostics 12, no. 2: 330. https://doi.org/10.3390/diagnostics12020330
APA StyleSergi, C. M., & Gilmour, S. (2022). Biliary Atresia: A Complex Hepatobiliary Disease with Variable Gene Involvement, Diagnostic Procedures, and Prognosis. Diagnostics, 12(2), 330. https://doi.org/10.3390/diagnostics12020330






