Accelerating the Laboratory Testing Capacity through Saliva Pooling Prior to Direct RT-qPCR for SARS-CoV-2 Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Specimen Collection and Viability Test
2.2. RT-qPCR for SARS-CoV-2 Detection
2.3. Validation of Pooling Strategy Pre- and Post-Heating
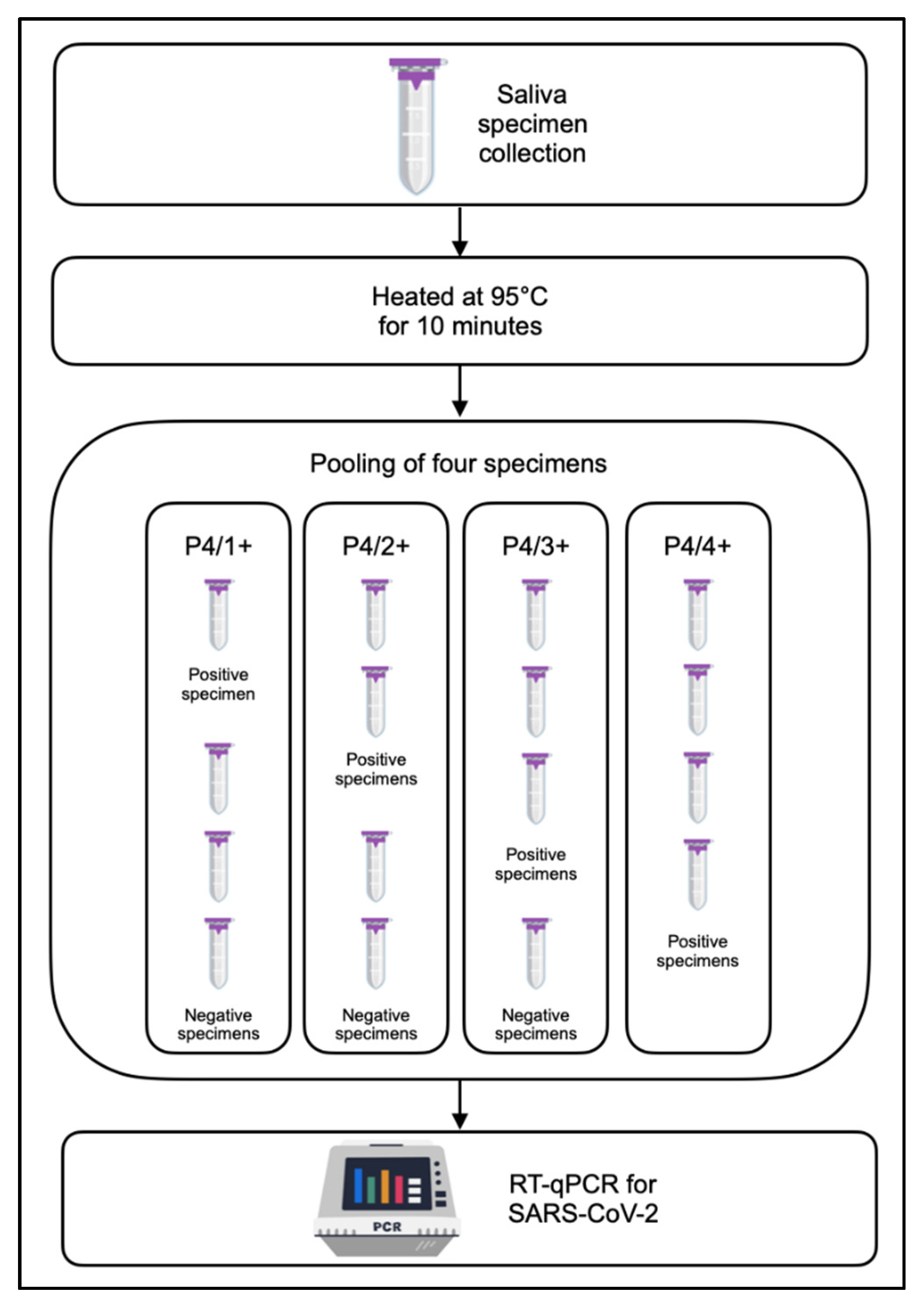
2.4. Pooling Approaches Using a Combination of Four Specimens
2.5. Compatibility of RNA-Extraction-Free Saliva across Other SARS-CoV-2 PCR Detection Kits
2.6. Data Management and Statistical Analysis
3. Results
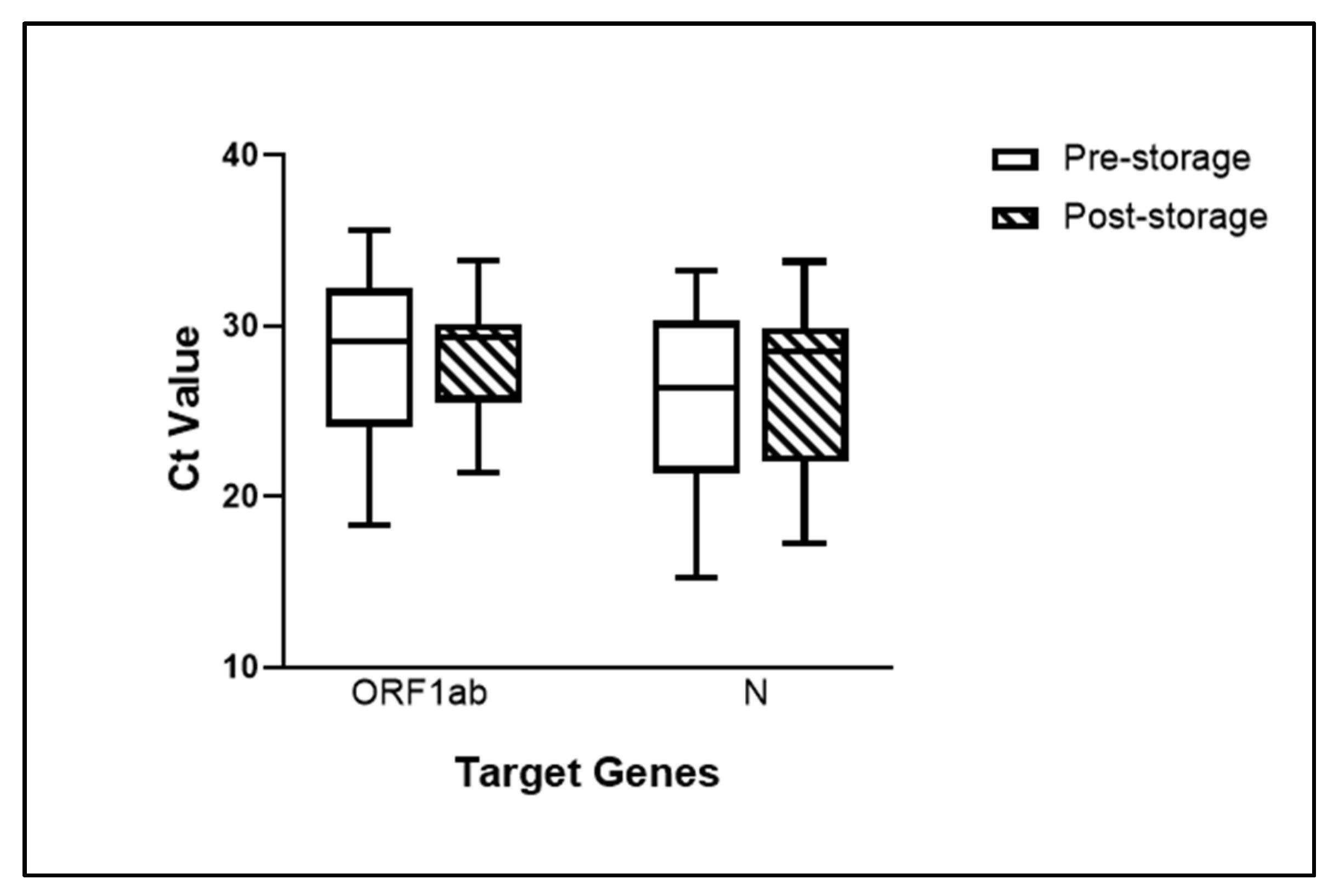
3.1. Saliva Specimens Stored at −80 °C Remained Viable
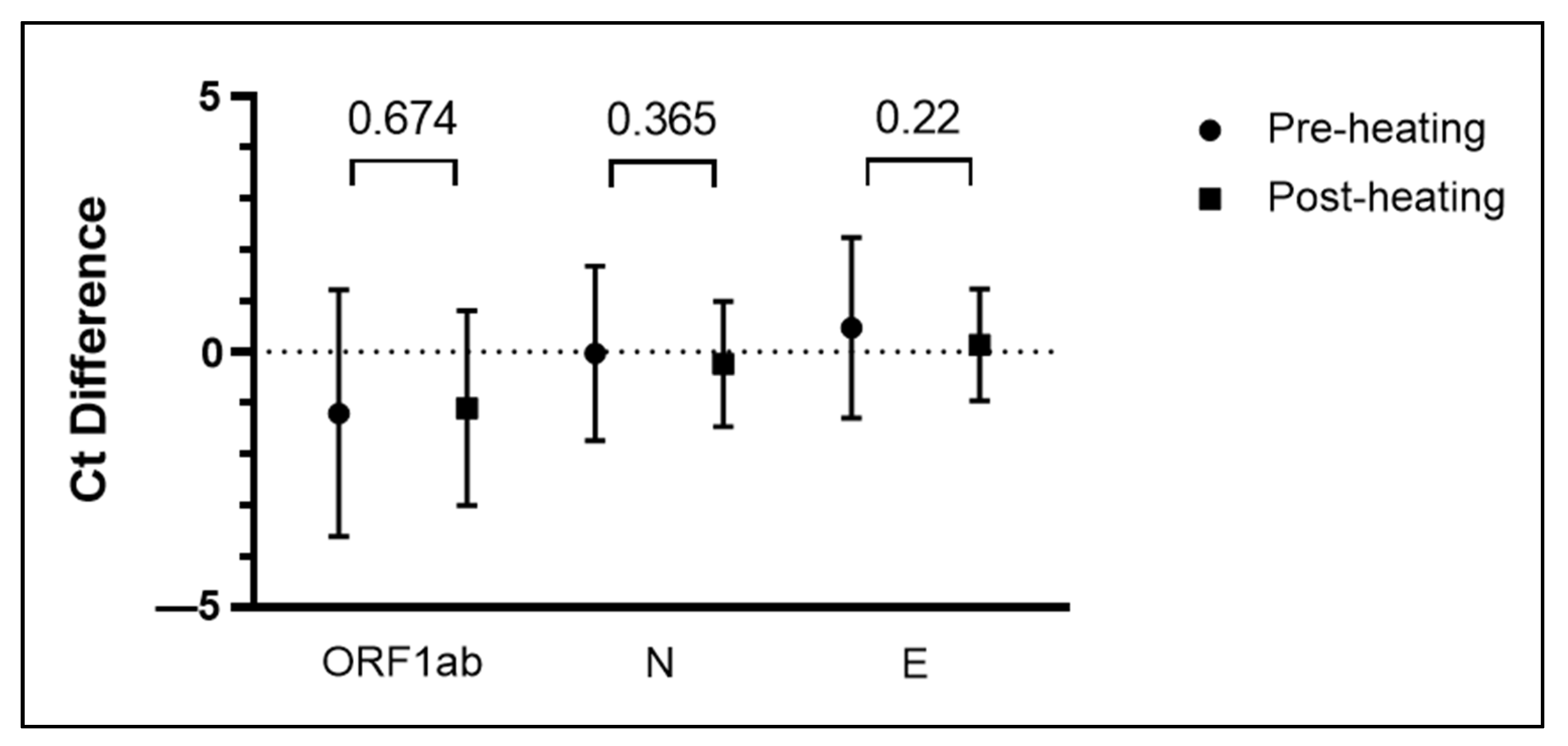
3.2. Specimen Pooling Can Be Performed Both Pre- and Post-Heating
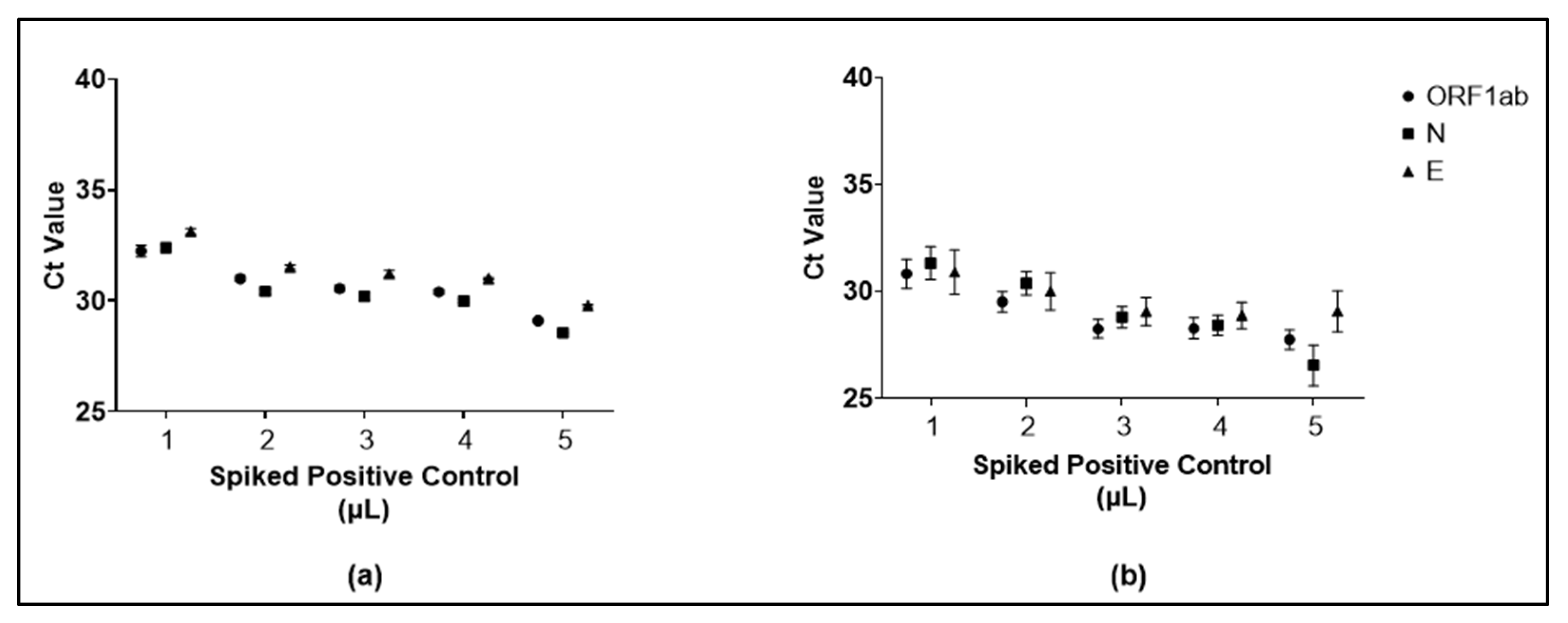
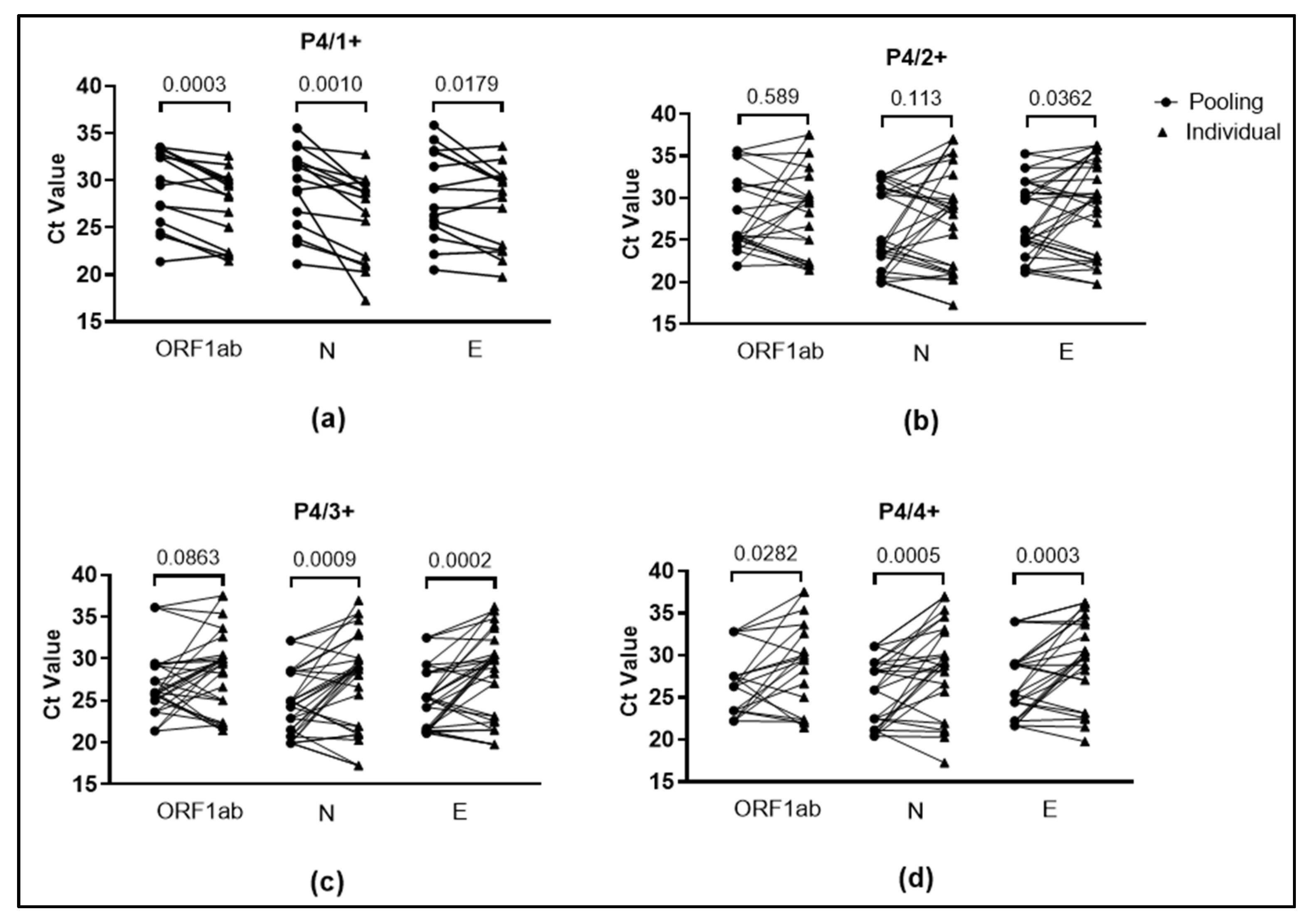
3.3. Validation of Pooling of Four Approach for SARS-CoV-2 Detection from Saliva Specimens
3.4. Saliva Pooling Approach Is Applicable in a Number of SARS-CoV-2 Commercial Detection Kits
4. Discussion
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. WHO COVID-19 Dashboard. Available online: https://covid19.who.int/ (accessed on 14 June 2022).
- WHO. WHO Indonesia Situation Report-11 Who.Int/Indonesia Situation Report-7 INDONESIA Situation Report 19; WHO: Geneva, Switzerland, 2020; Volume 19. [Google Scholar]
- Ritchie, H.; Mathieu, E.; Rodés-Guirao, L.; Appel, C.; Giattino, C.; Ortiz-Ospina, E. Coronavirus Pandemic (COVID-19). Available online: https://ourworldindata.org/coronavirus-testing#testing-for-covid-19-background-the-our-world-in-data-covid-19-testing-dataset (accessed on 24 May 2022).
- Williams, E.; Isles, N.; Chong, B.; Bond, K.; Yoga, Y.; Druce, J.; Catton, M.; Ballard, S.A.; Howden, B.P.; Williamson, D.A. Detection of SARS-CoV-2 in Saliva: Implications for Specimen Transport and Storage. J. Med. Microbiol. 2020, 70, 2–4. [Google Scholar] [CrossRef] [PubMed]
- To, K.K.W.; Tsang, O.T.Y.; Yip, C.C.Y.; Chan, K.H.; Wu, T.C.; Chan, J.M.C.; Leung, W.S.; Chik, T.S.H.; Choi, C.Y.C.; Kandamby, D.H.; et al. Consistent Detection of 2019 Novel Coronavirus in Saliva. Clin. Infect. Dis. 2020, 71, 841–843. [Google Scholar] [CrossRef] [PubMed]
- Mahendra, C.; Kaisar, M.M.M.; Vasandani, S.R.; Surja, S.S.; Tjoa, E.; Chriestya, F.; Junusmin, K.I.; Widowati, T.A.; Irwanto, A.; Ali, S. Wide Application of Minimally Processed Saliva on Multiple RT-QPCR Kits for SARS-CoV-2 Detection in Indonesia. Front. Cell. Infect. Microbiol. 2021, 11, 691538. [Google Scholar] [CrossRef] [PubMed]
- Sahajpal, N.S.; Mondal, A.K.; Ananth, S.; Njau, A.; Ahluwalia, P.; Newnam, G.; Lozoya-Colinas, A.; Hud, N.V.; Kota, V.; Ross, T.M.; et al. Salivastat: Direct-Pcr and Pooling of Saliva Samples Collected in Healthcare and Community Setting for Sars-Cov-2 Mass Surveillance. Diagnostics 2021, 11, 904. [Google Scholar] [CrossRef]
- da Costa Fernandes, P.A.; da Conceicao Ferreira, F.A.; Morais, O.M.; Ramos, C.M.T.; Fernandes, É.M.R.; da Rocha, S.A.A.; Rocha, R.J.A.; Monteiro, V.J.P.; Vilar, P.S.G.; Romão, A.M.; et al. Performance of Saliva as a Specimen to Detect SARS-CoV-2. J. Clin. Virol. 2021, 142, 104913. [Google Scholar] [CrossRef]
- Brotons, P.; Perez-Argüello, A.; Launes, C.; Torrents, F.; Subirats, M.P.; Saucedo, J.; Claverol, J.; Garcia-Garcia, J.J.; Rodas, G.; Fumado, V.; et al. Validation and Implementation of a Direct RT-QPCR Method for Rapid Screening of SARS-CoV-2 Infection by Using Non-Invasive Saliva Samples. Int. J. Infect. Dis. 2021, 110, 363–370. [Google Scholar] [CrossRef]
- Gallian, P.; Lhomme, S.; Piquet, Y.; Sauné, K.; Abravanel, F.; Assal, A.; Tiberghien, P.; Izopet, J. Hepatitis e Virus Infections in Blood Donors, France. Emerg. Infect. Dis. 2014, 20, 1914–1917. [Google Scholar] [CrossRef]
- Paixão, P.; Almeida, S.; Gouveia, P.; Binda, S.; Caroppo, S.; Barbi, M. Diagnosis of Congenital Cytomegalovirus Infection by Detection of Viral DNA in Urine Pools. J. Virol. Methods 2005, 128, 1–5. [Google Scholar] [CrossRef]
- Dorfman, R. The Detection of Defective Members of Large Populations. Ann. Math. Stat. 1943, 14, 436–440. [Google Scholar] [CrossRef]
- Barat, B.; Das, S.; de Giorgi, V.; Henderson, D.K.; Kopka, S.; Lau, A.F.; Miller, T.; Moriarty, T.; Palmore, T.N.; Sawney, S.; et al. Pooled Saliva Specimens for SARS-CoV-2 Testing. J. Clin. Microbiol. 2021, 59, 1–8. [Google Scholar] [CrossRef]
- Denny, T.N.; Andrews, L.; Bonsignori, M.; Cavanaugh, K.; Datto, M.B.; Deckard, A.; DeMarco, C.T.; DeNaeyer, N.; Epling, C.A.; Gurley, T.; et al. Implementation of a Pooled Surveillance Testing Program for Asymptomatic SARS-CoV-2 Infections on a College Campus—Duke University, Durham, North Carolina, August 2–October 11, 2020. MMWR. Morb. Mortal. Wkly. Rep. 2020, 69, 1743–1747. [Google Scholar] [CrossRef]
- Joachim, A.; Dewald, F.; Suárez, I.; Zemlin, M.; Lang, I.; Stutz, R.; Marthaler, A.; Bosse, H.M.; Lübke, N.; Münch, J.; et al. Pooled RT-QPCR Testing for SARS-CoV-2 Surveillance in Schools—A Cluster Randomised Trial. eClinicalMedicine 2021, 39, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Mahmoud, S.A.; Ibrahim, E.; Thakre, B.; Teddy, J.G.; Raheja, P.; Ganesan, S.; Zaher, W.A. Evaluation of Pooling of Samples for Testing SARS-CoV- 2 for Mass Screening of COVID-19. BMC Infect. Dis. 2021, 21, 360. [Google Scholar] [CrossRef]
- Mendoza, R.P.; Bi, C.; Cheng, H.T.; Gabutan, E.; Pagapas, G.J.; Khan, N.; Hoxie, H.; Hanna, S.; Holmes, K.; Gao, N.; et al. Implementation of a Pooled Surveillance Testing Program for Asymptomatic SARS-CoV-2 Infections in K-12 Schools and Universities. EClinicalMedicine 2021, 38, 101028. [Google Scholar] [CrossRef] [PubMed]
- Moreno-Contreras, J.; Espinoza, M.A.; Sandoval-Jaime, C.; Cantú-Cuevas, M.A.; Madrid-González, D.A.; Barón-Olivares, H.; Ortiz-Orozco, O.D.; Muñoz-Rangel, A.V.; Guzmán-Rodríguez, C.; Hernández-de la Cruz, M.; et al. Pooling Saliva Samples as an Excellent Option to Increase the Surveillance for SARS-CoV-2 When Re-Opening Community Settings. PLoS ONE 2022, 17, e0263114. [Google Scholar] [CrossRef] [PubMed]
- Mastrianni, D.; Falivena, R.; Brooks, T.; McDermott, B.; Tan, J.; Vandell, R.; Holland, M. Pooled Testing for SARS-CoV-2 in Hospitalized Patients. J. Hosp. Med. 2020, 15, 538–539. [Google Scholar] [CrossRef]
- Watkins, A.E.; Fenichel, E.P.; Weinberger, D.M.; Vogels, C.B.F.; Brackney, D.E.; Casanovas-Massana, A.; Campbell, M.; Fournier, J.; Bermejo, S.; Datta, R.; et al. Pooling Saliva to Increase SARS-CoV-2 Testing Capacity. medRxiv Prepr. Serv. Health Sci. 2020, 27, 1184–1187. [Google Scholar] [CrossRef]
- Rainey, A.; Pierce, A.; Deng, X.; Actis, L.A.; Smith, P.; Kiss, A.J.; Wilson, T.J. Validation and Deployment of a Direct Saliva Real-Time RT-PCR Test on Pooled Samples for COVID-19 Surveillance Testing. PLoS ONE 2021, 16, e0261956. [Google Scholar] [CrossRef]
- Deckert, A.; Bärnighausen, T.; Kyei, N.N.A. Simulation of Pooled-Sample Analysis Strategies for Covid-19 Mass Testing. Bull. World Health Organ. 2020, 98, 590–598. [Google Scholar] [CrossRef]
- Bhattarai, K.R.; Kim, H.R.; Chae, H.J. Compliance with Saliva Collection Protocol in Healthy Volunteers: Strategies for Managing Risk and Errors. Int. J. Med. Sci. 2018, 15, 823–831. [Google Scholar] [CrossRef]
- Nemoda, Z.; Horvat-Gordon, M.; Fortunato, C.K.; Beltzer, E.K.; Scholl, J.L.; Granger, D.A. Assessing Genetic Polymorphisms Using DNA Extracted from Cells Present in Saliva Samples. BMC Med. Res. Methodol. 2011, 11, 170. [Google Scholar] [CrossRef] [PubMed]
- Gulec, E.Y.; Cesur, N.P.; Yesilyurt Fazlioğlu, G.; Kazezoğlu, C. Effect of Different Storage Conditions on COVID-19 RT-PCR Results. J. Med. Virol. 2021, 93, 6575–6581. [Google Scholar] [CrossRef] [PubMed]
- Rhee, C.; Kanjilal, S.; Baker, M.; Klompas, M. Duration of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2) Infectivity: When Is It Safe to Discontinue Isolation? Clin. Infect. Dis. 2021, 72, 1467–1474. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Li, X.; Guo, Z.; Wang, Z.; Zhang, K.; Li, C.; Wang, C.; Zhang, S. Influence of Storage Conditions on SARS-CoV-2 Nucleic Acid Detection in Throat Swabs. J. Infect. Dis. 2020, 222, 203–205. [Google Scholar] [CrossRef] [PubMed]
- Van, T.T.; Miller, J.; Warshauer, D.M.; Reisdorf, E.; Jernigan, D.; Humes, R.; Shulta, P.A. Pooling Nasopharyngeal/Throat Swab Specimens to Increase Testing Capacity for Influenza Viruses by PCR. J. Clin. Microbiol. 2012, 50, 891–896. [Google Scholar] [CrossRef]
- Hill, J.A.; HallSedlak, R.; Magaret, A.; Huang, M.L.; Zerr, D.M.; Jerome, K.R.; Boeckh, M. Efficient Identification of Inherited Chromosomally Integrated Human Herpesvirus 6 Using Specimen Pooling. J. Clin. Virol. 2016, 77, 71–76. [Google Scholar] [CrossRef]
- Abdalhamid, B.; Bilder, C.R.; Garrett, J.L.; Iwen, P.C. Cost Effectiveness of Sample Pooling to Test for SARS-CoV-2. J. Infect. Dev. Ctries. 2020, 14, 1136–1137. [Google Scholar] [CrossRef]
- Watkins, A.E.; Fenichel, E.P.; Weinberger, D.M.; Vogels, C.B.F.; Brackney, D.E.; Casanovas-Massana, A.; Campbell, M.; Fournier, J.; Bermejo, S.; Datta, R.; et al. Increased SARS-CoV-2 Testing Capacity with Pooled Saliva Samples. Emerg. Infect. Dis. 2021, 27, 1184–1187. [Google Scholar] [CrossRef]
- Abdalhamid, B.; Bilder, C.R.; McCutchen, E.L.; Hinrichs, S.H.; Koepsell, S.A.; Iwen, P.C. Assessment of Specimen Pooling to Conserve SARS CoV-2 Testing Resources. Am. J. Clin. Pathol. 2020, 153, 715–718. [Google Scholar] [CrossRef]
- Jeong, H.; Lee, J.; Cheon, S.; Sohn, K.M.; Kim, J.; Kym, S.; Kim, Y.S. Experimental and Mathematical Optimization of a Pooling Test for Detection of SARS-CoV-2 in a Population with Low Viral Load. Infect. Chemother. 2021, 53, 118–127. [Google Scholar] [CrossRef]
- Reolo, M.J.Y.; Eleazar, C.S.R.; Sonio, J.P.; Solon, R.T.; Villamor, J.L.B.; Loedin, A.K.; Moore, K.J.M. Saliva “Treat-and-Heat” Triplex Reverse Transcription Loop-Mediated Isothermal Amplification Assay for SARS-CoV-2. J. Biomol. Tech. 2021, 2, 186–198. [Google Scholar] [CrossRef] [PubMed]
- Rubio, C.P.; Franco-martínez, L.; Sánchez, C.; Torres-cantero, A.; Martinez-morata, I.; Bernal, E.; Alcaraz, M.J. Evaluation of different sample treatments options in a simple and safe procedure for the detection of SARS-COV-2 in saliva. Int. J. Infect. Dis. 2021, 108, 413–418. [Google Scholar] [CrossRef] [PubMed]
- Ochert, A.S.; Boulter, A.W.; Birnbaum, W.; Johnson, N.W.; Teo, C.G. Inhibitory Effect of Salivary Fluids on PCR: Potency and Removal. Genome Res. 1994, 3, 365–368. [Google Scholar] [CrossRef] [PubMed]
- Kandel, C.; Zheng, J.; McCready, J.; Serbanescu, M.A.; Racher, H.; Desaulnier, M.; Powis, J.E.; Vojdani, K.; Finlay, L.; Sheldrake, E.; et al. Detection of Sars-Cov-2 from Saliva as Compared to Nasopharyngeal Swabs in Outpatients. Viruses 2020, 12, 1314. [Google Scholar] [CrossRef] [PubMed]
| Post-Storage Testing | Pre-Storage Testing | Total | |
|---|---|---|---|
| (+) | (−) | ||
| (+) | 24 | 0 | 24 |
| (−) | 3 | 20 | 23 |
| Total | 27 | 20 | 47 |
| Overall agreement | 93.62% | ||
| Pools | Concordance to Individual Result (n) | Pools (n) | Overall Agreement (%) | |
|---|---|---|---|---|
| Concordant | Discordant | |||
| P4/1+ | 19 | 1 | 20 | 95.00 |
| P4/2+ | 16 | 0 | 16 | 100.00 |
| P4/3+ | 12 | 0 | 12 | 100.00 |
| P4/4+ | 7 | 0 | 7 | 100.00 |
| Total | 54 | 1 | 55 | 98.18 |
| Commercial Kit # | Concordant to Individuals (n) | Discordant to Individuals (n) | Invalid Specimens (n) | Overall Agreement (%) |
|---|---|---|---|---|
| XABT * | 38 | 1 | 0 | 97.43 |
| Ardent | 35 | 4 | 0 | 89.74 |
| Tianlong | 38 | 1 | 0 | 97.43 |
| SD Biosensor | 34 | 3 | 2 | 87.17 |
| BioSewoom | 34 | 5 | 0 | 87.17 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kaisar, M.M.M.; Jonnatan, S.; Widowati, T.A.; Kristin, H.; Vasandani, S.R.; Mahendra, C.; Ali, S. Accelerating the Laboratory Testing Capacity through Saliva Pooling Prior to Direct RT-qPCR for SARS-CoV-2 Detection. Diagnostics 2022, 12, 3160. https://doi.org/10.3390/diagnostics12123160
Kaisar MMM, Jonnatan S, Widowati TA, Kristin H, Vasandani SR, Mahendra C, Ali S. Accelerating the Laboratory Testing Capacity through Saliva Pooling Prior to Direct RT-qPCR for SARS-CoV-2 Detection. Diagnostics. 2022; 12(12):3160. https://doi.org/10.3390/diagnostics12123160
Chicago/Turabian StyleKaisar, Maria Mardalena Martini, Sheila Jonnatan, Tria Asri Widowati, Helen Kristin, Suraj Rajan Vasandani, Caroline Mahendra, and Soegianto Ali. 2022. "Accelerating the Laboratory Testing Capacity through Saliva Pooling Prior to Direct RT-qPCR for SARS-CoV-2 Detection" Diagnostics 12, no. 12: 3160. https://doi.org/10.3390/diagnostics12123160
APA StyleKaisar, M. M. M., Jonnatan, S., Widowati, T. A., Kristin, H., Vasandani, S. R., Mahendra, C., & Ali, S. (2022). Accelerating the Laboratory Testing Capacity through Saliva Pooling Prior to Direct RT-qPCR for SARS-CoV-2 Detection. Diagnostics, 12(12), 3160. https://doi.org/10.3390/diagnostics12123160






