Abstract
A post-operative manifest refractive error as close as possible to target is key when performing cataract surgery with intraocular lens (IOL) implantation, given that residual astigmatism and refractive errors negatively impact patients’ vision and satisfaction. This review explores refractive outcomes prior to modern biometry; advances in biometry and its impact on patients’ vision and refractive outcomes after cataract surgery; key factors that affect prediction accuracy; and residual refractive errors and the impact on visual outcomes. There are numerous pre-, intra-, and post-operative factors that can influence refractive outcomes after cataract surgery, leaving surgeons with a small “error budget” (i.e., the source and sum of all influencing factors). To mitigate these factors, precise measurement and correct application of ocular biometric data are required. With advances in optical biometry, prediction of patient post-operative refractory status has become more accurate, leading to an increased proportion of patients achieving their target refraction. Alongside improvements in biometry, advancements in microsurgical techniques, new IOL technologies, and enhancements to IOL power calculations have also positively impacted patients’ refractory status after cataract surgery.
1. Introduction
Cataract surgery with intraocular lens (IOL) implantation is one of the most common ophthalmic procedures in clinical practice; however, post-operative refractive outcomes remain a key area of concern for surgeons [1]. Improvements in surgical technique, new IOL technologies, enhanced biometric methods, and advanced methods of IOL power calculation have led to modern cataract surgery being a refined procedure that enables most patients to achieve high-quality post-operative vision [1]. In line with advances in cataract surgery, there is an increasing patient expectation of excellent post-operative outcomes and high demand for spectacle independence, particularly in developed countries [1,2,3]. However, unsatisfactory visual outcomes due to residual refractive errors may occur and remain an important cause of visual disability and poor quality of life [1,2,3]. For example, refractive surprise (i.e., any deviation from intended target) after cataract surgery, is unsatisfactory for both the patient and the surgeon, and correction of large refractive errors requires additional procedures [1]. There are a plethora of pre-, intra-, and post-operative factors that can influence refractive outcomes, leaving surgeons with little room for error (i.e., a small “error budget”): for this reason, a high level of diagnostic precision, including meticulous and accurate collection of measurements and appropriate application of ocular biometric data, is required.
In this review, we will examine key factors that may affect error prediction accuracy, and how residual refractive error impacts visual outcomes. We will also discuss how refractive outcomes have improved with the evolution of ocular biometry, and how modern technologies have positively impacted patients’ refractory status following cataract surgery.
2. Refractive Outcomes Prior to the Advent of Modern Optical Biometry
The first great advancement in ocular biometry was the development of ultrasound A-scan biometry, first described in the 1960s as a method that allowed visualization along an ultrasonic path to ensure alignment with a patient’s visual axis [4]. Ultrasound A-scan biometry values are obtained either by immersion or applanation methods: the immersion technique places a saline-filled scleral shell between the patient’s eye and an ultrasound probe, whereas the applanation method requires placing the ultrasound probe directly on the central cornea [5]. Prospective studies have demonstrated that the immersion technique has better reliability and fewer post-operative refractive errors compared with the applanation method [5,6]. Mean ± standard deviation axial lengths (AL) measured using applanation A-scan biometry can vary by 0.14 ± 0.12 mm compared with 0.03 ± 0.04 mm for immersion [5]. In addition, AL acquired by applanation can be 0.1–0.3 mm shorter than AL acquired by immersion [6,7]. The greater variability of AL measurements with the applanation technique could translate into larger post-operative refractive errors, as was demonstrated in a prospective, randomized trial of 288 patients in which the mean absolute prediction error (MAE) was 0.53 ± 0.48 diopters (D) for the applanation group and 0.43 ± 0.38 D for the immersion group [6]. From the safety outcomes perspective, direct contact of an ultrasound probe with the patient’s eye not only requires topical anesthesia [8], but also risks corneal epithelial injury, infection, and causes patient discomfort [9]. In terms of refractive outcomes, direct contact with the central cornea risks creating corneal compressions that may introduce errors in the biometric readings acquired during applanation A-scan biometry [5,6], in turn leading to errors in the predicted refraction [6]. Despite these drawbacks, applanation A-scan biometry was widely used and, together with immersion A-scan biometry, the introduction of biometric techniques greatly improved refractive outcomes [10,11].
In the late 1990s, technologic advances in biometry continued with the introduction of optical biometry—a technique based on partial coherence interferometry (PCI) [12]. Compared with ultrasound biometry, which uses a 10-MHz sound wave [5], optical biometry uses infrared light technology [13], offering enhanced resolution (~12 μm) and more than 10-times greater precision (<10 μm) [12,14]. As a non-contact technique, optical biometry is less time-consuming than ultrasound biometry because it does not require local anesthesia or pupil dilation, in turn reducing patient discomfort (Figure 1) [8,12]. In addition, the non-contact approach eliminates variations due to corneal compressions, as is commonly seen with applanation A-scan biometry [9]. These advantages translate into better post-operative refractive outcomes when compared with applanation [8,12,15] and immersion biometry [16]. Prospective, randomized studies have shown that patients who underwent optical biometry using PCI achieved an MAE of 0.30–0.52 D compared with 0.62–0.94 D with applanation biometry [8,15]. Within these studies, 87–100% of patients in the PCI groups and 71–80% of patients in the applanation groups were within ±1.0 D of their target refraction [8,15]. In addition, 93% of patients in the PCI group and 85% of patients in the immersion group were within ±1.0 D of their target refraction (p = 0.04) [16].
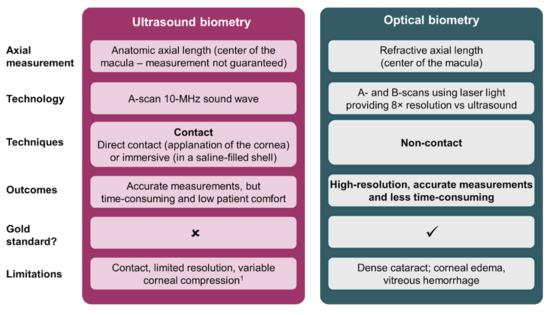
Figure 1.
Overview of ultrasound and optical biometry techniques [5,8,9,17,18,19]. 1 Only applicable to applanation biometry, corneal compression is not observed in immersion biometry.
The impact of optical biometry on refractive outcomes after cataract surgery can be appreciated by observing the steadily increasing percentage of patients achieving within ±1.0 D or ±0.5 D of their target refraction in studies between 1992 and 2017 [10,11,20,21,22,23,24]. Between 1992 and 2006, studies showed that 72.3–87.0% of patients achieved a deviation from the target refraction of ±1.0 D [10,11,20,21], which increased to 89.6–97.3% between 2007 and 2017 [22,23,24]. Between 1996 and 2005, studies showed that 44.6–58.4% of patients achieved a deviation from the target refraction of ±0.5 D [10,11], a proportion that increased to 61.2–88.0% between 2007 and 2017 [22,23,24,25,26]. It is therefore evident that, since its introduction in the 1980s [12], optical biometry using PCI has considerably improved refractive outcomes in clinical practice (Table 1) [11]. The magnitude of these improvements is, perhaps, best described by results from a prospective, multicenter, comparative, non-randomized study of 23,244 patients in the Swedish National Cataract Register that reported the outcomes of cataract extraction performed from 2000 through 2005 [11]. This study showed that there was a significant difference in MAE over 6 years (p < 0.0001), with the MAE consistently reducing between 2000 (~0.67 D) and 2004 (~0.51 D), likely driven by the gradual uptake of optical biometers in ophthalmology departments [11].

Table 1.
Target refraction prediction errors across various studies.
Innovations in optical biometry technology have continued to emerge with the introduction of biometers that use swept-source optical coherence tomography (SS-OCT), such as IOLMaster 700 (Carl Zeiss Meditec AG, Jena, Germany), ARGOS (Movu, Inc., San José, CA, USA), and OA-2000 (Tomey, Nagoya, Japan) [27]. SS-OCT is a non-invasive, high-speed method that collects thousands of scans from an extended imaging axial range within 1 second and generates 2- or 3-dimensional data with high lateral resolution and axial resolution [28]. This principle is at the core of the ARGOS, IOLMaster 700, and OA-2000 biometers, which use a ~1060-nm wavelength swept-source technology [28]. SS-OCT biometers provide OCT images of the entire eye by scanning up to 2000 times per second and providing measurements of AL, anterior chamber depth (ACD), central corneal thickness, lens thickness, aqueous depth, pupil size, corneal diameter, and keratometry [27,28]. SS-OCT biometers utilize either composite or segmented methods to obtain measurements of the eye, of which segmented analysis has been shown to be more accurate than composite analysis for eyes with long AL [29]. Furthermore, the longer wavelength utilized by SS-OCT biometers can penetrate dense cataracts, thereby providing accurate measurements for a broad range of patients [27,30].
3. Pre-, Intra-, and Post-Operative Factors That Affect Refractive Outcomes
In this section, we review the main factors that affect current refractive outcomes.
3.1. Pre-Operative Factors
Pre-operative biometric data, derived from measuring the physical dimensions of the eye, are necessary to determine the refractive power of the IOL to be implanted [2,31]. Key biometric parameters involved in IOL power calculations, including AL, corneal power, and ACD, are detailed in the following sections and outlined in Figure 2.
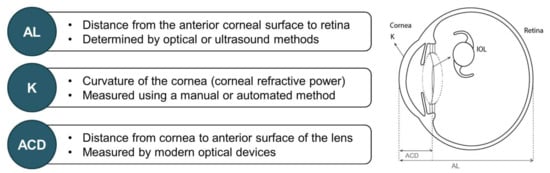
Figure 2.
Key biometric parameters involved in IOL power calculation [2,3,31,32,33,34,35]. ACD = anterior chamber depth; AL = axial length; IOL = intraocular lens; K = keratometry.
3.1.1. Axial Length
AL measurement is one of the most critical steps in IOL power calculation: this parameter should ideally be accurate within 0.1 mm because such a small error equates to a post-operative refraction error of ~0.27 D [33]. Measurements of AL using SS-OCT are more accurate than ultrasound biometry, with a median accuracy of 0.05 mm and 0.12 mm, respectively [36].
3.1.2. Corneal Power
Corneal power is another important measure to optimize refractive outcomes, as keratometric errors of 0.5 D in corneal power can lead to an error of 0.5 D in post-operative refraction [3]. Keratometry can be performed using manual or automated methods: the manual method takes four measurements 3.2 mm from the corneal center; whereas the automated method takes four to six radial measurements 2.6 mm from the center of the cornea [3]. Keratometry has two limitations: first, it measures only the radius of the anterior corneal surface’s curvature and considers a population average effect, when in actuality, the posterior corneal surface/aqueous interface contributes uniquely to the net corneal power. In addition, irregular corneas do not have a consistent anterior surface curvature; therefore, detailed topography (>5000 points of measurement) must be employed in such cases, to characterize overall refractive power [3,37].
3.1.3. Pre-Operative Corneal Astigmatism
Pre-operative corneal astigmatism is a very common condition and can lead to residual refractive errors after cataract surgery [1,3]. Corneal astigmatism of ≥0.5 D occurs in approximately 78% of eyes (based on real-world assessment of 110,468 eyes) and ≥1.0 D in approximately 42% of eyes [38]. Corneal astigmatism can be classified based on the location of the steepest meridian—in eyes that have with-the-rule (WTR) astigmatism the vertical meridian is steepest and conversely, the horizontal meridian is steepest in against-the-rule (ATR) astigmatism [39]. Corneal astigmatism can be corrected by the placement of incision on the steeper axis, peripheral corneal relaxing incisions, and use of a toric IOL [3]. A retrospective analysis of corneal topography data from 641 eyes concluded that the magnitude of corneal astigmatism decreases from the center to the mid-periphery, and the extent of the difference depends on the size and the type of corneal astigmatism [40]. As a change in corneal power from the center to the periphery of the cornea could potentially lead to suboptimal refractive correction in some cases, as indicated by ray-tracing simulations, more research is required to assess the impact of mid-peripheral astigmatism on patients’ visual function [40,41].
Mounting evidence suggests that posterior corneal astigmatism is clinically relevant when assessing the effect of astigmatism on refractive outcomes. In a prospective, observational study of 493 patients, posterior corneal astigmatism reduced total corneal astigmatism by approximately 13% and total corneal astigmatism differed from anterior corneal astigmatism by >0.5 D in around one-third of eyes [42]. In a case series that analyzed 715 eyes, the posterior cornea was steeper along the vertical meridian in >80% of eyes and, being a minus lens, caused a plus refractive power horizontally (ATR refractive astigmatism) [43]. Additionally, the level of posterior astigmatism varied according to the level of anterior corneal astigmatism—approximately 0.5 D in corneas that had WTR anterior astigmatism and 0.3 D for those with ATR anterior astigmatism [43,44]. Although complex, the relationship between anterior and posterior corneal astigmatism follows distinct trends: the posterior cornea tends to partly compensate for increasing amounts of WTR anterior astigmatism, and is relatively constant in eyes with increasing amounts of ATR astigmatism [43]. Overall, calculating posterior corneal power based on a fixed ratio between anterior and posterior curvatures may introduce errors of up to 0.5 D [39].
3.1.4. Anterior Chamber Depth
Even though ACD measurement is required to increase the accuracy of the IOL power prediction curve with modern formulae [3], incorrect assessment of this parameter is the largest source of refractive error [45]. An estimated 1-mm error in post-operative ACD equates to a refractive error of 1.44 D for regular eyes [45]. It is worth noting that different definitions of ACD exist within the literature depending on the biometer used: for example, some biometers measure ACD from the corneal endothelium to the anterior lens surface, others from the corneal epithelium to the anterior lens surface [3,46].
3.1.5. IOL Power Calculations
Calculation of the IOL power is fundamental to enable patients to achieve desired refractive outcomes [32]. Accurate IOL power calculations rely on numerous factors, such as accurate pre-operative biometric measurements, precise prediction of effective lens position (ELP), appropriate IOL formula selection, and optimization of the IOL constant [2,45]. No single formula is suitable for all eyes, and each formula requires a different selection of biometric data [3]. Even though IOL constants provided by manufacturers allow for an initial estimation of best-suited IOL power [47], optimization is required to minimize systematic errors, and may also improve the accuracy of IOLs manufacturing [48].
3.1.6. Other Pre-Operative Considerations
Previous corneal refractive surgery should be considered when obtaining biometric measurements and selecting the appropriate IOL formula because keratometric values change after surgery [3]. Inaccurate biometric measurements and inappropriate IOL formula selection may lead to refractive surprise: hyperopic surprise often occurs in patients with previous myopic correction, and myopic surprise in patients with previous hyperopic correction [49]. Conversely, good refractive outcomes can be achieved, even in complicated cases, if a customized approach to biometric assessment and IOL implantation is used [50].
3.2. Intra-Operative Factors
Surgical technique is the key intra-operative factor that may influence refractive outcomes after cataract surgery. Indeed, predictability of refractive outcomes has improved because of refinements in surgical procedures [25]. Optimal refractive outcomes can be achieved by employing good surgical technique, aiming for a low rate of posterior capsular rupture, having a capsulorhexis size smaller than the optical diameter, and having an in-the-bag IOL placement [2]. Continuous curvilinear capsulorhexis ensures positional stability and enhances refractive predictability [2,25], while small incisions without sutures and the use of foldable IOLs reduce the incidence of complications and surgically induced astigmatism (SIA) [51], and complete ophthalmic viscosurgical device removal reduces the likelihood of IOL misalignment [52]. Non-intentional SIA can be prevented by pre-operative assessment of corneal hysteresis and biomechanical properties of the cornea, followed by microincision surgery using corneal topography data and standard IOL power formulae [3]. The magnitude of SIA depends on the incision size and site during surgery [53,54]. Small incision sizes of 2.0–2.2 mm are recommended with increasing frequency as IOL delivery methods advance [55,56]. However, results from a prospective study of 58 eyes undergoing IOL implantation found that the final wound size was ~2.4 mm due to wound stretch during implantation, irrespective of the incision size prior to implantation [57]. This wound enlargement might, in some cases, be caused by injector nozzle tip damage during the implantation process [58]. Additionally, eyes with greater incision enlargement tended to have higher SIA, suggesting that a clean corneal incision prior to implantation may be preferential to stretching the corneal incision [57].
3.3. Post-Operative Factors
Post-operatively, the astigmatism-correction power of toric IOLs may be reduced as a consequence of off-axis rotation [59]: a 1° rotation results in 3.3% loss of astigmatism correction, and a ≥30° rotation leads to no reduction in astigmatism magnitude but a large change in axis [60]. ELP, that is, the position of the IOL in the eye (specifically, the distance between the principal object plane of the IOL and the principal image plane of the cornea) [61] differs for each IOL design and displacement can significantly affect refraction and IOL power predictions [62,63]. Forward deviation of an IOL leads to myopia, and conversely backward deviation leads to hyperopia [62]. Decentration or tilt may also affect post-operative refractive errors by inducing increased astigmatism and coma aberrations [64,65]. In an optical bench study, it was found that aspheric monofocal lenses were less negatively affected by decentration than aspheric diffractive bifocal or trifocal lenses, with mean optical quality reduction of <10% for 1-mm decentration at physiologic pupil sizes. The optical quality at all distances for diffractive bifocal and trifocal lenses was significantly reduced if decentration was more than 0.75 mm, with intermediate focus showing the least reduction [66]. In a prospective, non-comparative case series, monofocal anterior capsulotomy-fixated IOLs had low levels of decentration and high in-the-bag stability over a 1-year period [67].
By examining refractive accuracy in cataract surgery, the post-operative refraction measurement itself is also a source of error. Multiple studies indicate that the 95% test-retest spherical equivalent refraction measurement is approximately ±0.5 D [68,69,70,71,72]. This means that even if all other error sources are eliminated, it is highly unlikely that 100% of eyes would achieve outcomes within 0.5 D of the intended target.
Despite these limitations, retrospective analyses suggest that for patients with substantial interocular anatomic symmetry (such as patients without prior monocular refractive surgery or certain pathologic conditions) first-eye refractive results can be used to refine the treatment plan for the second eye and improve outcomes [73,74].
4. How Modern-Day Biometry Has Changed Refractive Outcomes
Modern-day optical biometry has improved refractive outcomes in several ways. A key example is represented by optical biometers, which allow a high success rate of AL measurements, ranging from 77.0–88.4% with PCI biometers, 79.0% with optical low-coherence reflectometry biometers, to 92.6–99.4% with SS-OCT biometers, with the best results to date reported using ARGOS (96.0–99.4%) [28,75,76,77,78]. Additionally, SS-OCT optical biometers allow for a high degree of repeatability and reproducibility for most biometric measurements and are compatible with a wide range of IOL power calculation formulae, resulting in good refractive outcomes [79,80,81]. SS-OCT biometers demonstrate excellent precision across a range of measurements, including AL, ACD, lens thickness, and anterior corneal radius of curvature [28,76] with low coefficient of variation and high intraclass correlation coefficient [76,77]. In instances where acquisition cannot be achieved with an optical biometer, such as dense cataracts, the enhanced retinal visualization mode of SS-OCT biometers can be utilized instead of ultrasound biometry: by using this mode, the sensitivity at the retina is enhanced by 10-times. In eyes with dense cataracts, ARGOS has proved successful at measuring AL [27,28] and AL acquisition rates are significantly higher with ARGOS (89.9%) and OA-2000 (80.8%) compared with IOLMaster 700 (63.6%) in eyes with grade IV cataract or higher [27]. With modern biometry, a prediction error of ±0.25 D can be achieved in 40–52% of patients, depending on the IOL formula used [80]. Measuring AL using multiple indices of refraction specific to each component of the eye increases the proportion of patients achieving ±0.5 D of the target refraction compared with a PCI optical biometer by up to 13.9% [82].
5. The Impact of Residual Refractive Error on Visual Outcomes
Results from patient-reported outcomes/satisfaction questionnaires have shown that a large proportion of patients (73–98%) were satisfied overall with their vision following cataract surgery and multifocal IOL implantation [83,84,85]. Nonetheless, a large proportion of patients reported glare, halos, and starburst symptoms (13–85%), and achievement of complete spectacle independence varied widely (31–95%) [83,84,85,86]. It is worth noting that the questionnaires used to evaluate patient satisfaction and reporting of the results vary within the literature and caution should be exercised when interpreting the outcomes and comparing such studies. Refractive errors are one of the main causes of poor vision, reported among 11–42% of patients assessed in population-based studies [87,88,89]. Residual refractive errors after cataract surgery can negatively impact patients’ uncorrected near, intermediate, and distance vision: in general, the larger the refractive error, the worse the patient’s vision [90,91,92,93]. Patients may experience blurred vision, with or without photic phenomena, as well as problems with reading in mesopic conditions following IOL implantation, ultimately impacting their quality of life, which can lead to their dissatisfaction [94].
Residual refractive errors can be adjusted with glasses or contact lenses [3]. Refractive surprise involving large errors in spherical or cylindrical power can be corrected with corneal-based laser refractive surgery [3] or lens-based procedures [1,3,95], each of which has advantages and limitations. Advantages of corneal-based laser refractive surgery are that additional intraocular surgery can be avoided, spherical and astigmatic refractive errors can be corrected [3]. However, drawbacks include the low availability of excimer lasers, high comparative cost, and the fact that correction of high residual refractive error depends on corneal thickness [3]. Lens-based procedures, such as in situ fine-tuning of light-adjustable IOLs, piggyback IOLs, supplementary IOLs, and IOL exchange can be used to correct large refractive errors where excimer lasers are not available [3]. In situ fine-tuning of light-adjustable IOLs is non-invasive, can be personalized to patient requirements and preferences, can adjust up to 2.0 D of sphere and cylinder in one procedure, and it is performed after complete healing has taken place and the IOL is locked in position (which increases refractive stability over time) [1,95,96]. This method has several disadvantages, some of which may result in additional visits to the ophthalmologist’s office (such as potential risk of macular burn due to ultraviolet light-based technology, requirement of pupil dilation of at least 6.5 mm, and repeat adjustments, which can also lead to dilation fatigue); in addition, it can only be performed on specific light-adjustable IOLs [95,97]. Piggyback and IOL exchange can correct large residual refractive spherical errors, do not change the corneal refractive power, and can be implanted via the original incision soon after initial surgery [3]. The development of an interface membrane between the IOLs is a disadvantage of piggyback IOL, and lens exchange can cause SIA due to wound enlargement while removing the original IOL [3]. Supplementary IOLs are designed for implantation into the ciliary sulcus and can be used to correct post-operative refractive errors without the requirement to exchange the IOL implanted into the capsular bag [98]. Ciliary sulcus-fixated IOLs can be prone to decentration or tilting, leading to decreased image quality and complications from ciliary-body contact. However, case reports suggest that supplementary IOLs have a high tolerance to misalignment and minimal light attenuation and can easily be removed or exchanged from the ciliary sulcus [98,99,100,101]. The impact of residual astigmatism could also be modestly influenced by the optics of the IOL. A recent study reported that diffractive extended depth of focus IOLs showed statistically significantly better uncorrected visual acuity (VA) compared with monofocal control; however, these differences were modest at best (1–2 letters for manifest refractive spherical equivalents within 1.0 D between groups and approaching borderline statistical significance) and hence, it is difficult to conclusively report if they are clinically meaningful [93]. Some studies that have conducted astigmatic defocus curve testing have shown that extended depth of focus IOLs may have a greater tolerance to residual refractive errors compared with trifocal and bifocal IOLs [102,103]. However, this type of testing is not straightforward and most studies in the literature do not maintain spherical equivalent when increasing astigmatic errors by more than 0.5 D and overall effect from different cylinder axis positions between manifest refraction and induced cylinder are also not addressed. Hence, a standardized way of conducting such tests is lacking.
The relationship between refractive error and uncorrected visual acuity (UCVA) is not straightforward, in the fact that different types of refractive error contribute differently to vision loss—for example, deterioration in distance VA is greater with myopic astigmatism (0.31 logarithm of the minimum angle of resolution [logMAR] per D of astigmatism) than with hyperopic astigmatism (0.23 logMAR per D of astigmatism) [104]. Changes in vision can be inferred from the changes in the magnitude of blur, which can be quantified using a blur strength metric that includes spherical equivalent, horizontal/vertical astigmatism, and oblique astigmatism [104].
In an interventional case series of 493 eyes undergoing unilateral cataract surgery and implantation of monofocal IOLs, it was found that uncorrected distance visual acuity (UDVA) worsened with increasing magnitude of myopic refractive error, was optimal at emmetropia, and worsened with increasing hyperopic refractive error. UDVA and uncorrected near visual acuity (UNVA) intersected in the refractive error range of −1.0 to −1.5 D. The fact that less than half of patients (37%) achieved a post-operative corrected distance visual acuity (CDVA) of 20/20 Snellen or better, a UDVA of 20/32 Snellen, and a UNVA of Jaeger 4 suggests that satisfactory UCVA cannot always be obtained for both distance and near vision with implantation of monofocal IOLs [92]. For patients requiring full spectacle independence, trifocal IOLs may therefore represent a more appropriate choice [105,106,107,108].
It is also important to accurately report the impact of residual refractive error on VA. Snellen and logMAR are two of the most commonly used charts for assessing VA [109,110]; however, as most logMAR charts have a “bottom line” of −0.3 logMAR and use a 0.1 logMAR progression of letter size, whereas Snellen charts are usually truncated at 6/5 or 20/15 and have irregular progression in letter size, transposing Snellen directly into logMAR is not considered accurate and caution should be exercised when converting data from Snellen charts into logMAR [110]. LogMAR charts, namely the Early Treatment Diabetic Retinopathy Study (ETDRS) chart, are widely used in clinical trials as they are generally accepted as the more accurate, sensitive, and reproducible test [109,110], and should therefore be favored over Snellen when reporting the impact of residual refractive errors on VA.
6. Conclusions
The main goals of modern cataract surgery are rehabilitation of patients’ vision and achievement of on-target refraction. Post-operative refractive errors and residual astigmatism negatively impact VA and patient satisfaction, and therefore are of key concern to cataract surgeons. Various pre-, intra-, and post-operative factors influence refractive outcomes after cataract surgery, and accurate assessment and optimization of these factors are essential to achieving desired vision. The introduction of ultrasound biometry in the 1960s greatly improved refractive outcomes and, since then, biometric technology has continued to evolve. Optical biometry offered enhanced resolution and greater precision, and the non-contact method eliminated inaccuracies due to corneal compression, in addition to being more comfortable for patients. With further refinements in optical biometry, the proportion of patients achieving their target refraction has steadily increased. Innovations in optical biometry have continued to emerge with the introduction of biometers that utilize SS-OCT. Current research is advancing OCT methods further, for example through utilization of a large number of optical probe beams simultaneously (the “hyper-parallel” approach) to capture accurate anatomical snapshots with no eye motion degradation. Improvements such as these, alongside advancements in microsurgical techniques, new IOL technologies, and enhancements to IOL power calculations, continue to positively impact patients’ refractory status after cataract surgery.
Author Contributions
R.K., G.A., G.Ł., G.P. and R.S. contributed equally to the conceptualization, data curation, writing and review of this article. All authors have read and agreed to the published version of the manuscript.
Funding
Medical writing and editorial support was provided by Chameleon Communications, with funding from Alcon Research LLC.
Acknowledgments
The authors thank Chameleon Communications for medical writing and editorial support, with funding from Alcon Laboratories, Inc.
Conflicts of Interest
R.K. reports grants, personal fees, and non-financial support from Alcon Laboratories, Inc.; personal fees and non-financial support from Kowa Company, Ltd.; grants, personal fees, and non-financial support from Hoya Surgical Optics GmbH; personal fees from Ophtec B.V.; grants and personal fees from Physiol s.a; grants, personal fees, and non-financial support from Rayner; grants, personal fees, and non-financial support from Johnson & Johnson; non-financial support from Acufocus, Inc.; personal fees and non-financial support from Teleon; personal fees and non-financial support from Santen Pharmaceutical Co, Ltd.; personal fees from Oculus; all outside the submitted work. G.A. reports grants, personal fees, and non-financial support from Teleon Surgical B.V.; grants and non-financial support from Klaus Tschira Foundation; grants, personal fees, and non-financial support from Alcon Laboratories, Inc.; grants, personal fees, and non-financial support from J&J Vision; grants, personal fees, and non-financial support from Hoya Surgical Optics GmbH; grants and non-financial support from Kowa Company, Ltd.; personal fees from Ophtec B.V.; grants and non-financial support from Physiol s.a.; grants and non-financial support from Acufocus, Inc.; grants, personal fees, and non-financial support from Rayner Intraocular Lenses Ltd.; grants from Sifi S.p.A; grants, personal fees, and non-financial support from Santen Pharmaceutical Co, Ltd.; grants, personal fees, and non-financial support from Oculus; all outside the submitted work. G.Ł. reports no conflicts of interest. G.P. is an employee of Alcon Research LLC. R.S. is an employee of Alcon Research LLC.
References
- Abdelghany, A.A.; Alio, J.L. Surgical options for correction of refractive error following cataract surgery. Eye Vis. 2014, 1, 2. [Google Scholar] [CrossRef]
- Aristodemou, P.; Cartwright, N.E.; Sparrow, J.M.; Johnston, R.L. Improving refractive outcomes in cataract surgery: A global perspective. World J. Ophthalmol. 2014, 4, 140–146. [Google Scholar] [CrossRef]
- Ladi, J.S. Prevention and correction of residual refractive errors after cataract surgery. J. Clin. Ophthalmol. Res. 2017, 5, 45. [Google Scholar] [CrossRef]
- Coleman, D.J.; Carlin, B. A new system for visual axis measurements in the human eye using ultrasound. Arch. Ophthalmol. 1967, 77, 124–127. [Google Scholar] [CrossRef] [PubMed]
- Ademola-Popoola, D.S.; Nzeh, D.A.; Saka, S.E.; Olokoba, L.B.; Obajolowo, T.S. Comparison of ocular biometry measurements by applanation and immersion A-scan techniques. J. Curr. Ophthalmol. 2015, 27, 110–114. [Google Scholar] [CrossRef]
- Hoffmann, P.; Hütz, W.; Eckhardt, H.; Heuring, A. Intraocular lens calculation and ultrasound biometry: Immersion and contact proceduresn. Klin. Mon. Augenheilkd. 1998, 213, 161–165. [Google Scholar] [CrossRef] [PubMed]
- Giers, U.; Epple, C. Comparison of A-scan device accuracy. J. Cataract. Refract. Surg. 1990, 16, 235–242. [Google Scholar] [CrossRef]
- Rajan, M.S.; Keilhorn, I.; Bell, J.A. Partial coherence laser interferometry vs conventional ultrasound biometry in intraocular lens power calculations. Eye 2002, 16, 552–556. [Google Scholar] [CrossRef] [PubMed]
- Moon, S.W.; Lim, S.H.; Lee, H.Y. Accuracy of biometry for intraocular lens implantation using the new partial coherence interferometer, AL-scan. Korean J. Ophthalmol. 2014, 28, 444–450. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Murphy, C.; Tuft, S.J.; Minassian, D.C. Refractive error and visual outcome after cataract extraction. J. Cataract. Refract. Surg. 2002, 28, 62–66. [Google Scholar] [CrossRef]
- Kugelberg, M.; Lundström, M. Factors related to the degree of success in achieving target refraction in cataract surgery: Swedish National Cataract Register study. J. Cataract. Refract. Surg. 2008, 34, 1935–1939. [Google Scholar] [CrossRef] [PubMed]
- Drexler, W.; Findl, O.; Menapace, R.; Rainer, G.; Vass, C.; Hitzenberger, C.; Fercher, A.F. Partial coherence interferometry: A novel approach to biometry in cataract surgery. Am. J. Ophthalmol. 1998, 126, 524–534. [Google Scholar] [CrossRef]
- Sahin, A.; Hamrah, P. Clinically relevant biometry. Curr. Opin. Ophthalmol. 2012, 23, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Hitzenberger, C.K.; Drexler, W.; Leitgeb, R.A.; Findl, O.; Fercher, A.F. Key developments for partial coherence biometry and optical coherence tomography in the human eye made in Vienna. Investig. Opthalmology Vis. Sci. 2016, 57, 460–474. [Google Scholar] [CrossRef]
- Roy, A.; Das, S.; Sahu, S.K.; Rath, S. Ultrasound biometry vs. IOL Master. Ophthalmology 2012, 119, 1937–1937.e2. [Google Scholar] [CrossRef] [PubMed]
- Landers, J.; Goggin, M. Comparison of refractive outcomes using immersion ultrasound biometry and IOLMaster biometry. Clin. Exp. Ophthalmol. 2009, 37, 566–569. [Google Scholar] [CrossRef]
- Nakhli, F.R. Comparison of optical biometry and applanation ultrasound measurements of the axial length of the eye. Saudi J. Ophthalmol. 2014, 28, 287–291. [Google Scholar] [CrossRef] [PubMed]
- Lee, W.W. Ultrasound vs. Optical Biometry. Available online: https://www.ophthalmologyweb.com/Tech-Spotlights/26583-Ultrasound-Vs-Optical-Biometry/ (accessed on 10 December 2021).
- Hill, W. Biometry Methods Explained. Available online: https://www.doctor-hill.com/iol-main/biometry_explained.html (accessed on 10 December 2021).
- Gale, R.P.; Saldana, M.; Johnston, R.L.; Zuberbuhler, B.; McKibbin, M. Benchmark standards for refractive outcomes after NHS cataract surgery. Eye 2009, 23, 149–152. [Google Scholar] [CrossRef]
- Lundström, M.; Stenevi, U.; Thorburn, W. The Swedish National Cataract Register: A 9-year review. Acta Ophthalmol. Scand. 2002, 80, 248–257. [Google Scholar] [CrossRef] [PubMed]
- Behndig, A.; Montan, P.; Stenevi, U.; Kugelberg, M.; Zetterström, C.; Lundström, M. Aiming for emmetropia after cataract surgery: Swedish National Cataract Register study. J. Cataract. Refract. Surg. 2012, 38, 1181–1186. [Google Scholar] [CrossRef]
- Hahn, U.; Krummenauer, F.; Kölbl, B.; Neuhann, T.; Schayan-Araghi, K.; Schmickler, S.; von Wolff, K.; Weindler, J.; Will, T.; Neuhann, I. Determination of valid benchmarks for outcome indicators in cataract surgery: A multicenter, prospective cohort trial. Ophthalmology 2011, 118, 2105–2112. [Google Scholar] [CrossRef]
- Lundström, M.; Dickman, M.; Henry, Y.; Manning, S.; Rosen, P.; Tassignon, M.-J.; Young, D.; Behndig, A.; Stenevi, U. Changing practice patterns in European cataract surgery as reflected in the European Registry of Quality Outcomes for Cataract and Refractive Surgery 2008 to 2017. J. Cataract Refract. Surg. 2021, 47, 373–378. [Google Scholar] [CrossRef] [PubMed]
- Aristodemou, P.; Cartwright, N.E.K.; Sparrow, J.M.; Johnston, R.L. First eye prediction error improves second eye refractive outcome. Opthalmology 2011, 118, 1701–1709. [Google Scholar] [CrossRef]
- Jivrajka, R.V.; Shammas, M.C.; Shammas, H.J. Improving the second-eye refractive error in patients undergoing bilateral sequential cataract surgery. Ophthalmology 2012, 119, 1097–1101. [Google Scholar] [CrossRef] [PubMed]
- Tamaoki, A.; Kojima, T.; Hasegawa, A.; Yamamoto, M.; Kaga, T.; Tanaka, K.; Ichikawa, K. Clinical evaluation of a new swept-source optical coherence biometer that uses individual refractive indices to measure axial length in cataract patients. Ophthalmic Res. 2019, 62, 11–23. [Google Scholar] [CrossRef] [PubMed]
- Shammas, J.H.; Ortiz, S.; Shammas, M.C.; Kim, S.H.; Chong, C. Biometry measurements using a new large-coherence–length swept-source optical coherence tomographer. J. Cataract Refract. Surg. 2016, 42, 50–61. [Google Scholar] [CrossRef] [PubMed]
- Goto, S.; Maeda, N.; Noda, T.; Ohnuma, K.; Koh, S.; Iehisa, I.; Nishida, K. Comparison of composite and segmental methods for acquiring optical axial length with swept-source optical coherence tomography. Sci. Rep. 2020, 10, 4474. [Google Scholar] [CrossRef]
- Hirnschall, N.; Varsits, R.; Doeller, B.; Findl, O. enhanced penetration for axial length measurement of eyes with dense cataracts using swept source optical coherence tomography: A consecutive observational study. Ophthalmol. Ther. 2018, 7, 119–124. [Google Scholar] [CrossRef]
- Lee, A.C.; Qazi, M.A.; Pepose, J.S. Biometry and intraocular lens power calculation. Curr. Opin. Ophthalmol. 2008, 19, 13–17. [Google Scholar] [CrossRef]
- Kaswin, G.; Rousseau, A.; Mgarrech, M.; Barreau, E.; Labetoulle, M. Biometry and intraocular lens power calculation results with a new optical biometry device: Comparison with the gold standard. J. Cataract. Refract. Surg. 2014, 40, 593–600. [Google Scholar] [CrossRef]
- Olsen, T. Calculation of intraocular lens power: A review. Acta Ophthalmol. Scand. 2007, 85, 472–485. [Google Scholar] [CrossRef]
- Millán, M.S.; Alba-Bueno, F.; Vega, F. New trends in intraocular lens imaging. In Proceedings of the 22nd Congress of the International Commission for Optics: Light for the Development of the World, Puebla, Mexico, 15–19 August 2011; Volume 8011, p. 80119. [Google Scholar]
- American Academy of Ophthalmology. Biometry for Intra-Ocular Lens (IOL) Power Calculation. Available online: https://eyewiki.aao.org/Biometry_for_Intra-Ocular_Lens_(IOL)_Power_Calculation (accessed on 10 December 2020).
- Tamaoki, A.; Kojima, T.; Hasegawa, A.; Yamamoto, M.; Kaga, T.; Tanaka, K.; Ichikawa, K. Evaluation of axial length measurement using enhanced retina visualization mode of the swept-source optical coherence tomography biometer in dense cataract. Ophthalmic Res. 2021, 64, 595–603. [Google Scholar] [CrossRef] [PubMed]
- Kanclerz, P.; Khoramnia, R.; Wang, X. Current developments in corneal topography and tomography. Diagnostics 2021, 11, 1466. [Google Scholar] [CrossRef] [PubMed]
- Day, A.C.; Dhariwal, M.; Keith, M.S.; Ender, F.; Vives, C.P.; Miglio, C.; Zou, L.; Anderson, D.F. Distribution of preoperative and postoperative astigmatism in a large population of patients undergoing cataract surgery in the UK. Br. J. Ophthalmol. 2018, 103, 993–1000. [Google Scholar] [CrossRef]
- Koch, D.D. The posterior cornea: Hiding in plain sight. Ophthalmology 2015, 122, 1070–1071. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Varadi, D.; Khoramnia, R.; Auffarth, G.U. Central and mid-peripheral corneal astigmatism in an elderly population: A retrospective analysis of Scheimpflug topography results. Sci. Rep. 2021, 11, 7968. [Google Scholar] [CrossRef]
- Łabuz, G.; Varadi, D.; Khoramnia, R.; Auffarth, G.U. Progressive-toric IOL design reduces residual astigmatism with increasing pupil size: A ray-tracing simulation based on corneal topography data. Biomed. Opt. Express 2021, 12, 1568–1576. [Google Scholar] [CrossRef]
- Ho, J.-D.; Tsai, C.-Y.; Liou, S.-W. Accuracy of corneal astigmatism estimation by neglecting the posterior corneal surface measurement. Am. J. Ophthalmol. 2009, 147, 788–795.e2. [Google Scholar] [CrossRef] [PubMed]
- Koch, D.D.; Ali, S.F.; Weikert, M.; Shirayama, M.; Jenkins, R.; Wang, L. Contribution of posterior corneal astigmatism to total corneal astigmatism. J. Cataract Refract. Surg. 2012, 38, 2080–2087. [Google Scholar] [CrossRef]
- Koch, D.D.; Jenkins, R.B.; Weikert, M.P.; Yeu, E.; Wang, L. Correcting astigmatism with toric intraocular lenses: Effect of posterior corneal astigmatism. J. Cataract Refract. Surg. 2013, 39, 1803–1809. [Google Scholar] [CrossRef] [PubMed]
- Norrby, S. Sources of error in intraocular lens power calculation. J. Cataract Refract. Surg. 2008, 34, 368–376. [Google Scholar] [CrossRef]
- Rabsilber, T.M.; Khoramnia, R.; Auffarth, G. Anterior chamber measurements using Pentacam rotating Scheimpflug camera. J. Cataract. Refract. Surg. 2006, 32, 456–459. [Google Scholar] [CrossRef] [PubMed]
- Schröder, S.; Leydolt, C.; Menapace, R.; Eppig, T.; Langenbucher, A. Determination of personalized IOL-constants for the Haigis formula under consideration of measurement precision. PLoS ONE 2016, 11, e0158988. [Google Scholar] [CrossRef]
- Sheard, R. Optimising biometry for best outcomes in cataract surgery. Eye 2014, 28, 118–125. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.H.; Karp, C.L.; Yoo, S.H.; Amescua, G.; Galor, A. Cataract surgery after refractive surgery. Int. Ophthalmol. Clin. 2016, 56, 169–180. [Google Scholar] [CrossRef]
- Khoramnia, R.; Auffarth, G.; Rabsilber, T.M.; Holzer, M.P. Implantation of a multifocal toric intraocular lens with a surface-embedded near segment after repeated LASIK treatments. J. Cataract. Refract. Surg. 2012, 38, 2049–2052. [Google Scholar] [CrossRef] [PubMed]
- Turczynowska, M.; Koźlik-Nowakowska, K.; Gaca-Wysocka, M.; Grzybowski, A. Effective ocular biometry and intraocular lens power calculation. Eur. Ophthalmic Rev. 2016, 10, 94–100. [Google Scholar] [CrossRef]
- Vale, C.; Menezes, C.; Firmino-Machado, J.; Rodrigues, P.; Lume, M.; Tenedório, P.; Menéres, P.; Brochado, M.D.C. Astigmatism management in cataract surgery with Precizon® toric intraocular lens: A prospective study. Clin. Ophthalmol. 2016, 10, 151–159. [Google Scholar] [CrossRef]
- Yoon, J.H.; Kim, K.-H.; Lee, J.Y.; Nam, D.H. Surgically induced astigmatism after 3.0 mm temporal and nasal clear corneal incisions in bilateral cataract surgery. Indian J. Ophthalmol. 2013, 61, 645–648. [Google Scholar] [CrossRef]
- Yang, J.; Wang, X.; Zhang, H.; Pang, Y.; Wei, R.-H. Clinical evaluation of surgery-induced astigmatism in cataract surgery using 2.2 mm or 1.8 mm clear corneal micro-incisions. Int. J. Ophthalmol. 2017, 10, 68–71. [Google Scholar] [CrossRef]
- Liu, J.; Wolfe, P.; Hernandez, V.; Kohnen, T. Comparative assessment of the corneal incision enlargement of 4 preloaded IOL delivery systems. J. Cataract. Refract. Surg. 2020, 46, 1041–1046. [Google Scholar] [CrossRef] [PubMed]
- Ong, H.S.; Subash, M.; Sandhu, A.; Wilkins, M. Intraocular lens delivery characteristics of the preloaded AcrySof IQ SN60WS/AcrySert Injectable Lens System. Am. J. Ophthalmol. 2013, 156, 77–81.e2. [Google Scholar] [CrossRef]
- Yildirim, T.M.; Łabuz, G.; Baur, I.D.; Poompokawat, P.; Knorz, M.C.; Auffarth, G.U.; Khoramnia, R. Corneal incision enlargement in two preloaded intraocular lens injectors: An intraindividual in vivo study. J. Refract. Surg. 2021, 37, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Khoramnia, R.; Yildirim, T.M.; Weindler, J.; Naujokaitis, T.; Dzhambazova, M.; Auffarth, G.U. Preloaded injectors used in a clinical study: Videographic assessment and laboratory analysis of injector nozzle damage. J. Cataract. Refract. Surg. 2021, 47, 1338–1344. [Google Scholar] [CrossRef]
- American Academy of Ophthalmology. Toric IOLs. Available online: http://eyewiki.aao.org/Toric_IOLs (accessed on 24 May 2021).
- American Academy of Ophthalmology. Toric IOLs: Four Options for Addressing Residual Astigmatism. Available online: https://www.aao.org/eyenet/article/toric-iols-four-options-addressing-residual-astigm (accessed on 24 May 2021).
- Gatinel, D.; Debellemanière, G.; Saad, A.; Dubois, M.; Rampat, R. Determining the theoretical effective lens position of thick intraocular lenses for machine learning–based IOL power calculation and simulation. Transl. Vis. Sci. Technol. 2021, 10, 27. [Google Scholar] [CrossRef]
- Erickson, P. Effects of intraocular lens position errors on postoperative refractive error. J. Cataract. Refract. Surg. 1990, 16, 305–311. [Google Scholar] [CrossRef]
- Shajari, M.; Sonntag, R.; Niermann, T.; Holland, D.; Kohnen, T.; Priglinger, S.; Mayer, W.J. Determining and comparing the effective lens position and refractive outcome of a novel rhexis-fixated lens to established lens designs. Am. J. Ophthalmol. 2020, 213, 62–68. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Auffarth, G.; Yan, W.; Yildirim, T.; Khoramnia, R. Simulations of decentration and tilt of a supplementary sulcus-fixated intraocular lens in a polypseudophakic combination using ray-tracing software. Photonics 2021, 8, 309. [Google Scholar] [CrossRef]
- Ashena, Z.; Maqsood, S.; Ahmed, S.N.; Nanavaty, M.A. Effect of intraocular lens tilt and decentration on visual acuity, dysphotopsia and wavefront aberrations. Vision 2020, 4, 41. [Google Scholar] [CrossRef]
- Tandogan, T.; Son, H.S.; Choi, C.Y.; Knorz, M.C.; Auffarth, G.U.; Khoramnia, R. Laboratory evaluation of the influence of decentration and pupil size on the optical performance of a monofocal, bifocal, and trifocal intraocular lens. J. Refract. Surg. 2017, 33, 808–812. [Google Scholar] [CrossRef]
- Auffarth, G.U.; Friedmann, E.; Breyer, D.; Kaymak, H.; Holland, D.; Dick, B.; Petzold, A.; Shah, S.; Ladaria, L.S.; Garcia, S.A.; et al. Stability and visual outcomes of the capsulotomy-fixated FEMTIS-IOL after automated femtosecond laser–assisted anterior capsulotomy. Am. J. Ophthalmol. 2021, 225, 27–37. [Google Scholar] [CrossRef]
- Goss, D.A.; Grosvenor, T. Reliability of refraction--a literature review. J. Am. Optom. Assoc. 1996, 67, 619–630. [Google Scholar]
- Zadnik, K.; Mutti, D.O.; Adams, A.J. The repeatability of measurement of the ocular components. Investig. Ophthalmol. Vis. Sci. 1992, 33, 2325–2333. [Google Scholar]
- Bullimore, M.A.; Fusaro, R.E.; Adams, C.W. The repeatability of automated and clinician refraction. Optom. Vis. Sci. 1998, 75, 617–622. [Google Scholar] [CrossRef] [PubMed]
- Sheedy, J.; Schanz, P.; Bullimore, M. Evaluation of an automated subjective refractor. Optom. Vis. Sci. 2004, 81, 334–340. [Google Scholar] [CrossRef]
- Smith, G. Refraction and visual acuity measurements: What are their measurement uncertainties? Clin. Exp. Optom. 2006, 89, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Turnbull, A.M.; Barrett, G.D. Using the first-eye prediction error in cataract surgery to refine the refractive outcome of the second eye. J. Cataract. Refract. Surg. 2019, 45, 1239–1245. [Google Scholar] [CrossRef]
- Leffler, C.T.; Wilkes, M.; Reeves, J.; Mahmood, M.A. Postoperative refraction in the second eye having cataract surgery. ISRN Ophthalmol. 2011, 2011, 273923. [Google Scholar] [CrossRef][Green Version]
- Higashiyama, T.; Mori, H.; Nakajima, F.; Ohji, M. Comparison of a new biometer using swept-source optical coherence tomography and a conventional biometer using partial coherence interferometry. PLoS ONE 2018, 13, e0196401. [Google Scholar] [CrossRef]
- Huang, J.; Chen, H.; Li, Y.; Chen, Z.; Gao, R.; Yu, J.; Zhao, Y.; Lu, W.; McAlinden, C.; Wang, Q. Comprehensive comparison of axial length measurement with three swept-source OCT-Based biometers and partial coherence interferometry. J. Refract. Surg. 2019, 35, 115–120. [Google Scholar] [CrossRef] [PubMed]
- Sabatino, F.; Matarazzo, F.; Findl, O.; Maurino, V. Comparative analysis of 2 swept-source optical coherence tomography biometers. J. Cataract Refract. Surg. 2019, 45, 1124–1129. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.M.; Lim, D.H.; Kim, H.J.; Chung, T.-Y. Comparison of two swept-source optical coherence tomography biometers and a partial coherence interferometer. PLoS ONE 2019, 14, e0223114. [Google Scholar] [CrossRef]
- Whang, W.-J.; Yoo, Y.-S.; Kang, M.-J.; Joo, C.-K. Predictive accuracy of partial coherence interferometry and swept-source optical coherence tomography for intraocular lens power calculation. Sci. Rep. 2018, 8, 13732. [Google Scholar] [CrossRef]
- Connell, B.J.; Kane, J. Comparison of the Kane formula with existing formulas for intraocular lens power selection. BMJ Open Ophthalmol. 2019, 4, e000251. [Google Scholar] [CrossRef]
- Omoto, M.K.; Torii, H.; Masui, S.; Ayaki, M.; Tsubota, K.; Negishi, K. Ocular biometry and refractive outcomes using two swept-source optical coherence tomography-based biometers with segmental or equivalent refractive indices. Sci. Rep. 2019, 9, 6557. [Google Scholar] [CrossRef] [PubMed]
- Shammas, H.J.; Shammas, M.C.; Jivrajka, R.V.; Cooke, D.L.; Potvin, R. Effects on IOL power calculation and expected clinical outcomes of axial length measurements based on multiple vs single refractive indices. Clin. Ophthalmol. 2020, 14, 1511–1519. [Google Scholar] [CrossRef] [PubMed]
- Bianchi, G.R. Spectacle independence after cataract surgery: A prospective study with a multifocal intraocular lens. Med. Hypothesis Discov. Innov. Ophthalmol. 2020, 9, 38–46. [Google Scholar] [PubMed]
- Hovanesian, J.A.; Lane, S.S.; Allen, Q.B.; Jones, M. Patient-reported outcomes/satisfaction and spectacle independence with blended or bilateral multifocal intraocular lenses in cataract surgery. Clin. Ophthalmol. 2019, 13, 2591–2598. [Google Scholar] [CrossRef] [PubMed]
- El Kouhen, N.; Mnasri, H.; Mouna, A.; Agapie, A.; Ferte, A.; Ameloot, F.; Perone, J.-M. Refractive outcome and patient satisfaction after cataract surgery with mutifocal intraocular lens implantation. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2999. [Google Scholar]
- Visser, N.; Nuijts, R.M.; de Vries, N.E.; Bauer, N.J. Visual outcomes and patient satisfaction after cataract surgery with toric multifocal intraocular lens implantation. J. Cataract. Refract. Surg. 2011, 37, 2034–2042. [Google Scholar] [CrossRef]
- Lavanya, R.; Wong, T.Y.; Aung, T.; Tan, D.T.H.; Saw, S.-M.; Tay, W.T.; Wang, J.J.; For the SiMES Team. Prevalence of cataract surgery and post-surgical visual outcomes in an urban Asian population: The Singapore Malay Eye Study. Br. J. Ophthalmol. 2009, 93, 299–304. [Google Scholar] [CrossRef] [PubMed]
- Kanthan, G.L.; Mitchell, P.; Burlutsky, G.; Wang, J.J. Intermediate- and longer-term visual outcomes after cataract surgery: The Blue Mountains Eye Study. Clin. Exp. Ophthalmol. 2010, 39, 201–206. [Google Scholar] [CrossRef] [PubMed]
- Keel, S.; Xie, J.; Foreman, J.; Taylor, H.R.; Dirani, M. Population-based assessment of visual acuity outcomes following cataract surgery in Australia: The National Eye Health Survey. Br. J. Ophthalmol. 2018, 102, 1419–1424. [Google Scholar] [CrossRef]
- Park, C.Y.; Chuck, R.S. Residual refractive error and visual outcome after cataract surgery using spherical versus aspheric IOLs. Ophthalmic Surg. Lasers Imaging Retin. 2011, 42, 37–43. [Google Scholar] [CrossRef] [PubMed]
- Fernández-Vega, L.; Alfonso, J.F.; Montés-Micó, R.; Amhaz, H. Visual acuity tolerance to residual refractive errors in patients with an apodized diffractive intraocular lens. J. Cataract. Refract. Surg. 2008, 34, 199–204. [Google Scholar] [CrossRef] [PubMed]
- Schuster, A.K.; Schlichtenbrede, F.C.; Harder, B.C.; Beutelspacher, S.C.; Jonas, J.B. Target refraction for best uncorrected distance and near vision in cataract surgery. Eur. J. Ophthalmol. 2014, 24, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Son, H.-S.; Kim, S.H.; Auffarth, G.U.; Choi, C.Y. Prospective comparative study of tolerance to refractive errors after implantation of extended depth of focus and monofocal intraocular lenses with identical aspheric platform in Korean population. BMC Ophthalmol. 2019, 19, 187. [Google Scholar] [CrossRef] [PubMed]
- de Vries, N.E.; Webers, C.A.; Touwslager, W.R.; Bauer, N.J.; de Brabander, J.; Berendschot, T.T.; Nuijts, R.M. Dissatisfaction after implantation of multifocal intraocular lenses. J. Cataract Refract. Surg. 2011, 37, 859–865. [Google Scholar] [CrossRef] [PubMed]
- Sales, C.; Manche, E.E. Managing residual refractive error after cataract surgery. J. Cataract. Refract. Surg. 2015, 41, 1289–1299. [Google Scholar] [CrossRef] [PubMed]
- von Mohrenfels, C.W.; Salgado, J.; Khoramnia, R.; Maier, M.; Lohmann, C.P. Clinical results with the light adjustable intraocular lens after cataract surgery. J. Refract. Surg. 2010, 26, 314–320. [Google Scholar] [CrossRef] [PubMed]
- Jobson Medical Information LLC. Answering Your Top 10 IOL-Formula Questions. Review of Ophthalmology Annual IOL Edition. Reviewofophthalmology.com. 2018. Available online: https://www.reviewofophthalmology.com/CMSDocuments/2018/01/rp0118i.pdf (accessed on 25 August 2021).
- Baur, I.D.; Auffarth, G.U.; Yildirim, T.M.; Mayer, C.S.; Khoramnia, R. Reversibility of the duet procedure: Bilateral exchange of a supplementary trifocal sulcus-fixated intraocular lens for correction of a postoperative refractive error. Am. J. Ophthalmol. Case Rep. 2020, 20, 100957. [Google Scholar] [CrossRef] [PubMed]
- Łabuz, G.; Auffarth, G.U.; Knorz, M.C.; Son, H.-S.; Yildirim, T.M.; Khoramnia, R. Trifocality achieved through polypseudophakia: Optical quality and light loss compared with a single trifocal intraocular lens. J. Refract. Surg. 2020, 36, 570–577. [Google Scholar] [CrossRef] [PubMed]
- Khoramnia, R.; Yildirim, T.M.; Son, H.-S.; Łabuz, G.; Mayer, C.S.; Auffarth, G.U. Reversible Trifokalität durch das Duett-Verfahren. Ophthalmologe 2020, 117, 999–1004. [Google Scholar] [CrossRef] [PubMed]
- Khoramnia, R.; Yildirim, T.M.; Baur, I.; Auffarth, G.U. Duet procedure to achieve reversible trifocality in a young patient with hereditary hyperferritinemia-cataract syndrome. Am. J. Ophthalmol. Case Rep. 2021, 21, 101026. [Google Scholar] [CrossRef] [PubMed]
- Ang, R.E. Comparison of tolerance to induced astigmatism in pseudophakic eyes implanted with small aperture, trifocal, or monofocal intraocular lenses. Clin. Ophthalmol. 2019, 13, 905–911. [Google Scholar] [CrossRef] [PubMed]
- Carones, F. Residual astigmatism threshold and patient satisfaction with bifocal, trifocal and extended range of vision intraocular lenses (IOLs). Open J. Ophthalmol. 2017, 7, 1–7. [Google Scholar] [CrossRef]
- Singh, A.; Pesala, V.; Garg, P.; Bharadwaj, S.R. Relation between uncorrected astigmatism and visual acuity in pseudophakia. Optom. Vis. Sci. 2013, 90, 378–384. [Google Scholar] [CrossRef]
- Kohnen, T.; Herzog, M.; Hemkeppler, E.; Schönbrunn, S.; De Lorenzo, N.; Petermann, K.; Böhm, M. Visual performance of a quadrifocal (trifocal) intraocular lens following removal of the crystalline lens. Am. J. Ophthalmol. 2017, 184, 52–62. [Google Scholar] [CrossRef]
- Ribeiro, F.; Ferreira, T.B. Comparison of clinical outcomes of 3 trifocal IOLs. J. Cataract. Refract. Surg. 2020, 46, 1247–1252. [Google Scholar] [CrossRef]
- Böhm, M.; Petermann, K.; Hemkeppler, E.; Kohnen, T. Defocus curves of 4 presbyopia-correcting IOL designs: Diffractive panfocal, diffractive trifocal, segmental refractive, and extended-depth-of-focus. J. Cataract Refract. Surg. 2019, 45, 1625–1636. [Google Scholar] [CrossRef]
- Tran, D.B.; Owyang, A.; Hwang, J.; Potvin, R. Visual acuity, quality of vision, and patient-reported outcomes after bilateral implantation with a trifocal or extended depth of focus intraocular lens. Clin. Ophthalmol. 2021, 15, 403–412. [Google Scholar] [CrossRef] [PubMed]
- Shamir, R.R.; Friedman, Y.G.; Joskowicz, L.; Mimouni, M.; Blumenthal, E.Z. The influence of varying the number of characters per row on the accuracy and reproducibility of the ETDRS visual acuity chart. Graefe’s Arch. Clin. Exp. Ophthalmol. 2016, 254, 971–976. [Google Scholar] [CrossRef] [PubMed]
- Elliott, D.B. The good (logMAR), the bad (Snellen) and the ugly (BCVA, number of letters read) of visual acuity measurement. Ophthalmic Physiol. Opt. 2016, 36, 355–358. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).