Evaluation of MMR Status and PD-L1 Expression Using Specimens Obtained by EUS-FNB in Patients with Pancreatic Ductal Adenocarcinoma (PDAC)
Abstract
:1. Introduction
2. Patients and Methods
2.1. Patients
2.2. EUS–FNB Technique for the Pathological Diagnosis
2.3. IHC
3. Results
3.1. Patient Characteristics
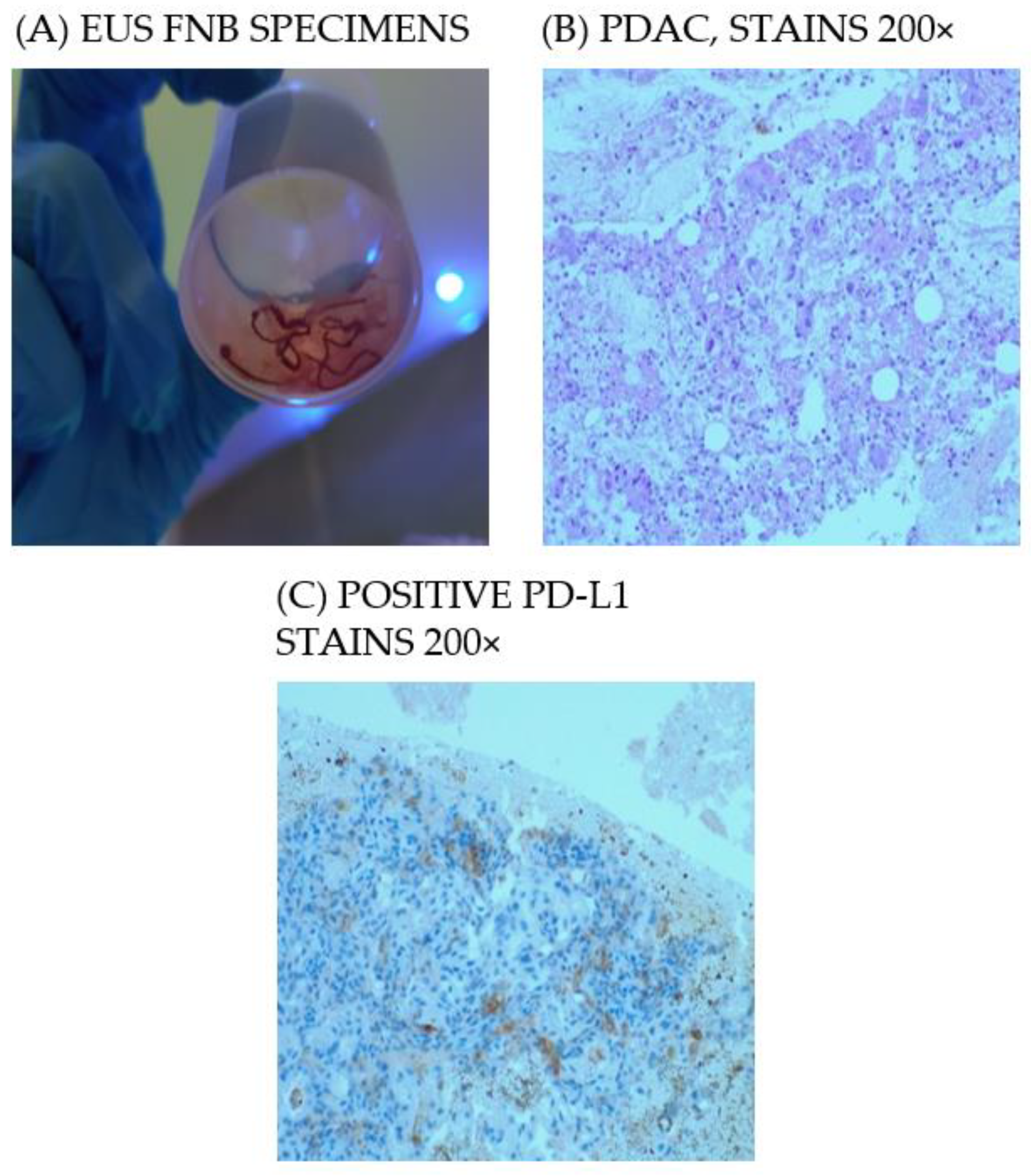
3.2. EUS FNB for the Pathological Diagnosis
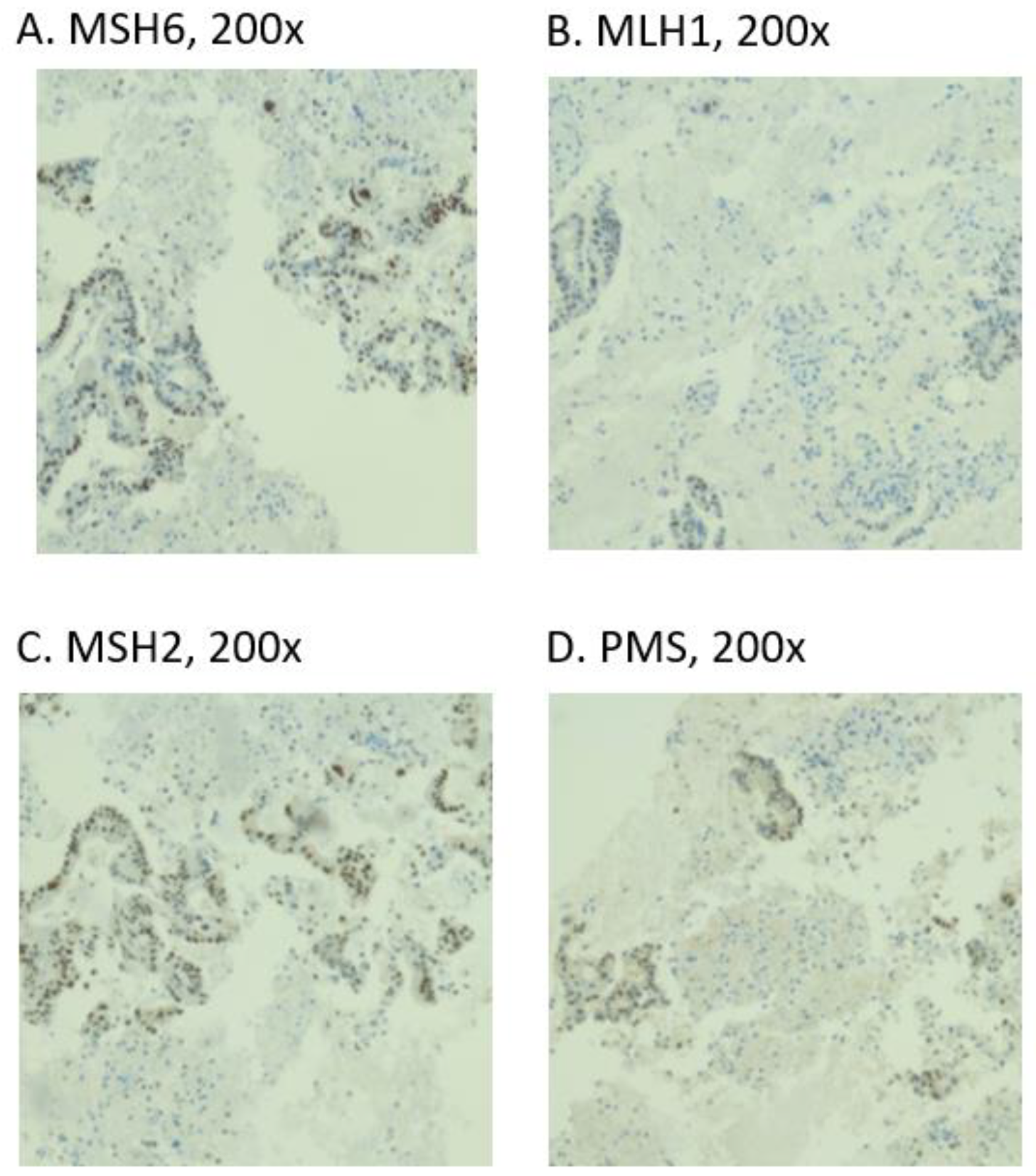
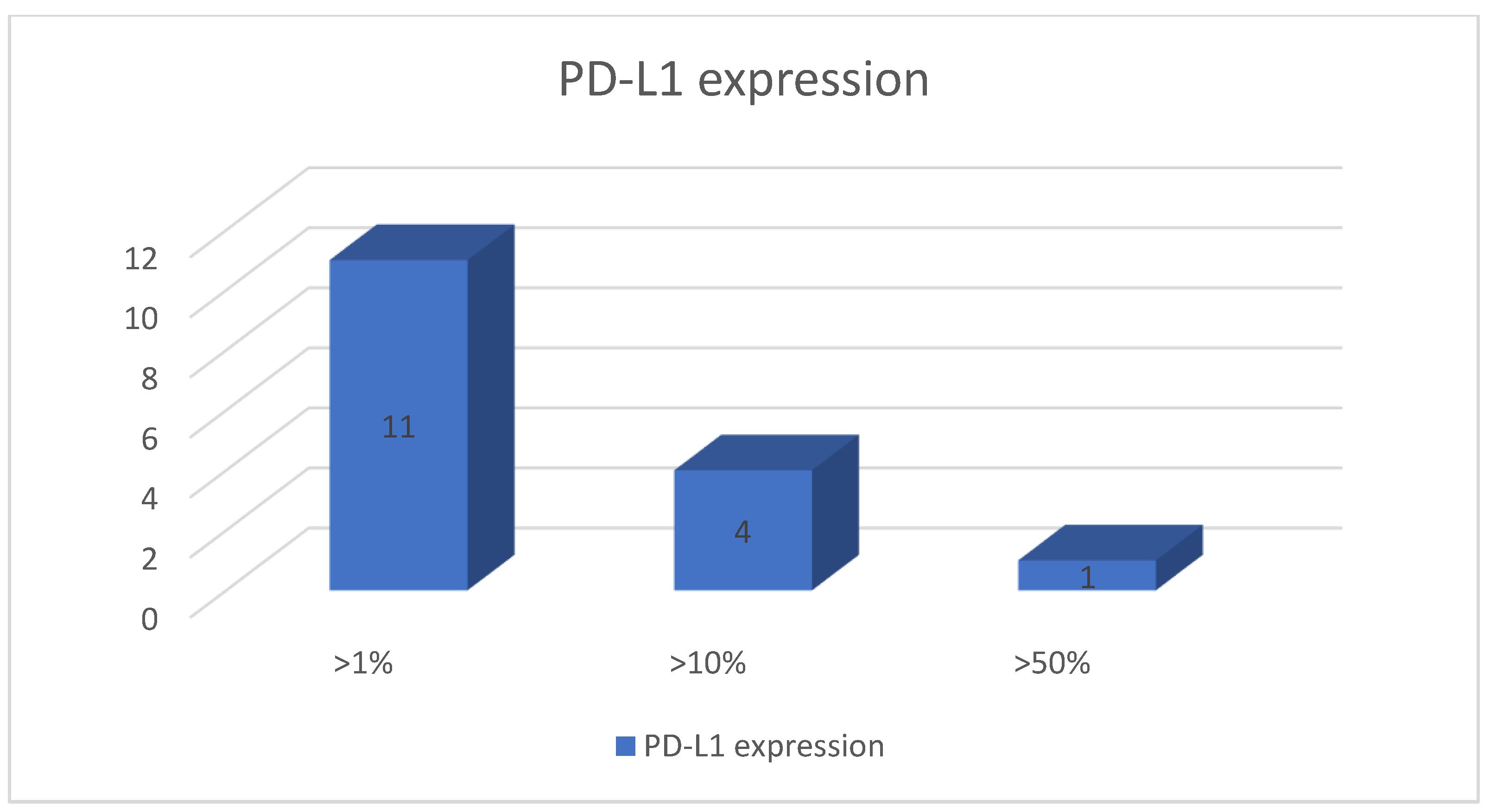
3.3. MMR Status and PD-L1 Expression
3.4. Immunotherapy Eligibility According to Biomarkers
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Siegel, R.L.; Mph, K.D.M.; Jemal, A. Cancer statistics. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Sanmamed, M.; Chen, L. Inducible expression of B7-H1 (PD-L1) and its selective role in tumor site immune modulation. Cancer J. 2014, 20, 256–261. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brahmer, J.R.; Tykodi, S.S.; Chow, L.Q.; Hwu, W.J.; Topalian, S.L.; Hwu, P.; Drake, C.G.; Camacho, L.H.; Kauh, J.; Odunsi, K.; et al. Safety and activity of anti–PD-L1 antibody in patients with advanced cancer. N. Engl. S. Afr. 2012, 366, 2455–2465. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, X.; Bao, Z.; Zhang, X.; Li, F.; Lai, T.; Cao, C.; Chen, Z.; Li, W.; Shen, H.; Ying, S. Effectiveness and safety of PD-1/PD-L1 inhibitors in the treatment of solid tumors: A systematic review and meta-analysis. Oncotarget 2017, 8, 59901–59914. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, Z.; Shia, J.; Stadler, Z.; Varghese, A.; Capanu, M.; Salo-Mullen, E.; Lowery, M.A.; Diaz, L.A.; Mandelker, D.; Yu, K.H.; et al. Evaluating mismatch repair deficiency in Pancreatic Adenocarcinoma: Challenges and recommendations. Clin. Cancer Res. 2018, 24, 1326–1336. [Google Scholar] [CrossRef] [Green Version]
- US Food and Drug Administration approves first cancer treatment for any solid tumors with specific biomarker. Cancer 2017, 123, 3652–3653. [CrossRef] [Green Version]
- Amin, M.B.; Edge, S.; Greene, F.; Byrd, D.R.; Brookland, R.K.; Washington, M.K.; Gershenwald, J.E.; Compton, C.C.; Hess, K.R. (Eds.) AJCC Cancer Staging Manual, 8th ed.; American Joint Commission on Cancer; Springer International Publishing: New York, NY, USA, 2017. [Google Scholar]
- Mace, T.A.; Shakya, R.; Pitarresi, J.R.; Swanson, B.; McQuinn, C.W.; Loftus, S.; Nordquist, E.; Cruz-Monserrate, Z.; Yu, L.; Young, G.; et al. IL-6 and PD-L1 antibody blockade combination therapy reduces tumour progression in murine models of pancreatic cancer. Gut 2018, 67, 320–332. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Li, X.; Wei, X.; Jiang, H.; Lan, C.; Yang, S.; Wang, H.; Yang, Y.; Tian, C.; Xu, Z. PD-L1 is a direct target of cancer-FOXP3 in pancreatic ductal adenocarcinoma (PDAC), and combined immunotherapy with antibodies against PD-L1 and CCL5 is effective in the treatment of PDAC. Signal Transduct. Target. Ther. 2020, 5, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Bournet, B. Role of endoscopic ultrasound in the molecular diagnosis of pancreatic cancer. World J. Gastroenterol. 2014, 20, 10758–10768. [Google Scholar] [CrossRef]
- Khan, M.; Grimm, I.; Ali, B.; Nollan, R.; Tombazzi, C.; Ismail, M.; Baron, T. A meta-analysis of endoscopic ultrasound–fine-needle aspiration compared to endoscopic ultrasound–fine-needle biopsy: Diagnostic yield and the value of onsite cytopathological assessment. Endosc. Int. Open 2017, 5, E363–E375. [Google Scholar] [CrossRef] [Green Version]
- Lee, B.; Cho, C.; Jung, M.; Jang, J.; Bae, H. Comparison of histologic core portions acquired from a core biopsy needle and a conventional needle in solid mass lesions: A prospective randomized trial. Gut Live 2017, 11, 559–566. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iacobuzio-Donahue, C.; Ryu, B.; Hruban, R.; Kern, S. Exploring the host desmoplastic response to pancreatic carcinoma. Am. J. Pathol. 2002, 160, 91–99. [Google Scholar] [CrossRef]
- Yoshizawa, N.; Yamada, R.; Sakuno, T.; Inoue, H.; Miura, H.; Takeuchi, T.; Nakamura, M.; Hamada, Y.; Katsurahara, M.; Tanaka, K.; et al. Comparison of endoscopic ultrasound-guided fine-needle aspiration and biopsy with 22-gauge and 25-gauge needles for the “precision medicine” of pancreatic cancer. Medicine 2018, 97, e11096. [Google Scholar] [CrossRef] [PubMed]
- Matsumoto, K.; Ohara, T.; Fujisawa, M.; Takaki, A.; Takahara, M.; Tanaka, N.; Kato, H.; Horiguchi, S.; Yoshida, R.; Umeda, Y.; et al. The relationship between the PD-L1 expression of surgically resected and fine-needle aspiration specimens for patients with pancreatic cancer. J. Gastroenterol. 2019, 54, 1019–1028. [Google Scholar] [CrossRef]
- VanderWalde, A.; Spetzler, D.; Xiao, N.; Gatalica, Z.; Marshall, J. Microsatellite instability status determined by next-generation sequencing and compared with PD-L1 and tumor mutational burden in 11,348 patients. Cancer Med. 2018, 7, 746–756. [Google Scholar] [CrossRef] [Green Version]
- Nomi, T.; Sho, M.; Akahori, T.; Hamada, K.; Kubo, A.; Kanehiro, H.; Nakamura, S.; Enomoto, K.; Yagita, H.; Azuma, M.; et al. Clinical Significance and Therapeutic Potential of the Programmed Death-1 Ligand/Programmed Death-1 Pathway in Human Pancreatic Cancer. Clin. Cancer Res. 2007, 13, 2151–2157. [Google Scholar] [CrossRef] [Green Version]
- Lie, J.W.; Lu, Y.; Shen, G.J. The relationship of B7-H1 with clinicopathological characteristics and prognosis of pancreatic carcinoma. Chin. J. Gen. Pract. 2016, 14, 571–574. [Google Scholar]
- Tessier-Cloutier, B.; Kalloger, S.E.; Al-Kandari, M.; Milne, K.; Gao, D.; Nelson, B.H.; Renouf, D.J.; Sheffield, B.S.; Schaeffer, D.F. Programmed cell death ligand 1 cut-point is associated with reduced disease specific survival in resected pancreatic ductal adenocarcinoma. BMC Cancer 2017, 17, 1–10. [Google Scholar] [CrossRef]
- Gao, H.-L.; Liu, L.; Qi, Z.-H.; Xu, H.-X.; Wang, W.-Q.; Wu, C.-T.; Zhang, S.-R.; Xu, J.-Z.; Ni, Q.-X.; Yu, X.-J. The clinicopathological and prognostic significance of PD-L1 expression in pancreatic cancer: A meta-analysis. Hepatobiliary Pancreat. Dis. Int. 2018, 17, 95–100. [Google Scholar] [CrossRef] [PubMed]
- Geboers, B.; Timmer, F.; Ruarus, A.; Pouw, J.; Schouten, E.; Bakker, J.; Puijk, R.; Nieuwenhuizen, S.; Dijkstra, M.; Tol, M.V.D.; et al. Irreversible electroporation and nivolumab combined with intratumoral administration of a toll-like receptor ligand, as a means of in vivo vaccination for metastatic pancreatic ductal adenocarcinoma (PANFIRE-III). A phase-I study protocol. Cancers 2021, 13, 3902. [Google Scholar] [CrossRef]
- Azad, A.; Lim, S.Y.; D’Costa, Z.; Jones, K.; Diana, A.; Sansom, O.J.; Kruger, P.; Liu, S.; McKenna, W.G.; Dushek, O.; et al. PD -L1 blockade enhances response of pancreatic ductal adenocarcinoma to radiotherapy. EMBO Mol. Med. 2017, 9, 167–180. [Google Scholar] [CrossRef] [PubMed]
- Gleeson, F.C.; Levy, M.J.; Roden, A.C.; Boardman, L.A.; Sinicrope, F.A.; McWilliams, R.R.; Zhang, L. EUS fine-needle pancreatic core biopsy can determine eligibility for tumor-agnostic immunotherapy. Endosc. Int. Open 2018, 6, E1278–E1282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
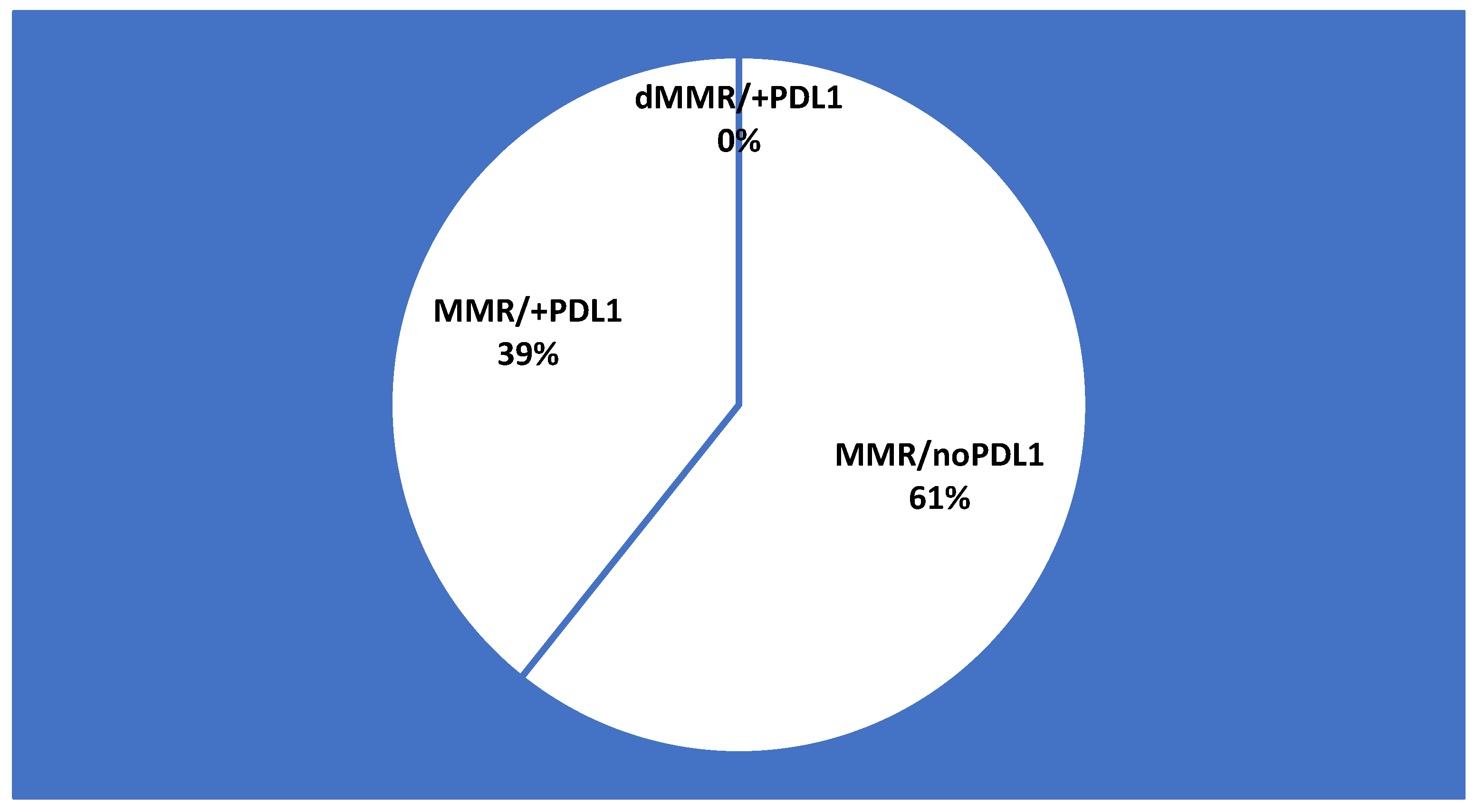
| Parameter | Number |
|---|---|
| Age, median (range) (years) | 63.9 (43–83) |
| Sex, male n% | 13 (52) |
| Tumor size, median (IQR) (mm) | 35.8 (16–60 mm) |
| Stage (AJCC classification) I/II/III/IV | 4/16/20/60 |
| 1 year survival rate PD-L1 positive/PD-L1 negative | 21.4 (6) 33.3/66.6 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Constantin, A.; Iovănescu, V.; Cazacu, I.M.; Ungureanu, B.S.; Copăescu, C.; Stroescu, C.; Bejinariu, N.; Săftoiu, A. Evaluation of MMR Status and PD-L1 Expression Using Specimens Obtained by EUS-FNB in Patients with Pancreatic Ductal Adenocarcinoma (PDAC). Diagnostics 2022, 12, 294. https://doi.org/10.3390/diagnostics12020294
Constantin A, Iovănescu V, Cazacu IM, Ungureanu BS, Copăescu C, Stroescu C, Bejinariu N, Săftoiu A. Evaluation of MMR Status and PD-L1 Expression Using Specimens Obtained by EUS-FNB in Patients with Pancreatic Ductal Adenocarcinoma (PDAC). Diagnostics. 2022; 12(2):294. https://doi.org/10.3390/diagnostics12020294
Chicago/Turabian StyleConstantin, Alina, Vlad Iovănescu, Irina Mihaela Cazacu, Bogdan Silviu Ungureanu, Cătălin Copăescu, Cezar Stroescu, Nona Bejinariu, and Adrian Săftoiu. 2022. "Evaluation of MMR Status and PD-L1 Expression Using Specimens Obtained by EUS-FNB in Patients with Pancreatic Ductal Adenocarcinoma (PDAC)" Diagnostics 12, no. 2: 294. https://doi.org/10.3390/diagnostics12020294
APA StyleConstantin, A., Iovănescu, V., Cazacu, I. M., Ungureanu, B. S., Copăescu, C., Stroescu, C., Bejinariu, N., & Săftoiu, A. (2022). Evaluation of MMR Status and PD-L1 Expression Using Specimens Obtained by EUS-FNB in Patients with Pancreatic Ductal Adenocarcinoma (PDAC). Diagnostics, 12(2), 294. https://doi.org/10.3390/diagnostics12020294








